Temporal Control of Morphogenic Factor Expression Determines Efficacy in Enhancing Regeneration
Abstract
:1. Introduction
2. Results
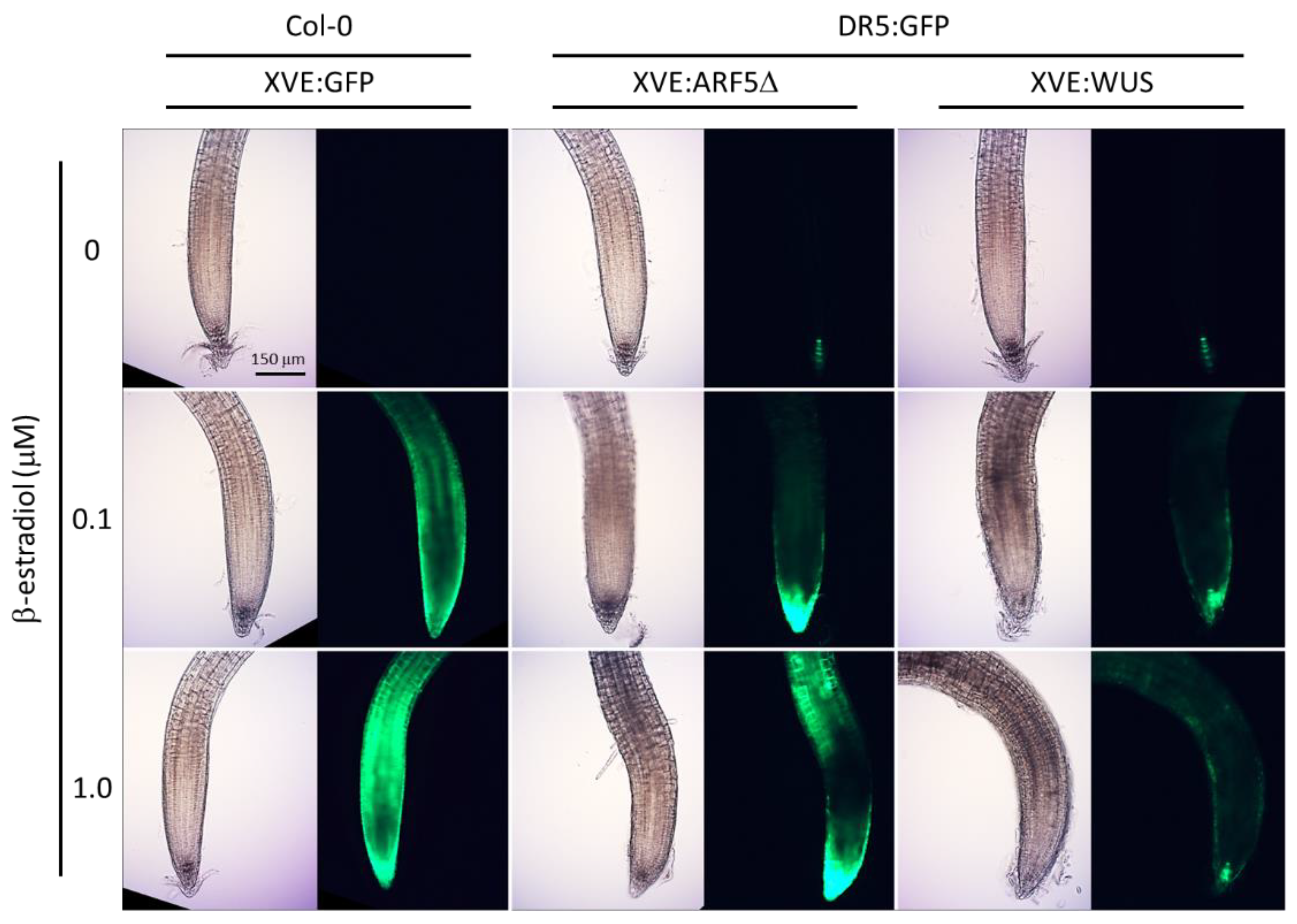
2.1. Ectopic Expression of WUS and ARF5Δ Induces DR5:GFP in Roots
2.2. Transient Expression of ARF5Δ and WUS Activates Auxin Signaling in Root Protoplasts
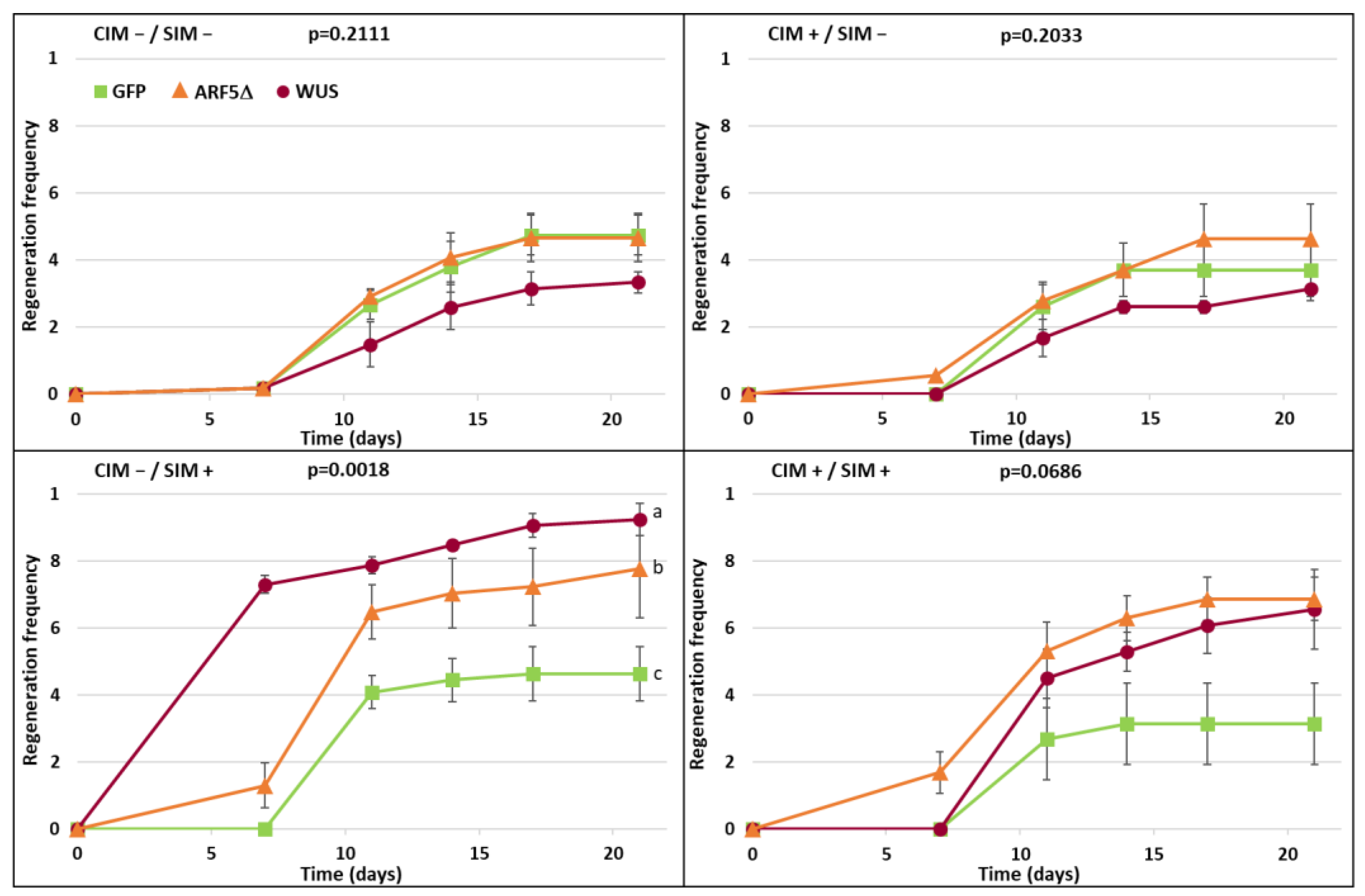
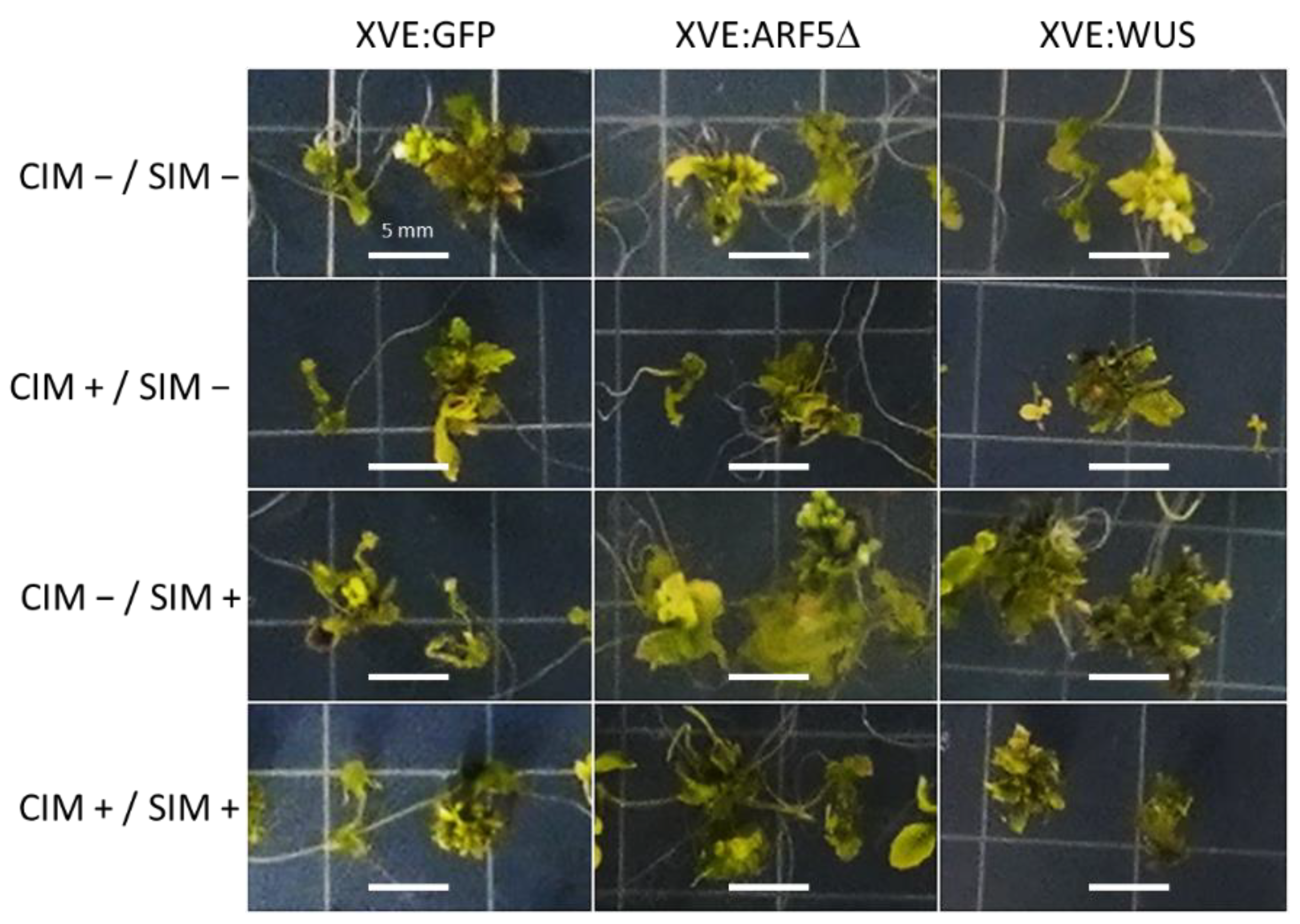
2.3. Effects of Conditional Expression of WUS and ARF5Δ on Regeneration Efficiency
3. Discussion
3.1. Morphogenic Factors WUS and ARF5Δ Both Enhance Auxin Signaling
3.2. Timing the Overexpression of Morphogenic Factors to Enhance Shoot Regeneration
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Microscopy
4.3. Protoplast Isolation, Transformation, and Treatment
4.4. Flow Cytometry
4.5. Regeneration Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atkins, P.A.; Voytas, D.F. Overcoming Bottlenecks in Plant Gene Editing. Curr. Opin. Plant Biol. 2020, 54, 79–84. [Google Scholar] [CrossRef]
- Gordon-Kamm, B.; Sardesai, N.; Arling, M.; Lowe, K.; Hoerster, G.; Betts, S.; Jones, T. Using Morphogenic Genes to Improve Recovery and Regeneration of Transgenic Plants. Plants 2019, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Zuo, J.; Niu, Q.-W.; Frugis, G.; Chua, N.-H. The WUSCHEL Gene Promotes Vegetative-to-Embryonic Transition in Arabidopsis. Plant J. 2002, 30, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Bouchabké-Coussa, O.; Obellianne, M.; Linderme, D.; Montes, E.; Maia-Grondard, A.; Vilaine, F.; Pannetier, C. Wuschel Overexpression Promotes Somatic Embryogenesis and Induces Organogenesis in Cotton (Gossypium Hirsutum L.) Tissues Cultured in Vitro. Plant Cell Rep. 2013, 32, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Herrera, A.; Ku Gonzalez, A.; Canche Moo, R.; Quiroz-Figueroa, F.R.; Loyola-Vargas, V.M.; Rodriguez-Zapata, L.C.; Burgeff D′Hondt, C.; Suárez-Solís, V.M.; Castaño, E. Expression of WUSCHEL in Coffea Canephora Causes Ectopic Morphogenesis and Increases Somatic Embryogenesis. Plant Cell Tiss. Organ. Cult. 2008, 94, 171–180. [Google Scholar] [CrossRef]
- Ckurshumova, W.; Smirnova, T.; Marcos, D.; Zayed, Y.; Berleth, T. Irrepressible MONOPTEROS/ARF5 Promotes de Novo Shoot Formation. New Phytol. 2014, 204, 556–566. [Google Scholar] [CrossRef]
- Krogan, N.T.; Ckurshumova, W.; Marcos, D.; Caragea, A.E.; Berleth, T. Deletion of MP/ARF5 Domains III and IV Reveals a Requirement for Aux/IAA Regulation in Arabidopsis Leaf Vascular Patterning. New Phytol. 2012, 194, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Ulmasov, T.; Murfett, J.; Hagen, G.; Guilfoyle, T.J. Aux/IAA Proteins Repress Expression of Reporter Genes Containing Natural and Highly Active Synthetic Auxin Response Elements. Plant Cell 1997, 9, 1963–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bargmann, B.O.; Birnbaum, K.D. Positive Fluorescent Selection Permits Precise, Rapid, and in-Depth Overexpression Analysis in Plant Protoplasts. Plant Physiol. 2009, 149, 1231–1239. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Miotk, A.; Šutiković, Z.; Ermakova, O.; Wenzl, C.; Medzihradszky, A.; Gaillochet, C.; Forner, J.; Utan, G.; Brackmann, K.; et al. WUSCHEL Acts as an Auxin Response Rheostat to Maintain Apical Stem Cells in Arabidopsis. Nat. Commun. 2019, 10, 5093. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.K.; Luijten, M.; Miyashima, S.; Lenhard, M.; Hashimoto, T.; Nakajima, K.; Scheres, B.; Heidstra, R.; Laux, T. Conserved Factors Regulate Signalling in Arabidopsis Thaliana Shoot and Root Stem Cell Organizers. Nature 2007, 446, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Savina, M.S.; Pasternak, T.; Omelyanchuk, N.A.; Novikova, D.D.; Palme, K.; Mironova, V.V.; Lavrekha, V.V. Cell Dynamics in WOX5-Overexpressing Root Tips: The Impact of Local Auxin Biosynthesis. Front. Plant Sci. 2020, 11, 1529. [Google Scholar] [CrossRef]
- Snipes, S.A.; Rodriguez, K.; DeVries, A.E.; Miyawaki, K.N.; Perales, M.; Xie, M.; Reddy, G.V. Cytokinin Stabilizes WUSCHEL by Acting on the Protein Domains Required for Nuclear Enrichment and Transcription. PLoS Genet. 2018, 14, e1007351. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Du, Q.; Tian, C.; Wang, Y.; Jiao, Y. Stochastic Gene Expression Drives Mesophyll Protoplast Regeneration. Sci. Adv. 2021, 7, eabg8466. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Kawamura, A.; Suzuki, T.; Segami, S.; Maeshima, M.; Polyn, S.; Veylder, L.D.; Sugimoto, K. Transcriptional Activation of Auxin Biosynthesis Drives Developmental Reprogramming of Differentiated Cells. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lowe, K.; Wu, E.; Wang, N.; Hoerster, G.; Hastings, C.; Cho, M.-J.; Scelonge, C.; Lenderts, B.; Chamberlin, M.; Cushatt, J.; et al. Morphogenic Regulators Baby Boom and Wuschel Improve Monocot Transformation. Plant Cell 2016, 28, 1998–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, K.; La Rota, M.; Hoerster, G.; Hastings, C.; Wang, N.; Chamberlin, M.; Wu, E.; Jones, T.; Gordon-Kamm, W. Rapid Genotype “Independent” Zea Mays L. (Maize) Transformation via Direct Somatic Embryogenesis. Vitro Cell. Dev. Biol.-Plant 2018, 54, 240–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richael, C.M.; Kalyaeva, M.; Chretien, R.C.; Yan, H.; Adimulam, S.; Stivison, A.; Weeks, J.T.; Rommens, C.M. Cytokinin Vectors Mediate Marker-Free and Backbone-Free Plant Transformation. Transgenic. Res. 2008, 17, 905–917. [Google Scholar] [CrossRef]
- Heidmann, I.; de Lange, B.; Lambalk, J.; Angenent, G.C.; Boutilier, K. Efficient Sweet Pepper Transformation Mediated by the BABY BOOM Transcription Factor. Plant Cell Rep. 2011, 30, 1107–1115. [Google Scholar] [CrossRef] [Green Version]
- Zuo, J.; Niu, Q.W.; Chua, N.H. An Estrogen Receptor-based Transactivator XVE Mediates Highly Inducible Gene Expression in Transgenic Plants. Plant J. 2000, 24, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Atta, R.; Laurens, L.; Boucheron-Dubuisson, E.; Guivarc’h, A.; Carnero, E.; Giraudat-Pautot, V.; Rech, P.; Chriqui, D. Pluripotency of Arabidopsis Xylem Pericycle Underlies Shoot Regeneration from Root and Hypocotyl Explants Grown in Vitro. Plant J. Cell Mol. Biol. 2009, 57, 626–644. [Google Scholar] [CrossRef]
- Wu, M.-F.; Yamaguchi, N.; Xiao, J.; Bargmann, B.; Estelle, M.; Sang, Y.; Wagner, D. Auxin-Regulated Chromatin Switch Directs Acquisition of Flower Primordium Founder Fate. Elife 2015, 4, e09269. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.D.; Grossniklaus, U. A Gateway Cloning Vector Set for High-Throughput Functional Analysis of Genes in Planta. Plant Physiol. 2003, 133, 462–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, M.; Inzé, D.; Depicker, A. GATEWAYTM Vectors for Agrobacterium-Mediated Plant Transformation. Trends Plant Sci. 2002, 7, 193–195. [Google Scholar] [CrossRef]
- Meng, W.J.; Cheng, Z.J.; Sang, Y.L.; Zhang, M.M.; Rong, X.F.; Wang, Z.W.; Tang, Y.Y.; Zhang, X.S. Type-B Arabidopsis Response Regulators Specify the Shoot Stem Cell Niche by Dual Regulation of Wuschel. Plant Cell 2017, 29, 1357–1372. [Google Scholar] [CrossRef] [Green Version]
- Aickin, M.; Gensler, H. Adjusting for Multiple Testing When Reporting Research Results: The Bonferroni vs Holm Methods. Am. J. Public Health 1996, 86, 726–728. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez, J.H.; Taylor, J.S.; Reed, K.M.; Wright, R.C.; Bargmann, B.O.R. Temporal Control of Morphogenic Factor Expression Determines Efficacy in Enhancing Regeneration. Plants 2021, 10, 2271. https://doi.org/10.3390/plants10112271
Gonzalez JH, Taylor JS, Reed KM, Wright RC, Bargmann BOR. Temporal Control of Morphogenic Factor Expression Determines Efficacy in Enhancing Regeneration. Plants. 2021; 10(11):2271. https://doi.org/10.3390/plants10112271
Chicago/Turabian StyleGonzalez, Juan H., Joseph S. Taylor, Kelsey M. Reed, R. Clay Wright, and Bastiaan O. R. Bargmann. 2021. "Temporal Control of Morphogenic Factor Expression Determines Efficacy in Enhancing Regeneration" Plants 10, no. 11: 2271. https://doi.org/10.3390/plants10112271
APA StyleGonzalez, J. H., Taylor, J. S., Reed, K. M., Wright, R. C., & Bargmann, B. O. R. (2021). Temporal Control of Morphogenic Factor Expression Determines Efficacy in Enhancing Regeneration. Plants, 10(11), 2271. https://doi.org/10.3390/plants10112271





