Characterizing Biomass Yield and Nutritional Value of Selected Indigenous Range Species from Arid Tunisia
Abstract
:1. Introduction
2. Results
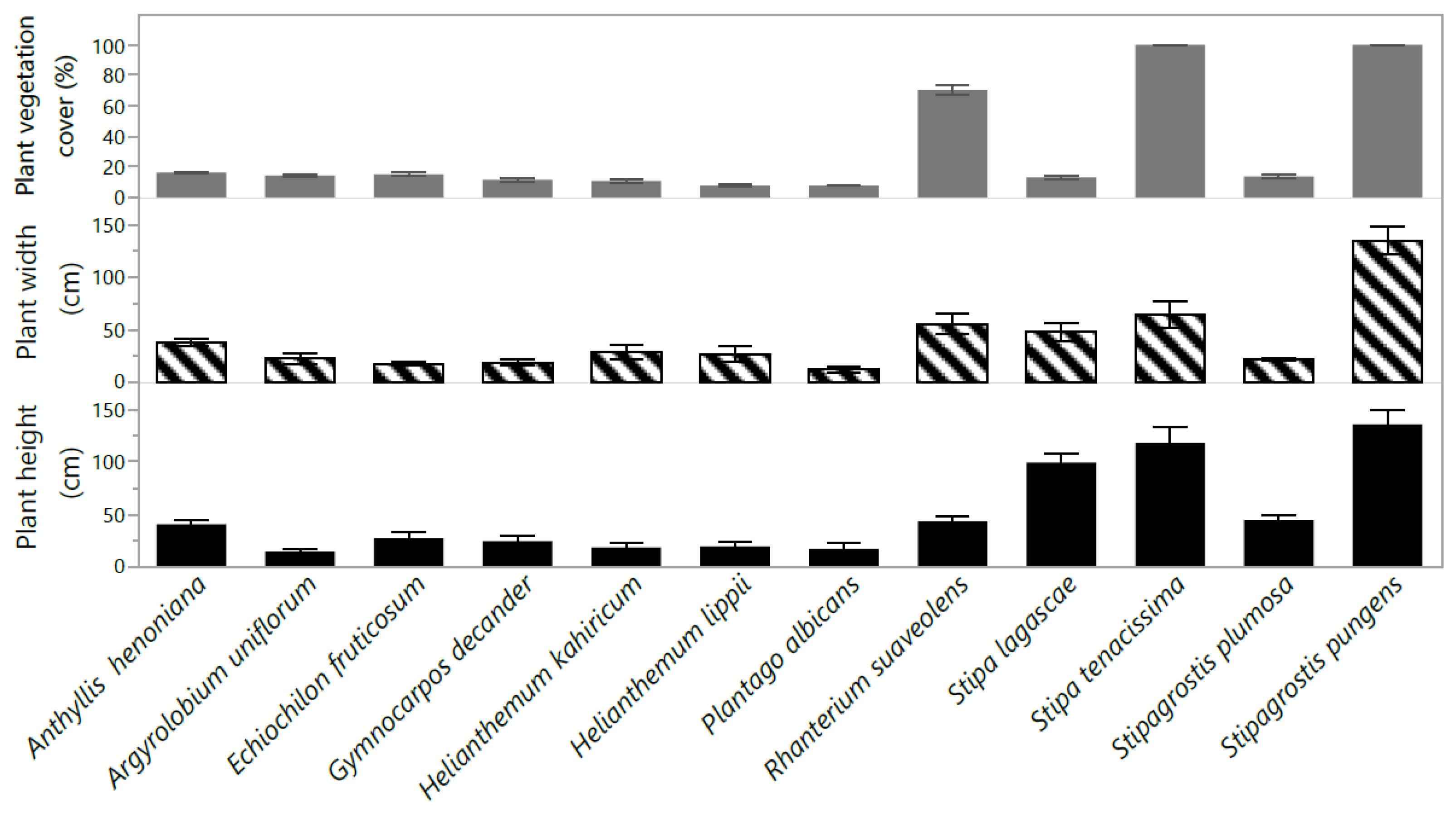
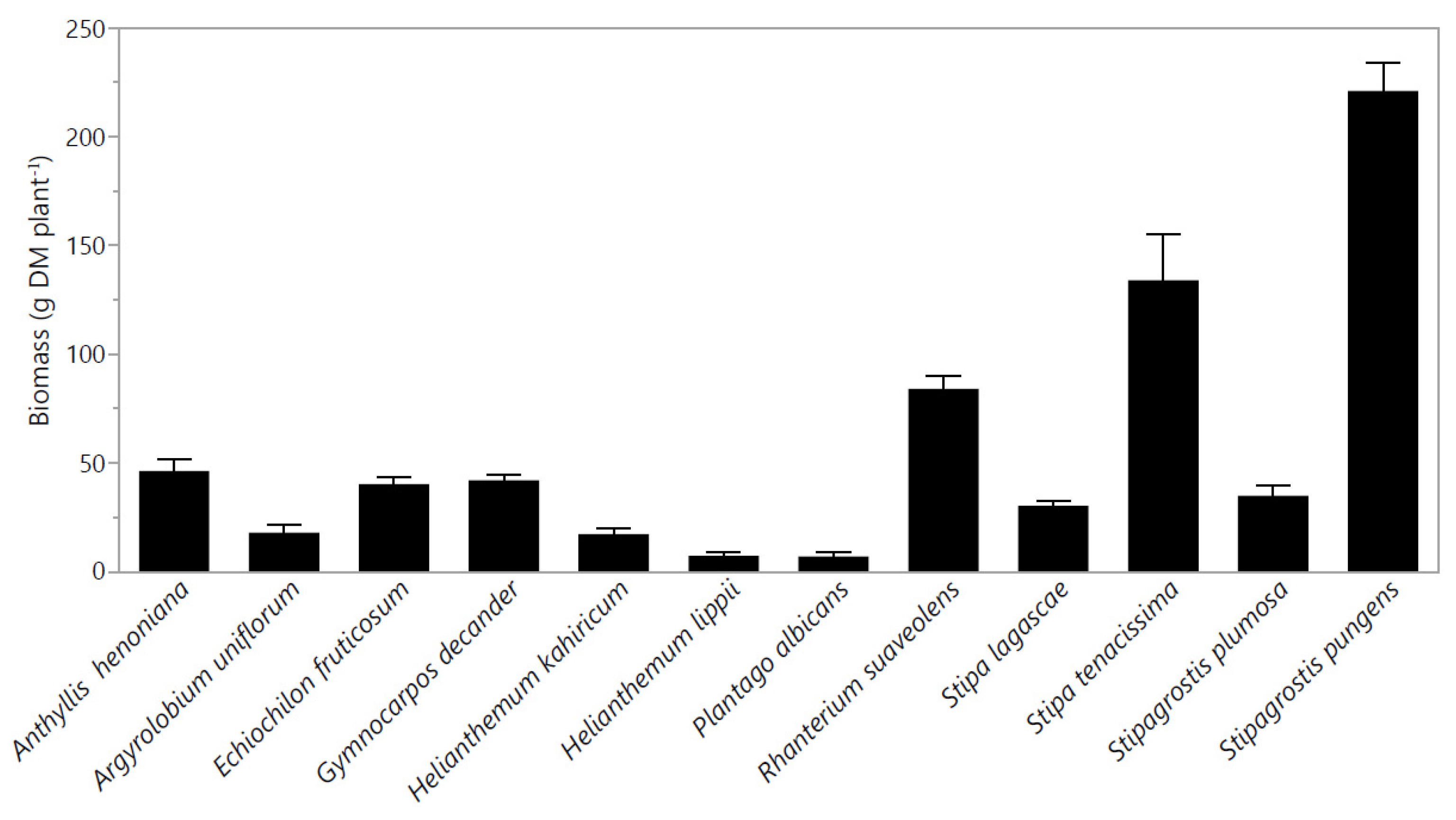
2.1. Plant Growth and Yield Attributes
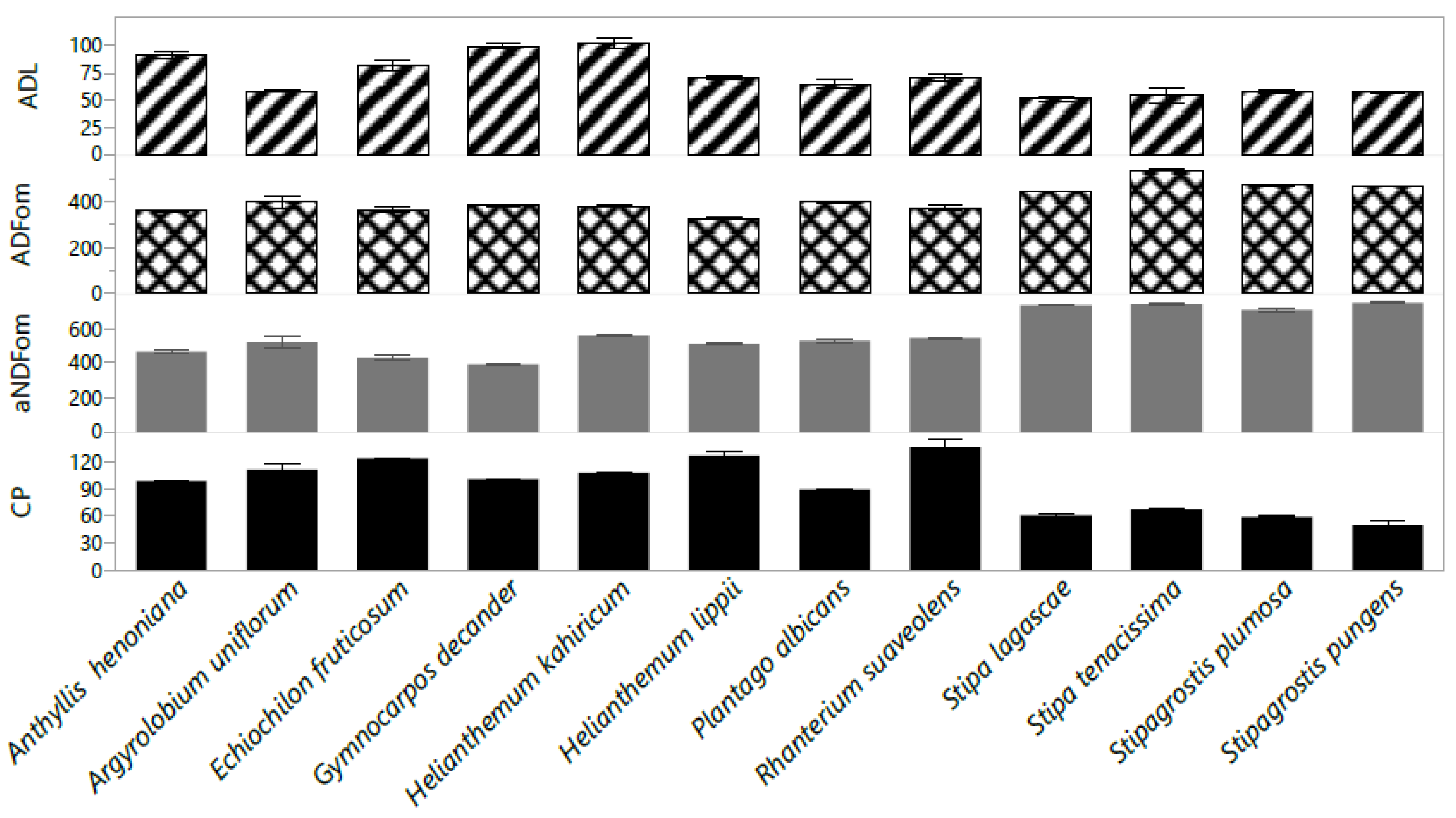
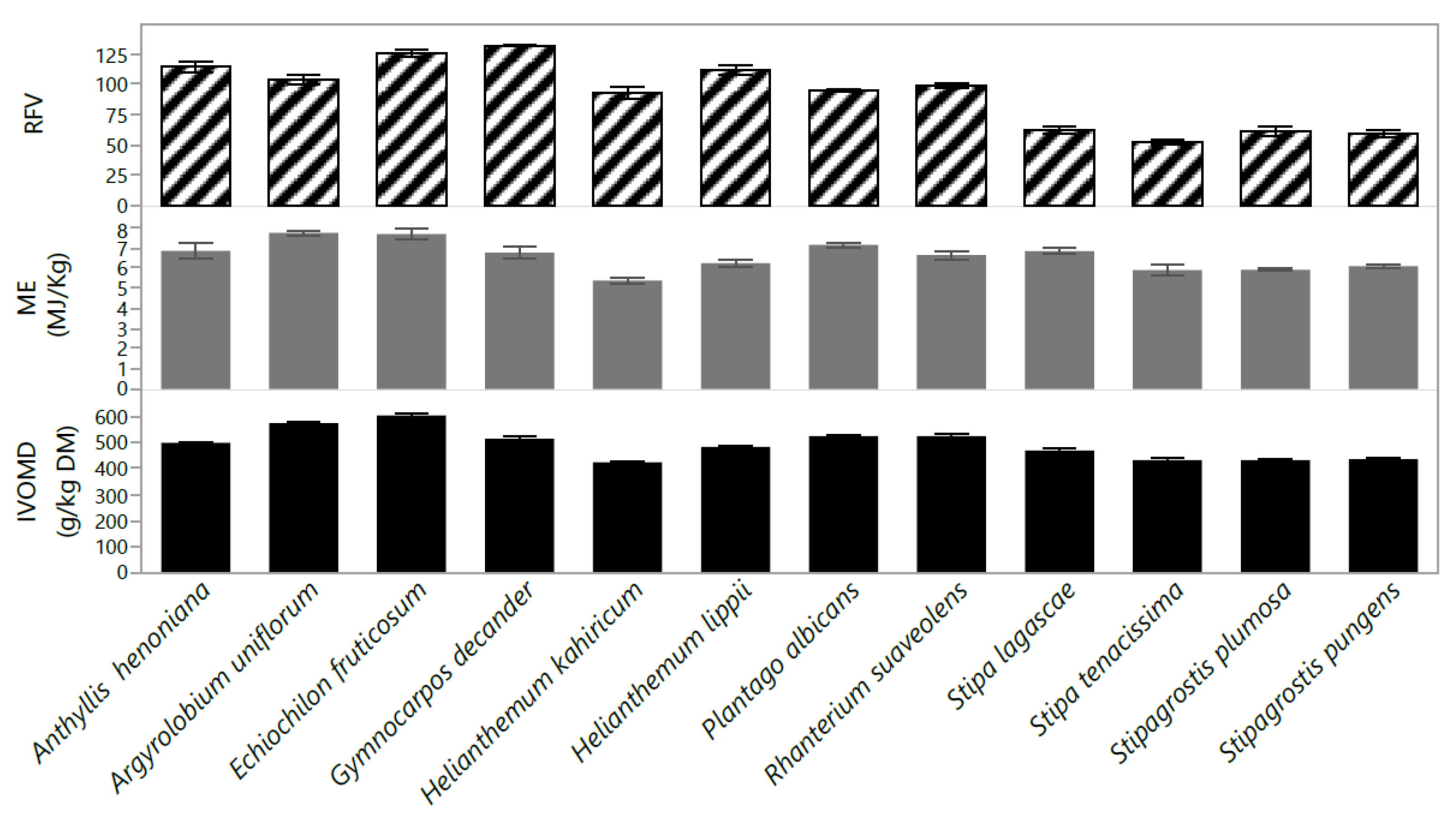
2.2. Chemical Composition
2.3. Mineral Composition
2.4. Classification of Species
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Species Collection
4.2.1. Anthyllis henoniana Coss. ex Batt.
4.2.2. Argyrolobium uniflorum (Deene.) Jaub. and Spach
4.2.3. Echiochilon fruticosum Desf.
4.2.4. Gymnocarpos decander Forssk.
4.2.5. Helianthemum kahiricum Delile
4.2.6. Helianthemum lippii (L.) Dum. Cours.
4.2.7. Plantago albicans L.
4.2.8. Rhanterium suaveolens Desf.
4.2.9. Stipa lagascae R. and Sch
4.2.10. Stipa tenacissima L.
4.2.11. Stipagrostis plumosa (L.) Munro ex T. Anderson
4.2.12. Stipagrostis pungens (Desf.) de Winter
4.3. Sampling Procedure
4.4. Proximate Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Are Grasslands under Threat? Brief Analysis of FAO Statistical Data on Pasture and Fodder Crops. 2008. Available online: http://www.fao.org/uploads/media/grass_stats_1.pdf (accessed on 29 July 2021).
- Gamoun, M.; Ouled Belgacem, A.; Louhaichi, M. Diversity of desert rangelands of Tunisia. Plant Divers. 2018, 40, 217–225. [Google Scholar] [CrossRef]
- Gamoun, M.; Louhaichi, M. Botanical composition and species diversity of arid and desert rangelands in Tataouine, Tunisia. Land 2021, 10, 313. [Google Scholar] [CrossRef]
- Gamoun, M. Management and resilience of Saharan rangelands: South Tunisia. Fourrages 2013, 216, 321–328. [Google Scholar]
- Abdullah, M.; Rafay, M.; Hussain, T.; Ahmad, H.; Tahir, U.; Rasheed, F.; Ruby, T.; Khalil, S. Nutritive potential and palatability preference of browse foliage by livestock in arid rangelands of Cholistan desert (Pakistan). Anim. Plant Sci. 2017, 27, 1656–1664. [Google Scholar]
- Louhaichi, M.; Salkini, A.K.; Petersen, S.L. Effect of small ruminant grazing on the plant community characteristics of semi-arid Mediterranean ecosystems. Int. J. Agric. Biol. 2009, 11, 681–689. [Google Scholar]
- Gondard, H.; Jauffret, S.; Aronson, J.; Lavorel, S. Plant functional types: A promising tool for management and restoration of degraded lands. Appl. Veg. Sci. 2003, 6, 223–234. [Google Scholar] [CrossRef]
- Ouled Belgacem, A.; Louhaichi, M. The vulnerability of native rangeland plant species to global climate change in the West Asia and North African regions. Clim. Change 2013, 119, 451–463. [Google Scholar] [CrossRef] [Green Version]
- Jauffret, S.; Lavorel, S. Are plant functional types relevant to describe degradation in arid, southern Tunisian steppes? J. Veg. Sci. 2003, 14, 399–408. [Google Scholar] [CrossRef]
- Ouled Belgacem, A.; Tarhouni, M.; Louhaichi, M. Effect of protection on plant dynamics in the Mediterranean arid zone of southern Tunisia: The case of Bou Hedma national park. Land Degrad. Dev. 2013, 24, 57–62. [Google Scholar] [CrossRef]
- Gamoun, M. Grazing intensity effects on the vegetation in desert rangelands of Southern Tunisia. J. Arid Land 2014, 6, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Ouled Belgacem, A.; Ben Salem, F.; Gamoun, M.; Chibani, R.; Louhaichi, M. Revival of traditional best practices for rangeland restoration under climate change in the dry areas: A case study from Southern Tunisia. Int. J. Clim. Chang. Strateg. Manag. 2019, 11, 643–659. [Google Scholar] [CrossRef]
- Tarhouni, M.; Ben Hmida, W.; Ouled Belgacem, A.; Louhaichi, M.; Neffati, M. Is long-term protection useful for the regeneration of disturbed plant communities in dry areas? Afr. J. Ecol. 2017, 55, 509–517. [Google Scholar] [CrossRef]
- Le Houérou, H.N. Browse in Africa: The current state of knowledge. In Proceedings of the International Symposium on Browse in Africa, Addis Ababa, Etiopia, 8–12 April 1980; International Livestock Center for Africa: Addis Ababa, Ethiopia, 1980. [Google Scholar]
- Crowder, L.V.; Cheddah, H.R. Tropical Grassland Husbandry; Longman Inc: New York, NY, USA, 1982. [Google Scholar]
- Le Houérou, H.N.; Haywood, M.; Claudin, J. Etude Phytoécologique du Hodna; AGS: DP/ALG/66/509; FAO: Rome, Italy, 1974. [Google Scholar]
- Floret, C.; Pontanier, R. Aridité climatique, aridité édaphique. Actual. Bot. 1984, 131, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Zaafouri, M.S. Contraintes du Milieu et Réponses de Quelques Espèces Arbustives Exotiques Introduites en Tunisie Présaharienne. Ph.D. Thesis, Aix-Marseille University, Marseille, France, 1993. [Google Scholar]
- Neffati, M.; Akrimi, N. Gene Bank of spontaneous plants of the desert and arid zones of Tunisia. Plant Genet. Resour. Newsl. 1996, 108, 26–32. [Google Scholar]
- Schut, A.G.T.; Gherardi, S.G.; Wood, D.A. Empirical models to quantify the nutritive characteristics of annual pastures in south-west Western Australia. Crop Pasture Sci. 2010, 61, 32–43. [Google Scholar] [CrossRef]
- Gwelo, F.A. Farmers’ Perceptions of Livestock Feeding and Rangeland Management; Dynamics of Soil, Forage and Cattle Blood Serum Mineral Levels in Two Communal Areas in the Eastern Cape, South Africa. Ph.D. Thesis, University of Fort Hare, Alice, South Africa, 2013. [Google Scholar]
- Singh, S.; Bhat, B.V.; Shukla, G.P.; Singh, K.; Gehrana, D. Variation in carbohydrate and protein fractions, energy, digestibility and mineral concentrations in stover of sorghum cultivars. Trop. Grassl. Forrajes Trop. 2018, 6, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Dehghani Bidgoli, R. Forage quality of Calligonum comosum in three phenological growth stages (Case study: Kashan rangelands, Iran). J. Rangel. Sci. 2018, 8, 309–314. [Google Scholar]
- Zhao, G.Q.; Wei, S.N.; Li, Y.F.; Jeong, E.C.; Kim, H.J.; Kim, J.G. Comparison of forage quality, productivity and β-carotene content according to maturity of forage rye (Secale cereale L.). J. Kor. Grassl. Forage. Sci. 2020, 40, 123–130. [Google Scholar] [CrossRef]
- Arrekhi, A.; Gharmakher, H.N.G.; Bachinger, J.; Bloch, R.; Hufnagel, J. Forage Quality of Salsola turcomanica (Litv) in Semi-arid region of Gomishan, Golestan Province, Iran. J. Rangel. Sci. 2021, 11, 74–86. [Google Scholar]
- Lee, M.A. A global comparison of the nutritive values of forage plants grown in contrasting environments. J. Plant Res. 2018, 131, 641–654. [Google Scholar] [CrossRef]
- Martiniello, P.; Teixeira da Silva, J.A. Physiological and bioagronomical aspects involved in growth and yield components of cultivated forage species in Mediterranean environments: A review. Eur. J. Plant Sci. Biotechnol. 2011, 5, 64–98. [Google Scholar]
- Newman, Y.C.; Lambert, B.; Muir, J.P. Defining forage quality. EDIS Publication SS-AGR-322; Agronomy Department, UF/IFAS Extension Service: Gainesville, FL, USA, 2009; Available online: http://publications.tamu.edu/FORAGE/PUB_forage_Defining%20Forage%20Quality.pdf (accessed on 29 July 2021).
- Asaadi, A.M.; Dadkhah, A.R. The study of forage quality of Haloxylon aphyllum and Eurotia ceratoides in different phenological stages. Res. J. Biol. Sci. 2010, 5, 470–475. [Google Scholar] [CrossRef]
- Waldron, B.L.; Greenhalgh, L.K.; ZoBell, D.R.; Olson, K.C.; Davenport, B.W.; Palmer, M.D. Forage kochia (Kochia prostrata) increases nutritional value, carrying capacity, and livestock performance on semiarid rangelands. Forage Grazinglands 2011, 9, 1–6. [Google Scholar] [CrossRef]
- Amiri, F.; Shari, A.R.M. Comparison of nutritive values of grasses and legume species using forage quality index. Songklanakarin J. Sci. Technol. 2012, 34, 577–586. [Google Scholar]
- Olowu, O.O.; Yaman Firincioğlu, S. Feed Evaluation methods: Performance, economy and environment. Eurasian J. Agric. Res. 2019, 3, 48–57. [Google Scholar]
- Hussain, F.; Durrani, M.J. Nutritional evaluation of some forage plants from Harboi rangeland, Kalat, Pakistan. Pak. J. Bot. 2009, 41, 1137–1154. [Google Scholar]
- Mahmoud, A.E.; Abbas, M.S.; Cieslak, A.; Szumacher-Strabel, M. Evaluation of chemical composition and in vitro dry and organic matter digestibility of some forage plant species derived from Egyptian rangelands. J. Anim. Plant Sci. 2017, 27, 1573–1581. [Google Scholar]
- Julian, A.A.M.; Scasta, J.D.; Stam, B.R.; Sebade, B.M.; Page, C.M.; Springer, B.E.; Renner, W.T.; Cunningham-Hollinger, H.; Stewart, W.C. Mineral element concentrations of common grass and shrub species on sheep winter range in Wyoming: Insights for mineral supplementation strategies. Transl. Anim. Sci. 2020, 4 (Suppl. 1), S11–S16. [Google Scholar] [CrossRef] [PubMed]
- Louhaichi, M.; Gamoun, M. Rhanterium suaveolens Desf: A Keystone Species Critical to Rangeland Structure and Functioning. Managing Rangelands: Promoting Native Shrub Species. 2018. Available online: https://hdl.handle.net/20.500.11766/8494 (accessed on 29 July 2021).
- Cook, C.W.; Stubbendieck, J. Range Research: Basic Problems and Techniques; Society for Range Management: Denver, CO, USA, 1986; p. 317. [Google Scholar]
- Njidda, A.A. Chemical composition, fibre fraction and anti-nutritional substances of semi-arid browse forages of North-Eastern Nigeria. Nig. J. Basic Appl. Sci. 2010, 18, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Salem, A.Z.M.; Salem, M.Z.M.; El-Adawy, M.M.; Robinson, P.H. Nutritive evaluations of some browse tree foliages during the dry season: Secondary compounds, feed intake and in vivo digestibility in sheep and goats. Anim. Feed Sci. 2006, 127, 251–267. [Google Scholar] [CrossRef]
- Salah, N.; Sauvant, D.; Archimède, H. Nutritional requirements of sheep, goats and cattle in warm climates: A meta-analysis. Animal 2014, 8, 1439–1447. [Google Scholar] [CrossRef]
- Tufarelli, V.; Cazzato, E.; Ficco, A.; Laudadio, V. Assessing nutritional value and in vitro digestibility of Mediterranean pasture species using yak (Bos grunniens) faeces as alternative microbial inoculum in a Daisy incubator. J. Food Agric. Environ. 2010, 8, 477–481. [Google Scholar]
- Boufennara, S.; Lopez, S.; Bousseboua, H.; Rodríguez, R.B.; Bouazza, L. Chemical composition and digestibility of some browse plant species collected from Algerian arid rangelands. Span. J. Agric. Res. 2012, 1, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Foroughbakhch, R.; Hernandez-Pinero, J.L.; Carrillo-Parra, A. Nutrient profile, floristic compositions and preference index of shrubs and herbs consumed by goats in semiarid region of Northeastern Mexico. J. Anim. Vet. Adv. 2012, 11, 1299–1305. [Google Scholar] [CrossRef] [Green Version]
- Bidgoli, R.D.; Ranjbarfordoei, A. Vegetation types and rangeland species nutritional values and forage quality indicators at various phonological stages. Int. J. Plant Physiol. Biochem. 2013, 5, 16–24. [Google Scholar]
- Elgersma, A.; Søegaard, K. Changes in nutritive value and herbage yield during extended growth intervals in grass–legume mixtures: Effects of species, maturity at harvest, and relationships between productivity. Grass Forage Sci. 2016, 73, 78–93. [Google Scholar] [CrossRef]
- Megersa, E.; Mengistu, A.; Asebe, G. Nutritional characterization of selected fodder species in Abol and Lare districts of Gambella region, Ethiopia. J. Nutr. Food Sci. 2017, 7, 1000581. [Google Scholar] [CrossRef]
- Muhakka, M.M.; Suwignyo, R.A.; Budianta, D. Nutritional values of swamp grasses as feed for Pampangan Buffaloes in South Sumatra, Indonesia. Biodiversitas 2020, 21, 956–967. [Google Scholar] [CrossRef]
- da Silva Pause, A.G.; de Souza França, A.F.; Okada, E.S.M.; Gandra, J.R.; Dupas, E.; de Oliveira, E.R.; Ferreira, J.L.; Marques, O.F.C. Nutritional value of Mombasa grass submitted to different grazing heights and nitrogen fertilization. Braz. J. Anim. Environ. Res. 2021, 4, 860–874. [Google Scholar] [CrossRef]
- Katongole, C.B.; Lumu, R.; Lindberg, J.E. Comparative chemical composition and rumen degradation of common reed and elephant grass in urban/peri-urban dairying systems in Uganda. Agroecol. Sustain. Food Syst. 2021, 45, 892–906. [Google Scholar] [CrossRef]
- Mosisa, A.; Nurfeta, A.; Bezabih, M.; Tolera, A.; Mengistu, S.; Yigrem, S.; Hassen, A. Assessment of botanical composition, biomass yield, nutritional quality and methane production of forages in selected grasslands, southern highlands of Ethiopia. Sci. Afr. 2021, 12, e00726. [Google Scholar] [CrossRef]
- Chakravarthi, M.K.; Reddy, Y.R.; Rao, K.S.; Ravi, A.; Punyakumari, B.; Ekambaram, B. A study on nutritive value and chemical composition of sorghum fodder. Int. J. Sci. Environ. Technol. 2017, 6, 104–109. [Google Scholar]
- Holechek, J.L.; Pieper, R.D.; Herbel, C.H. Range Management Principles and Practices; Prentice Hall: Englewood Cliff, NJ, USA, 2001. [Google Scholar]
- Bezabih, M.; Pellikaan, W.F.; Tolera, A.; Khan, N.A.; Hendriks, W.H. Chemical composition and in vitro total gas and methane production of forage species from the Mid Rift Valley grasslands of Ethiopia. Grass Forage Sci. 2014, 69, 635–643. [Google Scholar] [CrossRef]
- Evitayani, L.; Warly, L.; Fariani, A.; Ichinohe, T.; Abdulrazak, S.A.; Hayashida, M.; Fujihara, T. Study on nutritive value of tropical forages in North Sumatra, Indonesia. Anim. Sci. J. 2005, 76, 461–468. [Google Scholar] [CrossRef]
- Andualem, D.; Negesse, T.; Tolera, A. Chemical composition, in vitro organic matter digestibility and kinetics of rumen dry matter degradability of morphological fractions of stinging nettle (Urticasimensis). Adv. Biol. Res. 2016, 10, 183–190. [Google Scholar] [CrossRef]
- Rahim, I.; Sultan, J.I.; Sharif, M.; Bilal, M.Q. Chemical composition, mineral profile, palatability and in vitro digestibility of shrubs. J. Anim. Plant. Sci. 2013, 23, 45–49. [Google Scholar]
- Castro, M.; Teixeira, A.; Fernández-Núñez, E. The nutritive value of different Mediterranean browse species used as animal feeds under oak silvopastoral systems in Northern Portugal. Agrofor. Syst. 2021, 95, 269–278. [Google Scholar] [CrossRef]
- Flores, M.P.; Robles Cruz, A.B.; Rodríguez, R.; Ventura, M.R.; Caravaca, F.P. Effect of season on chemical composition and in vitro digestibility of six native forage shrubs species grazed by goats in protected areas in Canary Islands, Spain. Int. J. Agric. Biol. 2020, 23, 49–53. [Google Scholar] [CrossRef]
- Akbag, H.I. Potential nutritive value of Anagyris foetida shrub for goats. Agrofor. Syst. 2021, 95, 191–200. [Google Scholar] [CrossRef]
- Chebli, Y.; El Otmani, S.; Chentouf, M.; Hornick, J.L.; Cabaraux, J.F. Temporal variations in chemical composition, in vitro digestibility, and metabolizable energy of plant species browsed by goats in southern mediterranean forest rangeland. Animals 2021, 11, 1441. [Google Scholar] [CrossRef] [PubMed]
- Hutjens, M.F. Revisiting feed efficiency and its economic impact. In Proceedings of the Four-State Dairy Nutrition and Management Conference, Midwest Plan Service, Iowa State University, Ames, IA, USA, 6 June 2005; pp. 177–182. [Google Scholar]
- Decruyenaere, V.; Buldgen, A.; Stilmant, D. Factors affecting intake by grazing ruminants and related quantification methods: A review. Biotechnol. Agron. Soc. Environ. 2009, 13, 559–573. [Google Scholar]
- Balamurugan, B.; Ramamoorthy, M.; Mandal, R.S.K.; Keerthana, J.; Gopalakrishnan, G.; Kavya, K.; Katiyar, R. Mineral an important nutrient for efficient reproductive health in dairy cattle. Int. J. Environ. Sci. Technol. 2017, 6, 694–701. [Google Scholar]
- Hadiya, K.K.; Derashri, H.J.; Devalia, B.R.; Jani, R.G. Effect of supplementation of minerals and enzymes on service period and postpartum plasma minerals profile in crossbred cows. Vet. World. 2010, 3, 173–176. [Google Scholar]
- Dambe, L.M.; Mogotsi, K.; Odubeng, M.; Kgosikoma, O.E. Nutritive value of some important indigenous livestock browse species in semi-arid mixed Mopane bushveld, Botswana. Livest. Res. Rural. Dev. 2015, 27, 1–10. [Google Scholar]
- Suttle, N.F. Mineral Nutrition of Livestock; CABI Publishing: Wallingford, UK, 2010. [Google Scholar]
- Zervas, G. Feeding of Ruminants; Stamoulis Publications: Athens, Greece, 2013. (In Greek) [Google Scholar]
- Mata-González, R.; Hovland, M.; Abdallah, M.A.B.; Martin, D.W.; Noller, J.S. Nutrient uptake and gas exchange of Great Basin plants provide insight into drought adaptations and coexistence. Plant Ecol. 2021, 14, 854–869. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Small Ruminants; National Academic Press: Washington, DC, USA, 2007; pp. 271–280. [Google Scholar]
- DGF-Direction Générale des Forêts de Tunis. Résultats du Deuxième Inventaire Forestier et Pastoralnational, Inventaire des Forêts par Télédétection (IFPN); Direction Générale des Forêts de Tunis: Tunis, Tunisia, 2010; p. 195. [Google Scholar]
- Derbel, S.; Chaieb, M. Growth establishment and phenology of four woody Saharan species. Afr. J. Ecol. 2013, 51, 307–318. [Google Scholar] [CrossRef]
- Aronson, J.; Floret, C.; Le Floc’h, E.; Ovalle, C.; Pontanier, R. Restoration and rehabilitation of degraded ecosystems in arid and semi-arid lands. II. Case studies from Southern Tunisia, Central Chile and Northern Cameroon. Restor. Ecol. 1993, 1, 168–187. [Google Scholar] [CrossRef]
- Le Houérou, H.N. The nature and causes of desertization. In Desertification; Glantz, M., Ed.; Westview Press: Boulder, CO, USA, 1977; pp. 16–38. [Google Scholar]
- Heneidy, S.Z. A Study of the Nutrient Content and Nutritive Values of Range of Plants at Omayed, Egypt. Master’s Thesis, Alexandria University, Alexandria, Egypt, 1986. [Google Scholar]
- Raynaud, C. Atlanthemum Raynaud, un nouveau genre pour la famille des Cistaceae. An. Jard. Bot. Madr. 1987, 44, 309–317. [Google Scholar]
- Ozenda, P. Flore et Végétation du Sahara, 3rd ed.; CNRS: Paris, France, 1977. [Google Scholar]
- Hsia, C.N.; Korban, S. Effect of growth regulators, dark treatment and light intensity on shoot organogenesis from leaf tissues of evergreen azalea. J. Hortic. Sci. Biotechnol. 1998, 73, 53–60. [Google Scholar] [CrossRef]
- Radice, S.; Caso, O.H. In vitro organogenesis in leaves of azaleas ‘Petrick’ and ‘Rex’. Sci. Hortic. 1990, 41, 343–347. [Google Scholar] [CrossRef]
- Le Floc’h, E. Contribution à une Étude Ethnobotanique de la Flore Tunisienne; Imprimerie Officielle de la République Tunisienne: Tunis, Tunisia, 1983. [Google Scholar]
- Slama, A.; Fortas, Z.; Boudabous, A.; Neffati, M. Cultivation of an edible desert truffle (Terfezia boudieri Chatin). Afr. J. Microbiol. Res. 2010, 4, 2350–2356. [Google Scholar]
- Ermeli, N.B.; Alsabri, S.G.; Bensaber, S.M.; Mohamed, S.B.; Zetrini, A.A.; Aburas, K.M.; Fitouri, S.R.; Jaeda, M.I.; Mrema, I.A.; Hermann, A.; et al. Screening of analgesic and anti-inflammatory activities for two Libyan medicinal plants: Helianthemum lippii and Launaea residifolia. J. Chem. Pharm. Res. 2012, 4, 4201–4205. [Google Scholar]
- Alsabri, S.G.; Rmeli, N.B.; Zetrini, A.A.; Mohamed, S.B.; Meshri, M.I.; Aburas, K.M.; Bensaber, S.M.; Mrema, I.A.; Mosbah, A.A.; Allahresh, K.A.; et al. Phytochemical, anti-oxidant, anti-microbial, anti-inflammatory and anti-ulcer properties of Helianthemum lippii. J. Pharmacogn. Phytochem. 2013, 2, 86–96. [Google Scholar]
- Heneidy, S.Z.; Halmy, M.W.A.; Bidak, L.M. The ethnobotanical importance and conservation value of native plants in eastern Arabian Peninsula. Feddes Repert 2017, 128, 105–128. [Google Scholar] [CrossRef]
- Kartini, S.P.; Thongpraditchote, S.; Siripong, P.; Vallisuta, O. Effects of Plantago major extracts and its chemical compounds on proliferation of cancer cells and cytokines production of lipopolysaccharide-activated THP-1 macrophages. Pharmacogn. Mag. 2017, 13, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Eldesoky, A.H.; Abdel-Rahman, R.F.; Ahmed, O.K.; Soliman, G.A.; Saeedan, A.S.; Elzorba, H.Y.; Elansary, A.A.; Hattori, M. Antioxidant and hepatoprotective potential of Plantago major growing in Egypt and its major phenylethanoid glycoside, acteoside. J. Food Biochem. 2018, 42, e12567. [Google Scholar] [CrossRef]
- Farcaș, A.D.; Moț, A.C.; Pârvu, A.E.; Toma, V.A.; Popa, M.A.; Mihai, M.C.; Sevastre, B.; Roman, L.; Vlase, L.; Pârvu, M. In vivo pharmacological and anti-inflammatory evaluation of Xerophyte Plantago sempervirens crantz. Oxid. Med. Cell. Longev. 2019, 2019, 13. [Google Scholar] [CrossRef]
- Scholz, H. Stipa tunetana, eine neue Artaus Tunesien, und das St. lagascae Aggregat (Gramineae). Willdenowia 1991, 26, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Neffati, M. Caractérisation Morpho-Biologique de Quelques Espèces Végétales Nord Africaines: Implication Pour L’amélioration Pastorale. Ph.D. Thesis, University of Gent, Gent, Belgium, 1994. [Google Scholar]
- Le Floc’h, E.; Neffati, M.; Chaieb, M.; Floret, C.; Pontanier, R. Rehabilitation experiment at Menzel Habib, Southern Tunisia. Arid Soil Res. Rehabil. 1999, 13, 357–368. [Google Scholar] [CrossRef]
- Boughalleb, F.; Abdellaoui, R.; Hadded, Z.; Neffati, M. Anatomical adaptations of the desert species Stipa lagascae against drought stress. Biologia 2015, 70, 1042–1052. [Google Scholar] [CrossRef]
- Ouled Belgacem, A.; Neffati, M.; Papanastasis, V.P.; Chaieb, M. Effects of seed age and seeding depth on growth of Stipa lagascae R. & Sch. seedlings. J. Arid Environ. 2006, 65, 682–687. [Google Scholar] [CrossRef]
- Danin, A. Plants of desert dunes. In Cloudsley—Adaptations of Organisms to the Desert; Thompson, J.L., Ed.; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Louhaichi, M.; Hassan, S.; Clifton, K.; Johnson, D.E. A reliable and non-destructive method for estimating forage shrub cover and biomass in arid environments using digital vegetation charting technique. Agrofor. Syst. 2018, 92, 1341–1352. [Google Scholar] [CrossRef] [Green Version]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Tilley, J.M.A.; Terry, R.A. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feeding stuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Rivera, J.D.; Parish, J.A. Interpreting Forage and Feed Analysis Reports. Extension Service of Mississippi State University, Cooperating with U.S. 2010. Available online: https://extension.msstate.edu/sites/default/files/publications/publications/p2620.pdf (accessed on 29 July 2021).
- Undersander, D.; Moore, J.E.; Schneider, N. Relative forage quality. Focus Forag. 2002, 4, 1–2. [Google Scholar] [CrossRef]
- Geng, Y.; Ranjitkar, S.; Yanet, Q. Nutrient value of wild fodder species and the implications for improving the diet of mithun (Bos frontalis) in Dulongjiang area, Yunnan Province. China Plant Divers. 2020, 42, 455–463. [Google Scholar] [CrossRef]
- Esbati, M.; Farzadmehr, J.; Foroughi, A.; Rahdari, M.R.; Rodrigo-Comino, J. Assessment of the nutritional value of Gundelia tournefortii during its growth stages as a key element in the Senowbar rangeland ecosystem, Northeast of Iran. Int. J. Environ. Sci. Technol. 2021, 18, 1731–1738. [Google Scholar] [CrossRef]
- Habte, M.; Eshetu, M.; Andualem, D.; Maryo, M.; Legesse, A. The inventory of camel feed resource and the evaluation of its chemical composition in south-east rangelands of Ethiopia. Vet. Med. Sci. 2021, 7, 1172–1184. [Google Scholar] [CrossRef]
- Stalling, C.C. Test Available for Measuring Forage Quality. Virginia Cooperative Extension Dairy Guideline 404–424. 2005. Available online: https://vtechworks.lib.vt.edu/handle/10919/48380 (accessed on 29 July 2021).
- Oddy, V.H.; Robards, G.E.; Low, S.G. Prediction of in vivo dry matter digestibility from the fiber nitrogen content of a feed. In Feed Information and Animal Production; Robards, G.E., Packham, R.G., Eds.; Commonwealth Agricultural Bureaux: Farnham Royal, UK, 1983; pp. 395–398. [Google Scholar]

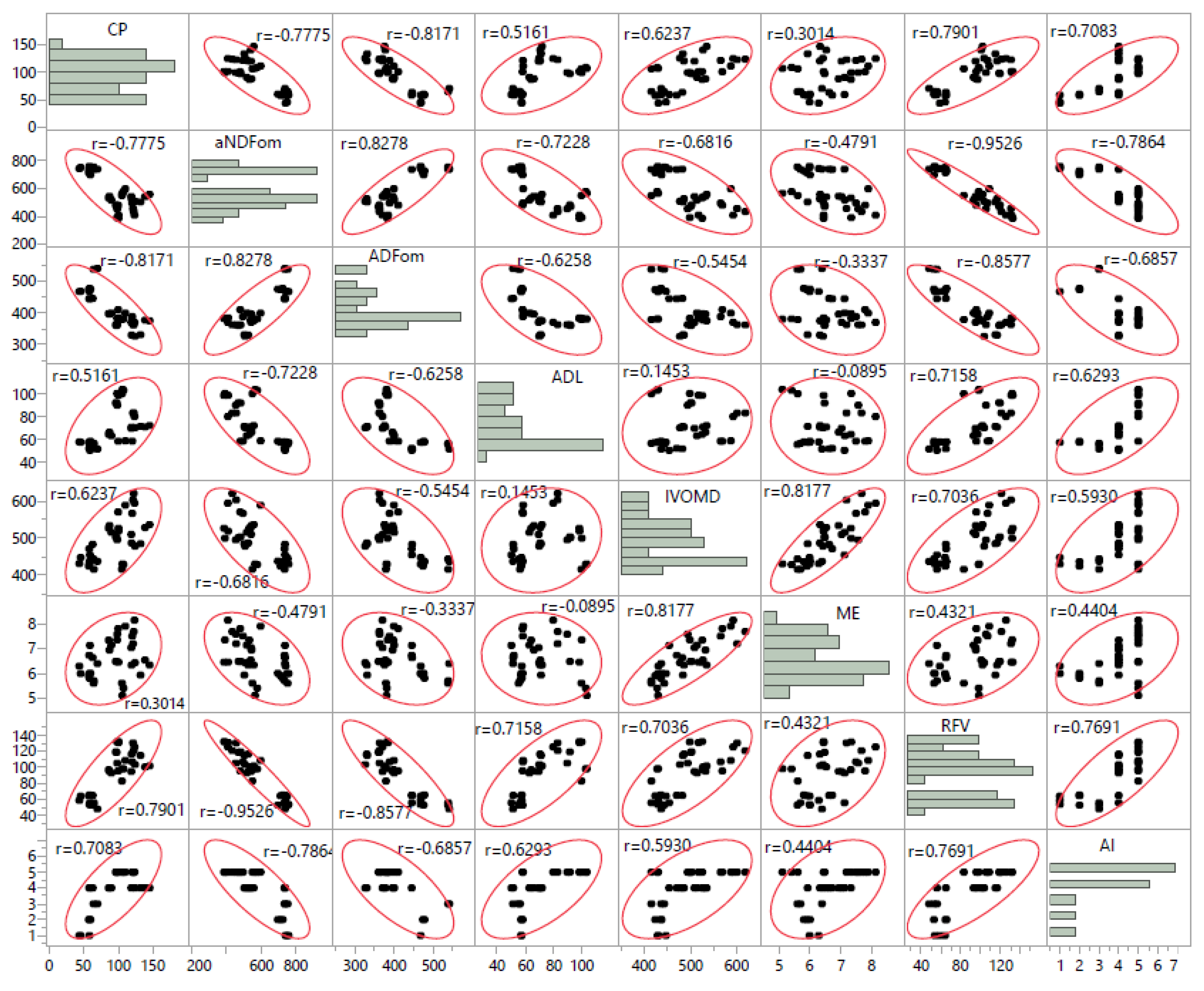
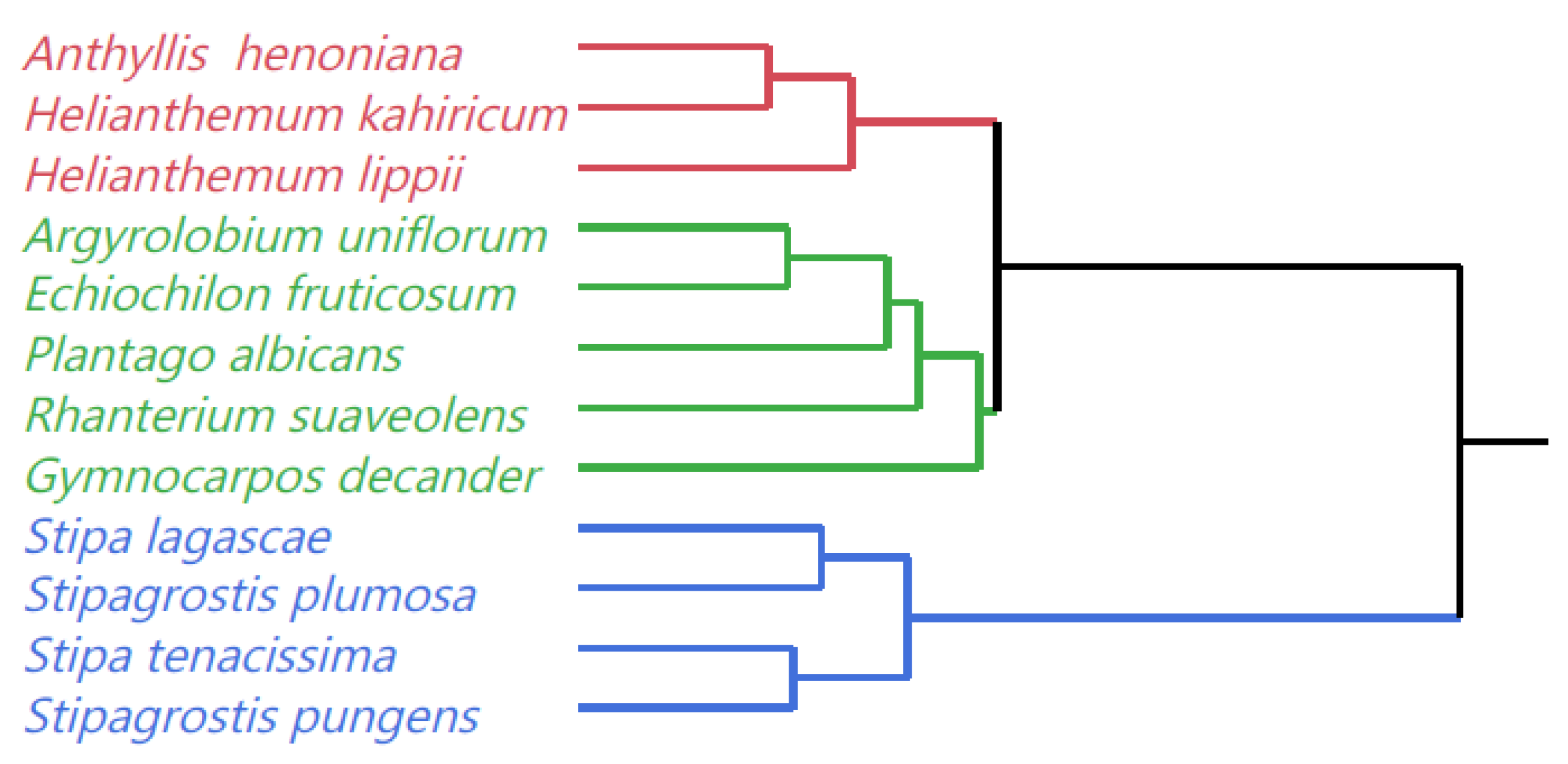
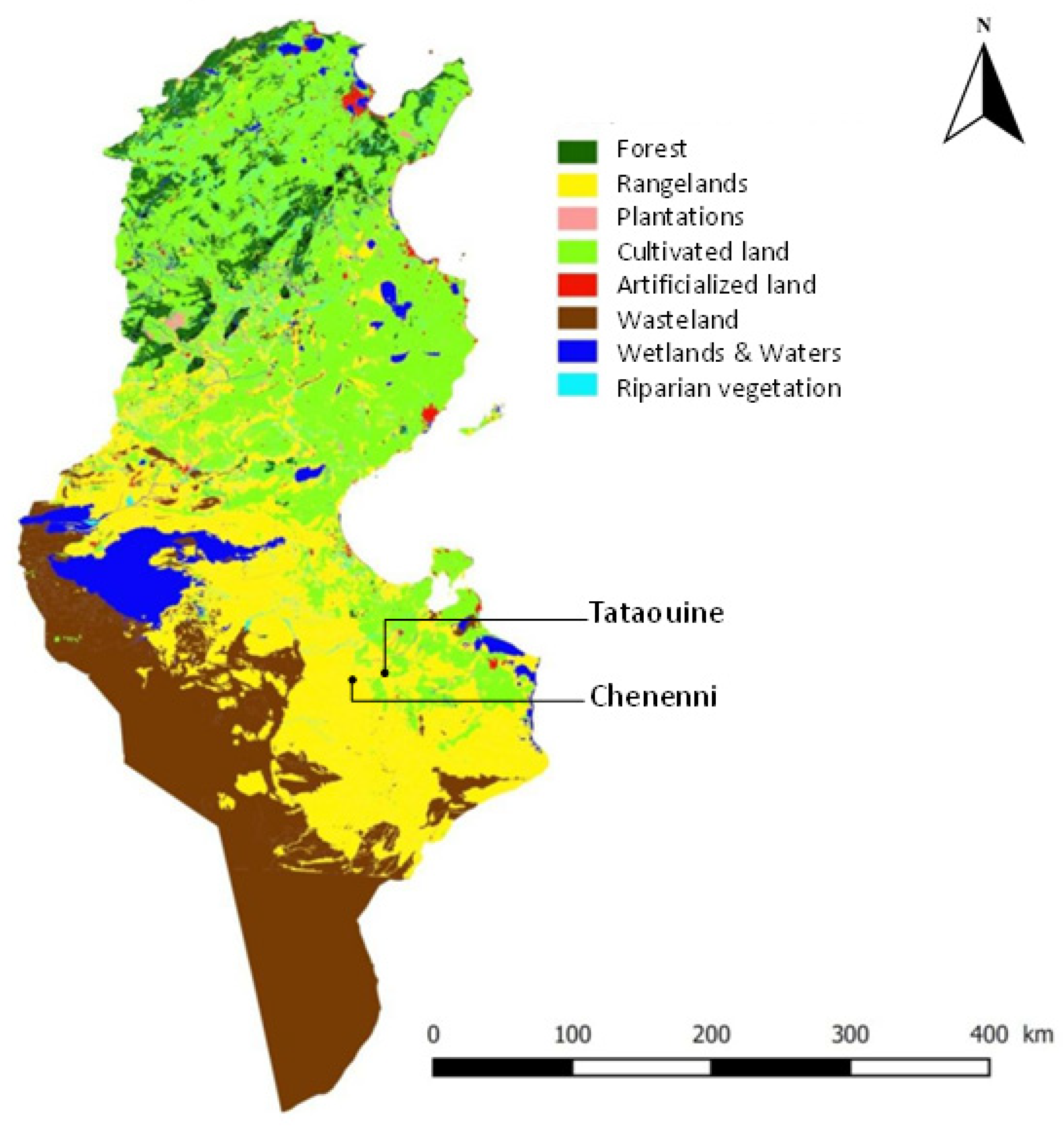
| Species | Fe (mg/kg DM) | Zn (mg/kg DM) | Cu (mg/kg DM) | Mn (mg/kg DM) | Na (g/kg DM) | Ca (g/kg DM) | Mg (g/kg DM) |
|---|---|---|---|---|---|---|---|
| A. henoniana | 1690.3 ± 2.76 b | 15 ± 0.03 h | 5 ± 0.05 j | 31 ± 0.06 h | 0.6 ± 0.01 g | 25.8 ± 0.13 d | 3.1 ± 0.06 cd |
| A. uniflorum | 1580.7 ± 2.12 c | 17.6 ± 0.03 e | 9.6 ± 0.03 c | 47.7 ± 0.12 d | 0.2 ± 0.01 k | 21.3 ± 0.25 e | 2.7 ± 0.35 de |
| E. fruticosum | 543.7 ± 2.1 j | 15.7 ± 0.02 f | 10.2 ± 0.01 b | 70.9 ± 0.02 b | 1.2 ± 0.02 c | 26.4 ± 0.16 c | 1.9 ± 0.06 f |
| G. decander | 1129.1 ± 2.08 f | 11.2 ± 0.02 k | 5.7 ± 0.02 h | 33.6 ± 0.03 e | 1.6 ± 0.01 b | 48 ± 0.06 a | 7.8 ± 0.18 a |
| H. kahiricum | 1500.9 ± 2.6 d | 15.3 ± 0.02 g | 7.2 ± 0.03 f | 33 ± 0.05 g | 0.6 ± 0.02 f | 19.8 ± 0.16 f | 2.7 ± 0.12 cde |
| H. lippii | 1277.1 ± 2.54 e | 27.3 ± 0.03 b | 7.7 ± 0.02 e | 71.6 ± 0.02 a | 0.5 ± 0.01 h | 20 ± 0.09 f | 3.1 ± 0.06 c |
| P. albicans | 2106.4 ± 0.03 a | 21.5 ± 0.03 c | 10.8 ± 0.03 a | 54.8 ± 0.02 c | 0.8 ± 0.02 d | 28.6 ± 0.14 b | 4.3 ± 0.15 b |
| R. suaveolens | 819.9 ± 1.06 g | 21.3 ± 0.02 d | 8.9 ± 0.12 d | 33.3 ± 0.14 f | 2.5 ± 0.01 a | 13.6 ± 0.2 g | 2.5 ± 0.03 e |
| S. lagascae | 387 ± 1.15 l | 12.6 ± 0.02 j | 3 ± 0.02 k | 23 ± 0.03 i | 0.5 ± 0.01 h | 4.4 ± 0.25 j | 0.8 ± 0.03 g |
| S. tenacissima | 619.9 ± 2.06 h | 21.5 ± 0.02 c | 2.8 ± 0.01 l | 20.6 ± 0.01 j | 0.7 ± 0.01 e | 5.5 ± 0.15 i | 1 ± 0.08 g |
| S. plumosa | 604.1 ± 2.1 i,** | 14.3 ± 0.03 i | 5.8 ± 0.01 g | 18.7 ± 0.02 k | 0.3 ± 0.01 i | 6.9 ± 0.03 h | 1.1 ± 0.06 g |
| S. pungens | 537.9 ± 2.1 k | 30.1 ± 0.05 a | 5.4 ± 0.02 i | 17.8 ± 0.12 l | 0.3 ± 0.01 j | 6.9 ± 0.06 h | 1.1 ± 0.09 g |
| Mean | 1066.41 | 18.6 | 6.8 | 38.0 | 0.81 | 18.9 | 2.7 |
| df | 11 | 11 | 11 | 11 | 11 | 11 | 11 |
| F value | 143831 | 46084 | 5188 | 83858 | 4142 | 6755 | 182 |
| Species | Family | Life Form | Acceptability Index |
|---|---|---|---|
| Anthyllis henoniana Coss. ex Batt. | Fabaceae | Chamaephyte | 4 |
| Argyrolobium uniflorum (Deene.) Jaub. and Spach. | Fabaceae | Chamaephyte | 5 |
| Echiochilon fruticosum Desf. | Boraginaceae | Chamaephyte | 5 |
| Gymnocarpos decander Forssk. | Caryophyllaceae | Chamaephyte | 5 |
| Helianthemum kahiricum Delile. | Cistaceae | Chamaephyte | 4 |
| Helianthemum lippii (L.) Dum. Cours. | Cistaceae | Chamaephyte | 5 |
| Plantago albicans L. | Plantaginaceae | Hemicryptophyte | 5 |
| Rhanterium suaveolens Desf. | Asteraceae | Chamaephyte | 2 |
| Stipa lagascae Roem. and Schult. | Poaceae | Hemicryptophyte | 4 |
| Stipa tenacissima L. | Poaceae | Hemicryptophyte | 1 |
| Stipagrostis plumosa (L.) Munro ex T. Anderson | Poaceae | Hemicryptophyte | 4 |
| Stipagrostis pungens (Desf.) de Winter. | Poaceae | Hemicryptophyte | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louhaichi, M.; Gamoun, M.; Hassan, S.; Abdallah, M.A.B. Characterizing Biomass Yield and Nutritional Value of Selected Indigenous Range Species from Arid Tunisia. Plants 2021, 10, 2031. https://doi.org/10.3390/plants10102031
Louhaichi M, Gamoun M, Hassan S, Abdallah MAB. Characterizing Biomass Yield and Nutritional Value of Selected Indigenous Range Species from Arid Tunisia. Plants. 2021; 10(10):2031. https://doi.org/10.3390/plants10102031
Chicago/Turabian StyleLouhaichi, Mounir, Mouldi Gamoun, Sawsan Hassan, and Mohamed A. B. Abdallah. 2021. "Characterizing Biomass Yield and Nutritional Value of Selected Indigenous Range Species from Arid Tunisia" Plants 10, no. 10: 2031. https://doi.org/10.3390/plants10102031
APA StyleLouhaichi, M., Gamoun, M., Hassan, S., & Abdallah, M. A. B. (2021). Characterizing Biomass Yield and Nutritional Value of Selected Indigenous Range Species from Arid Tunisia. Plants, 10(10), 2031. https://doi.org/10.3390/plants10102031









