Influence of Iron Addition (Alone or with Calcium) to Elements Biofortification and Antioxidants in Pholiota nameko
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Spawn
2.2. Substrate Preparation
2.3. Studies on Total Concentration of Elements
2.3.1. Procedure
2.3.2. Instrumentation
2.3.3. Analytical Method Validation
2.4. Determination of Phenolic Compounds and Organic Acids
2.5. Determination of Total Phenolic (TP) Content
2.6. Statistical Analysis
3. Results
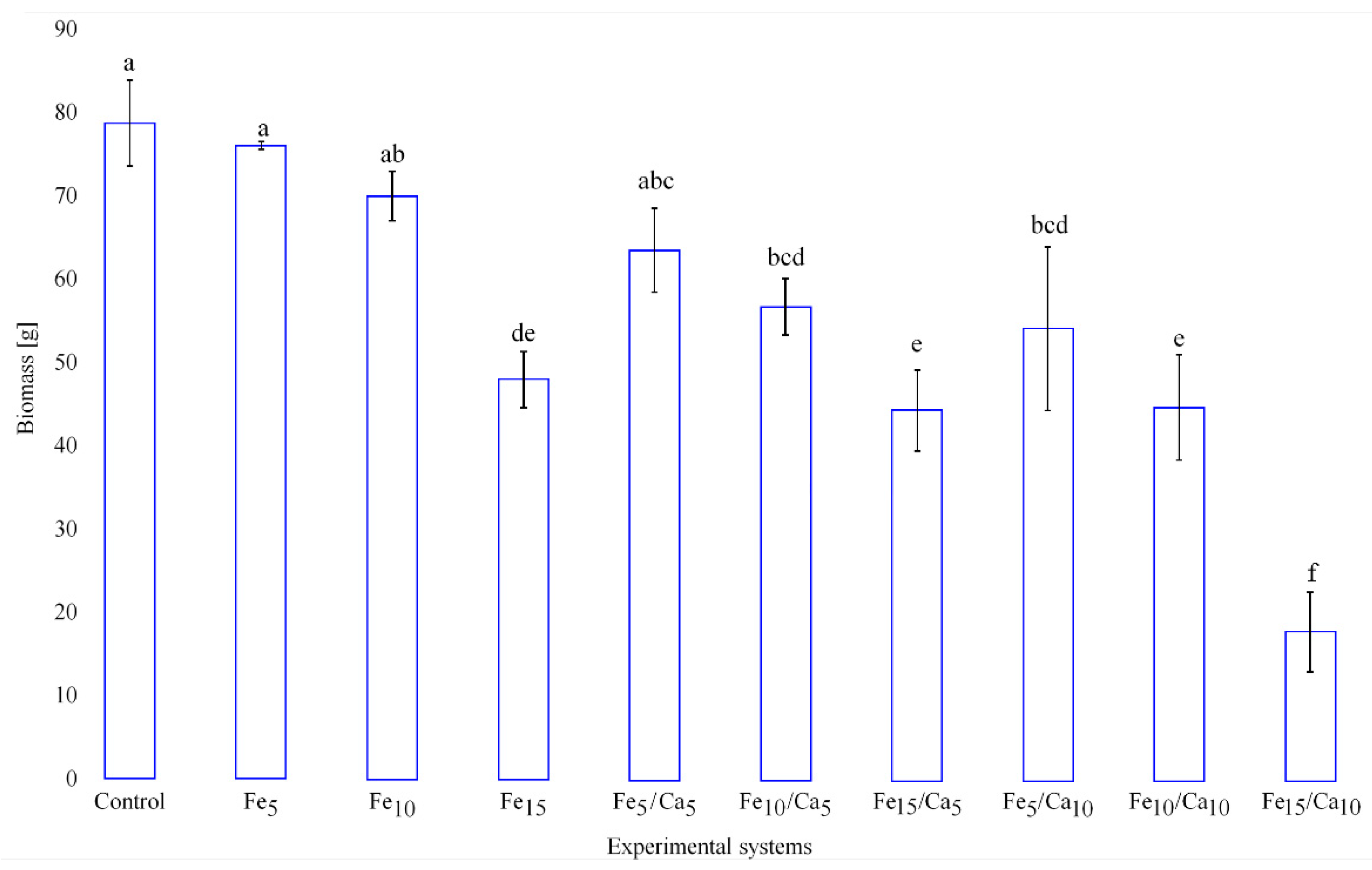
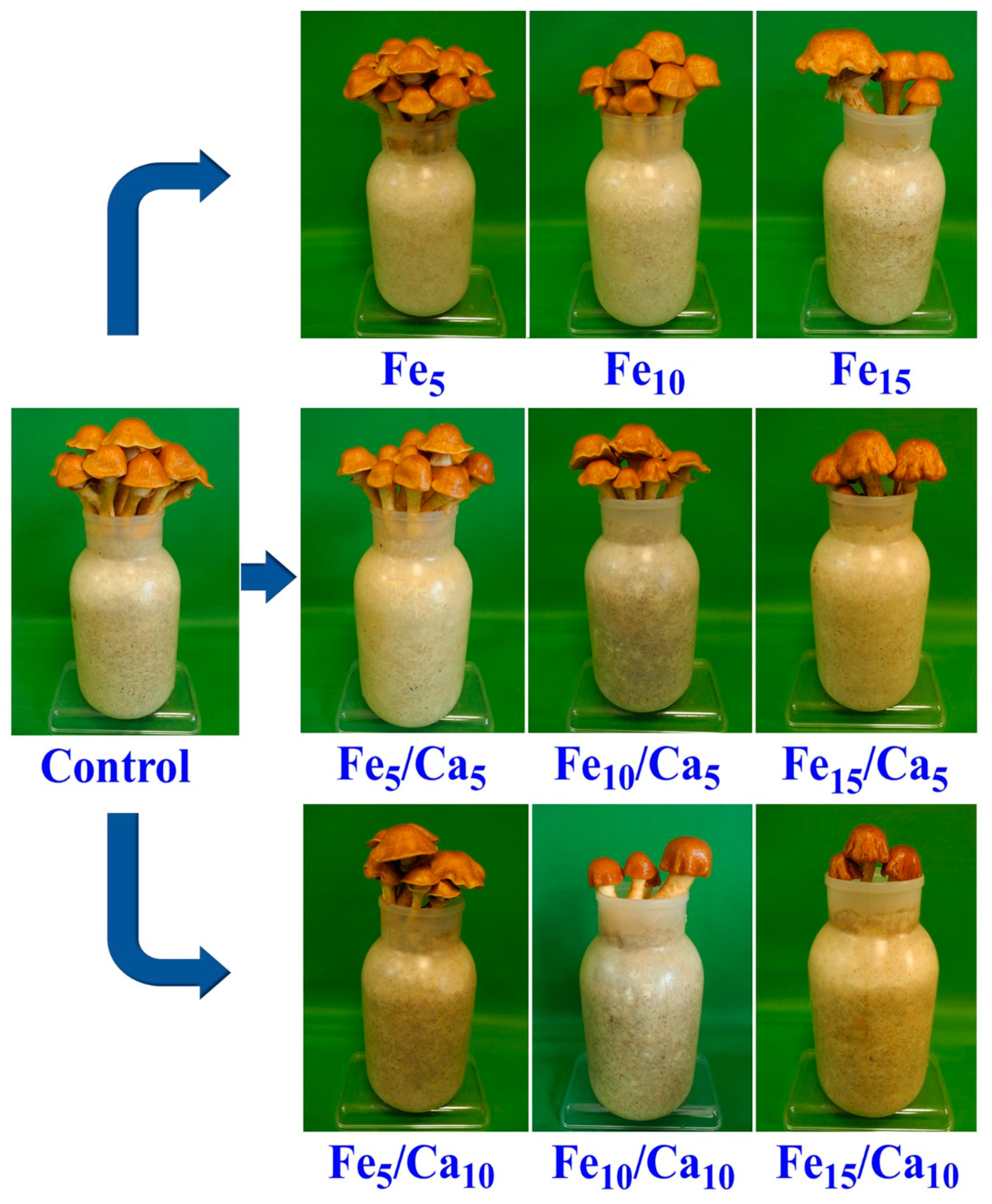
3.1. Mycelium Growth and Mushroom Yield
3.2. Mineral Composition of Mushroom Bodies
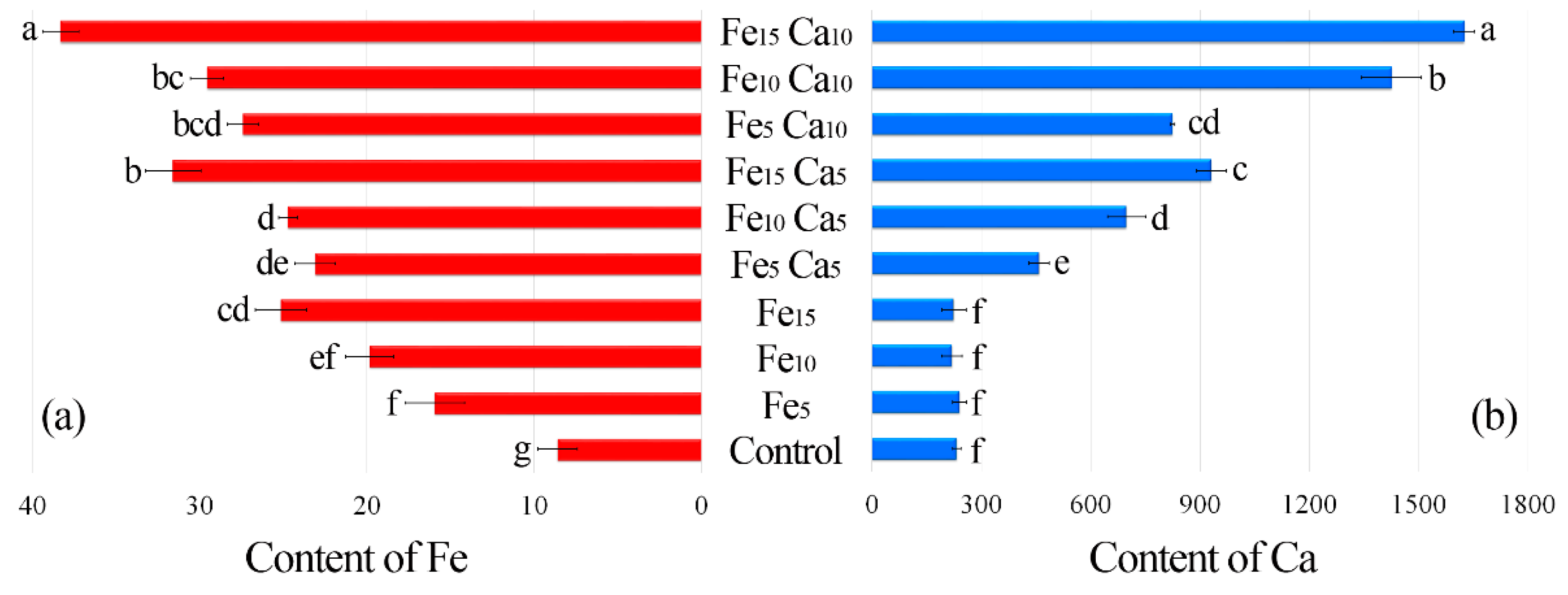
3.2.1. Content of Iron and Calcium
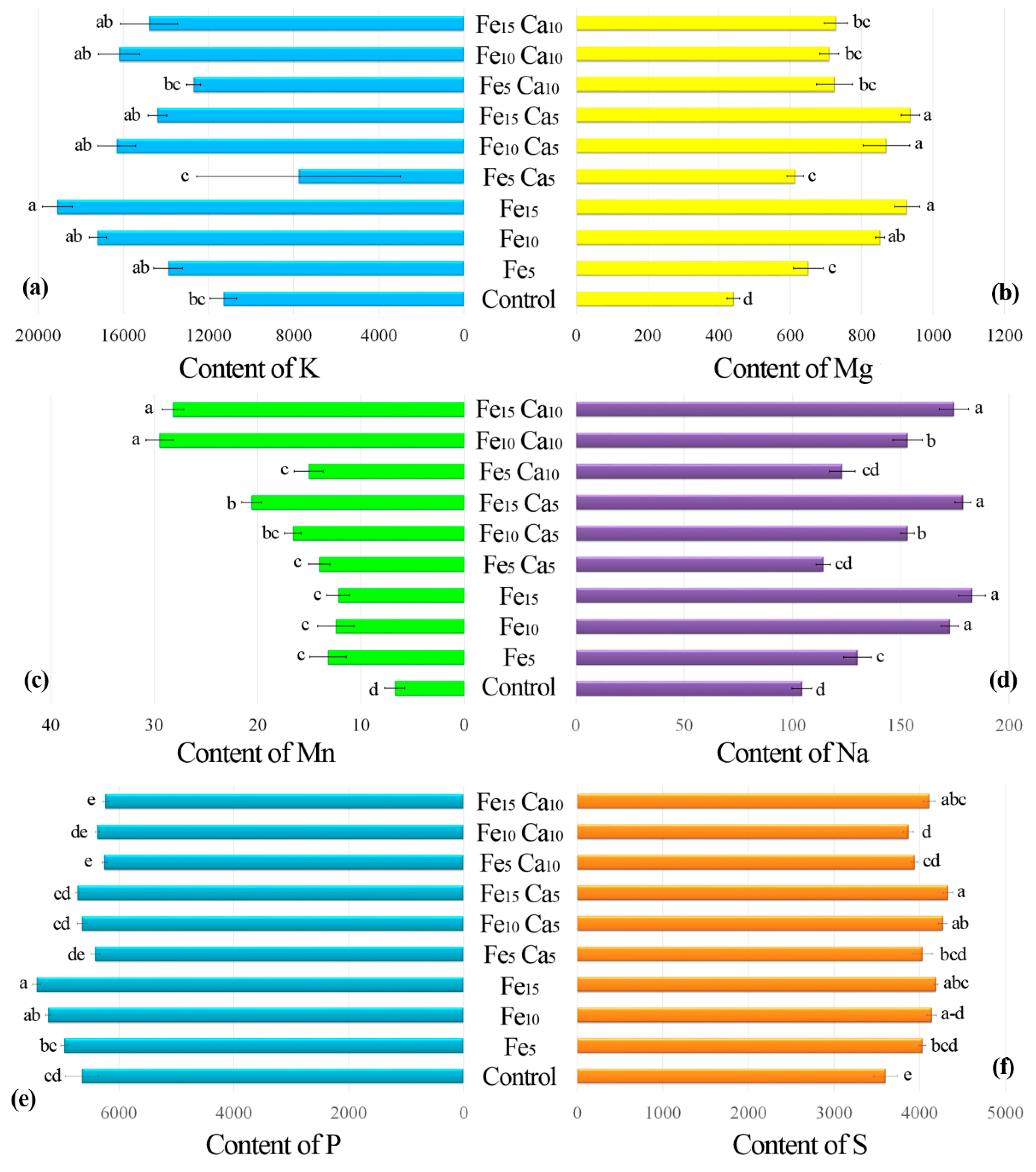
3.2.2. Content of Other Elements
3.3. Profile of Phenolic Compounds
3.4. Profile of Low Molecular Weight Organic Acids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nguyen, P.H.; Scott, S.; Headey, D.; Singh, N.; Tran, L.M.; Menon, P.; Ruel, M.T. The Double Burden of Malnutrition in India: Trends and Inequalities (2006–2016). PLoS ONE 2021, 16, e0247856. [Google Scholar] [CrossRef]
- Barzegar, F.; Kamankesh, M.; Mohammadi, A. Recent Development in Formation, Toxic Effects, Human Health and Analytical Techniques of Food Contaminants. Food Rev. Int. 2021. [Google Scholar] [CrossRef]
- Sellamuthu, K.M.; Malathi, P. Biofortification of Crops to Overcome Malnutrition in India. Biot. Res. Today 2021, 3, 402–405. [Google Scholar]
- Poniedziałek, B.; Perkowska, K.; Rzymski, P. Food Fortification. In Vitamins and Minerals Biofortification of Edible Plants; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 27–44. [Google Scholar] [CrossRef]
- Olson, R.; Gavin-Smith, B.; Ferraboschi, C.; Kraemer, K.; Davaasambuu, G. Food Fortification: The Advantages, Disadvantages and Lessons from Sight and Life Programs. Nutrients 2021, 13, 1118. [Google Scholar] [CrossRef]
- Wakeel, A.; Farooq, M.; Bashir, K.; Ozturk, L. Micronutrient Malnutrition and Biofortification: Recent Advances and Future Perspectives. In Plant Macronutrient Use Efficiency Molecular and Genomic Perspectives in Crop Plants; Elsevier: Amsterdam, The Netherlands, 2018; pp. 225–243. [Google Scholar] [CrossRef]
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Mineral Biofortification of Vegetables as a Tool to Improve Human Diet. Foods 2021, 10, 223. [Google Scholar] [CrossRef]
- Riaz, N.; Guerinot, M.L. All Together Now: Regulation of the Iron Deficiency Response. J. Exp. Bot. 2021, 72, 2045–2055. [Google Scholar] [CrossRef]
- Henjum, S.; Groufh-Jacobsen, S.; Stea, T.H.; Tonheim, L.E.; Almendingen, K. Iron Status of Vegans, Vegetarians and Pescatarians in Norway. Biomolecules 2021, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, V. Iron Homeostasis and Metabolism: Two Sides of a Coin. Adv. Exp. Med. Biol. 2021, 1301, 25–40. [Google Scholar] [CrossRef]
- Pasricha, S.-R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron Deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Man, Y.; Xu, T.; Adhikari, B.; Zhou, C.; Wang, Y.; Wang, B. Iron Supplementation and Iron-Fortified Foods: A Review. Crit. Rev. Food Sci. Nutr. 2021, 1–22. [Google Scholar] [CrossRef]
- Quintaes, K.D.; Barberá, R.; Cilla, A. Iron Bioavailability in Iron-Fortified Cereal Foods: The Contribution of in Vitro Studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 2028–2041. [Google Scholar] [CrossRef] [PubMed]
- Beinner, M.A.; Lamounier, J.A.; Tomaz, C. Effect of Iron-Fortified Drinking Water of Daycare Facilities on the Hemoglobin Status of Young Children. J. Am. Coll. Nutr. 2013, 24, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, S.; Mcmahon, D.J. Manufacture and Quality of Iron-Fortified Yogurt. J. Dairy Sci. 1997, 80, 3114–3122. [Google Scholar] [CrossRef]
- Larson, L.M.; Cyriac, S.; Djimeu, E.W.; Mbuya, M.N.N.; Neufeld, L.M. Can Double Fortification of Salt with Iron and Iodine Reduce Anemia, Iron Deficiency Anemia, Iron Deficiency, Iodine Deficiency, and Functional Outcomes? Evidence of Efficacy, Effectiveness, and Safety. J. Nutr. 2021, 151, 15S–28S. [Google Scholar] [CrossRef]
- Hurrell, R.F. Efficacy and safety of iron fortification. In Food Fortification in a Globalized World; Academic Press: London, UK, 2018; pp. 196–212. [Google Scholar] [CrossRef]
- Chopra, H.; Mishra, A.K.; Baig, A.A.; Mohanta, T.K.; Mohanta, Y.K.; Baek, K.-H. Narrative Review: Bioactive Potential of Various Mushrooms as the Treasure of Versatile Therapeutic Natural Product. J. Fungi 2021, 7, 728. [Google Scholar] [CrossRef]
- Friedman, M. Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods 2016, 5, 80. [Google Scholar] [CrossRef] [Green Version]
- Yue, G.G.-L.; Lau, C.B.-S.; Leung, P.-C. Medicinal Plants and Mushrooms with Immunomodulatory and Anticancer Properties—A Review on Hong Kong’s Experience. Molecules 2021, 26, 2173. [Google Scholar] [CrossRef]
- Oyetayo, V.O.; Ogidi, C.O.; Bayode, S.O.; Enikanselu, F.F. Evaluation of Biological Efficiency, Nutrient Contents and Antioxidant Activity of Pleurotus pulmonarius Enriched with Zinc and Iron. Indian Phytopathol. 2021, 1–10. [Google Scholar] [CrossRef]
- Windisch, W. Interaction of Chemical Species with Biological Regulation of the Metabolism of Essential Trace Elements. Anal. Bioanal. Chem. 2001, 372, 421–425. [Google Scholar] [CrossRef]
- Stamets, P. Growing Gourmet and Medicinal Mushrooms Paul Stamets; Ten Speed Press: Berkeley, CA, USA, 2000; ISBN 1580081754. [Google Scholar]
- Gąsecka, M.; Magdziak, Z.; Siwulski, M.; Jasińska, A.; Budzyńska, S.; Rzymski, P.; Kalač, P.; Niedzielski, P.; Pankiewicz, J.; Mleczek, M. Effect of Thymus Vulgaris Post-Extraction Waste and Spent Coffee Grounds on the Quality of Cultivated Pleurotus Eryngii. J. Food Process. Preserv. 2020, 44, e14648. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Falniowski, A. The Numerical Methods in Taxonomy, 1st ed.; Metody Numeryczne w Taksonomii; Wydawnictwo Uniwersytetu Jagiellońskiego: Kraków, Poland, 2003; ISBN 83-233-1745-3. [Google Scholar]
- Galili, T. Dendextend: An R Package for Visualizing, Adjusting and Comparing Trees of Hierarchical Clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. The Global Prevalence of Anaemia in 2011; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Benson, C.S.; Shah, A.; Stanworth, S.J.; Frise, C.J.; Spiby, H.; Lax, S.J.; Murray, J.; Klein, A.A. The Effect of Iron Deficiency and Anaemia on Women’s Health. Anaesthesia 2021, 76, 84–95. [Google Scholar] [CrossRef]
- Lynch, S.R. Why Nutritional Iron Deficiency Persists as a Worldwide Problem. J. Nutr. 2011, 141, 763S–768S. [Google Scholar] [CrossRef] [Green Version]
- Hurrell, R.F. Iron Fortification Practices and Implications for Iron Addition to Salt. J. Nutr. 2021, 151, 3S–14S. [Google Scholar] [CrossRef] [PubMed]
- Zięba, P.; Kała, K.; Włodarczyk, A.; Szewczyk, A.; Kunicki, E.; Sękara, A.; Muszyńska, B. Selenium and Zinc Biofortification of Pleurotus Eryngii Mycelium and Fruiting Bodies as a Tool for Controlling Their Biological Activity. Molecules 2020, 25, 889. [Google Scholar] [CrossRef] [Green Version]
- Rzymski, P.; Niedzielski, P.; Siwulski, M.; Mleczek, M.; Budzyńska, S.; Gąsecka, M.; Poniedziałek, B. Lithium Biofortification of Medicinal Mushrooms Agrocybe Cylindracea and Hericium Erinaceus. J. Food Sci. Technol. 2017, 54, 2387–2393. [Google Scholar] [CrossRef] [PubMed]
- Gąsecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P. Phenolic Composition and Antioxidant Properties of Pleurotus Ostreatus and Pleurotus Eryngii Enriched with Selenium and Zinc. Eur. Food Res. Technol. 2015, 242, 723–732. [Google Scholar] [CrossRef] [Green Version]
- Almeida, S.M.; Umeo, S.H.; Marcante, R.C.; Yokota, M.E.; Valle, J.S.; Dragunski, D.C.; Colauto, N.B.; Linde, G.A. Iron Bioaccumulation in Mycelium of Pleurotus Ostreatus. Braz. J. Microbiol. 2015, 46, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Yokota, M.E.; Frison, P.S.; Marcante, R.C.; Jorge, L.F.; Valle, J.S.; Dragunski, D.C.; Colauto, N.B.; Linde, G.A. Iron Translocation in Pleurotus Ostreatus Basidiocarps: Production, Bioavailability, and Antioxidant Activity. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Umeo, S.H.; Faria, M.G.I.; Vilande, S.S.S.; Dragunski, D.C.; do Valle, J.S.; Colauto, N.B.; Linde, G.A. Iron and Zinc Mycelial Bioaccumulation in Agaricus Subrufescens Strains. Semina: Ciências Agrárias 2019, 40, 2513–2522. [Google Scholar] [CrossRef] [Green Version]
- Meniqueti, A.B.; Ruiz, S.P.; Faria, M.G.I.; Valle, J.S.; Gonçalves, A.C., Jr.; Dragunski, D.C.; Colauto, N.B.; Linde, G.A. Iron-Enriched Mycelia of Edible and Medicinal Basidiomycetes. Environ. Technol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Verna, G.; Sila, A.; Liso, M.; Mastronardi, M.; Chieppa, M.; Cena, H.; Campiglia, P. Iron-Enriched Nutritional Supplements for the 2030 Pharmacy Shelves. Nutrients 2021, 13, 378. [Google Scholar] [CrossRef] [PubMed]
- Scheid, S.S.; Faria, M.G.I.; Velasquez, L.G.; do Valle, J.S.; Gonçalves, A.C.; Dragunski, D.C.; Colauto, N.B.; Linde, G.A. Iron Biofortification and Availability in the Mycelial Biomass of Edible and Medicinal Basidiomycetes Cultivated in Sugarcane Molasses. Sci. Rep. 2020, 10, 12875. [Google Scholar] [CrossRef] [PubMed]
- Meniqueti, A.B.; Ruiz, S.P.; Faria, M.G.I.; do Valle, J.S.; Gonçalves, A.C., Jr.; Dragunski, D.C.; Colauto, N.B.; Linde, G.A. Iron Bioaccumulation in Lentinus Crinitus Mycelia Cultivated in Agroindustrial Byproducts. Waste Biomass Valorization 2021, 12, 4965–4974. [Google Scholar] [CrossRef]
- Gasecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P.; Kozak, L. The Effect of Selenium on Phenolics and Flavonoids in Selected Edible White Rot Fungi. LWT Food Sci. Technol. 2015, 63, 726–731. [Google Scholar] [CrossRef]
- Lin, S.; Ching, L.T.; Ke, X.; Cheung, P.C.K. Comparison of the Composition and Antioxidant Activities of Phenolics from the Fruiting Bodies of Cultivated Asian Culinary-Medicinal Mushrooms. Int. J. Med. Mushrooms 2016, 18, 871–881. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, N.; Li, J.; Xu, R.; Wang, T.; Guo, L.; Ma, M.; Fan, M.; Wei, X. Selenium-Enriched Lactobacillus Plantarum Improves the Antioxidant Activity and Flavor Properties of Fermented Pleurotus Eryngii. Food Chem. 2021, 345. [Google Scholar] [CrossRef]
- Vieira, P.A.F.; Gontijo, D.C.; Vieira, B.C.; Fontes, E.A.; de Assunção, L.S.; Leite, J.; Oliveira, M.G.D.A.; Kasuya, M.C.M. Antioxidant Activities, Total Phenolics and Metal Contents in Pleurotus Ostreatus Mushrooms Enriched with Iron, Zinc or Lithium. LWT Food Sci. Technol. 2013, 54, 421–425. [Google Scholar] [CrossRef]
- Li, W.; Chen, W.; Yang, Y.; Zhang, J.; Feng, J.; Yu, H.; Zhou, S.; Li, X.; Liu, Y. Effects of Culture Substrates on Taste Component Content and Taste Quality of Lentinula Edodes. Int. J. Food Sci. Technol. 2017, 52, 981–991. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, Z.; Liu, Y.F.; Zhou, S.; Zhang, J.S. Determination of Seven Organic Acids in Edible Fungi by Reversed-Phase High Performance Liquid Chromatography. Mycosystema 2013, 32, 1064–1070. [Google Scholar]
- Ferreira, I.; Barros, L.; Abreu, R. Antioxidants in Wild Mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, B.; Valentão, P.; Baptista, P.; Seabra, R.M.; Andrade, P.B. Phenolic Compounds, Organic Acids Profiles and Antioxidative Properties of Beefsteak Fungus (Fistulina Hepatica). Food Chem. Toxicol. 2007, 45, 1805–1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magdziak, Z.; Siwulski, M.; Mleczek, M. Characteristics of Organic Acid Profiles in 16 Species of Wild Growing Edible Mushrooms. J. Environ. Sci. Health Part B 2017, 52, 784–789. [Google Scholar] [CrossRef]
- Karaman, M.; Stahl, M.; Vulić, J.; Vesić, M.; Čanadanović-Brunet, J. Wild-Growing Lignicolous Mushroom Species as Sources of Novel Agents with Antioxidative and Antibacterial Potentials. Int. J. Food Sci. Nutr. 2014, 65, 311–319. [Google Scholar] [CrossRef]
- Cardoso, R.V.C.; Carocho, M.; Fernandes, Â.; Pinela, J.; Stojković, D.; Soković, M.; Zied, D.C.; Cobos, J.D.V.; González-Paramás, A.M.; Ferreira, I.C.F.R.; et al. Antioxidant and Antimicrobial Influence on Oyster Mushrooms (Pleurotus Ostreatus) from Substrate Supplementation of Calcium Silicate. Sustainability 2021, 13, 5019. [Google Scholar] [CrossRef]
- Stojković, D.S.; Kovačević-Grujičić, N.; Reis, F.S.; Davidović, S.; Barros, L.; Popović, J.; Petrović, I.; Pavić, A.; Glamočlija, J.; Ćirić, A.; et al. Chemical Composition of the Mushroom Meripilus Giganteus Karst. and Bioactive Properties of Its Methanolic Extract. LWT Food Sci. Technol. 2017, 79, 454–462. [Google Scholar] [CrossRef] [Green Version]
- National Health Service Vitamins and Minerals—NHS. Available online: https://www.nhs.uk/conditions/vitamins-and-minerals/ (accessed on 2 October 2021).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Iron. EFSA J. 2015, 13, 115. [Google Scholar] [CrossRef]



| System | 4-HBA | Gallic | Sinapic | Syringic | TP [mg g−1 GAE *] |
|---|---|---|---|---|---|
| Control | bDL | 14.8 ab | bDL | 9.18 e | 0.810 e |
| Fe5 | 27.6 d | 11.1 c | bDL | bDL | 1.02 de |
| Fe10 | 24.9 c | 13.0 bc | bDL | 11.7 de | 1.03 cde |
| Fe15 | 37.5 b | 13.4 bc | bDL | 21.8 c | 1.44 a |
| Fe5Ca5 | 43.2 a | 17.9 a | bDL | 11.1 e | 1.26 ab |
| Fe10Ca5 | bDL | bDL | bDL | 31.7 b | 1.22 a–d |
| Fe15Ca5 | bDL | bDL | bDL | 15.5 d | 1.16 bcd |
| Fe5Ca10 | bDL | bDL | 1.78 | bDL | 1.19 bcd |
| Fe10Ca10 | bDL | bDL | bDL | bDL | 1.17 bcd |
| Fe15Ca10 | bDL | bDL | bDL | 50.8 a | 1.25 abc |
| System | Acetic | Citric | Succinic | Sum |
|---|---|---|---|---|
| Control | bDL | 1.05 e | 6.89 cd | 7.93 bc |
| Fe5 | bDL | bDL | 3.60 de | 3.60 cd |
| Fe10 | bDL | bDL | 1.98 e | 1.98 d |
| Fe15 | bDL | bDL | 4.89 cde | 4.90 bcd |
| Fe5Ca5 | bDL | 1.19 d | 6.98 cd | 8.16 bc |
| Fe10Ca5 | bDL | bDL | 6.12 cd | 6.12 bc |
| Fe15Ca5 | bDL | 1.63 a | 36.4 a | 38.0 a |
| Fe5Ca10 | bDL | 1.30 c | 8.83 c | 10.1 b |
| Fe10Ca10 | 8.32 | 1.47 b | 28.6 b | 38.3 a |
| Fe15Ca10 | bDL | 1.10 e | 5.39 cde | 6.46 bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budzyńska, S.; Siwulski, M.; Magdziak, Z.; Budka, A.; Gąsecka, M.; Kalač, P.; Rzymski, P.; Niedzielski, P.; Mleczek, M. Influence of Iron Addition (Alone or with Calcium) to Elements Biofortification and Antioxidants in Pholiota nameko. Plants 2021, 10, 2275. https://doi.org/10.3390/plants10112275
Budzyńska S, Siwulski M, Magdziak Z, Budka A, Gąsecka M, Kalač P, Rzymski P, Niedzielski P, Mleczek M. Influence of Iron Addition (Alone or with Calcium) to Elements Biofortification and Antioxidants in Pholiota nameko. Plants. 2021; 10(11):2275. https://doi.org/10.3390/plants10112275
Chicago/Turabian StyleBudzyńska, Sylwia, Marek Siwulski, Zuzanna Magdziak, Anna Budka, Monika Gąsecka, Pavel Kalač, Piotr Rzymski, Przemysław Niedzielski, and Mirosław Mleczek. 2021. "Influence of Iron Addition (Alone or with Calcium) to Elements Biofortification and Antioxidants in Pholiota nameko" Plants 10, no. 11: 2275. https://doi.org/10.3390/plants10112275
APA StyleBudzyńska, S., Siwulski, M., Magdziak, Z., Budka, A., Gąsecka, M., Kalač, P., Rzymski, P., Niedzielski, P., & Mleczek, M. (2021). Influence of Iron Addition (Alone or with Calcium) to Elements Biofortification and Antioxidants in Pholiota nameko. Plants, 10(11), 2275. https://doi.org/10.3390/plants10112275













