Preharvest Spray Hexanal Formulation Enhances Postharvest Quality in ‘Honeycrisp’ Apples by Regulating Phospholipase D and Calcium Sensor Proteins Genes
Abstract
:1. Introduction
2. Results
2.1. Effect of Preharvest Spray on Quality Parameters and Phytohormones at Harvest
2.2. Effect of Preharvest Spray on Ethylene and Phospholipase D Enzyme at Cold Storage
2.2.1. Ethylene Production
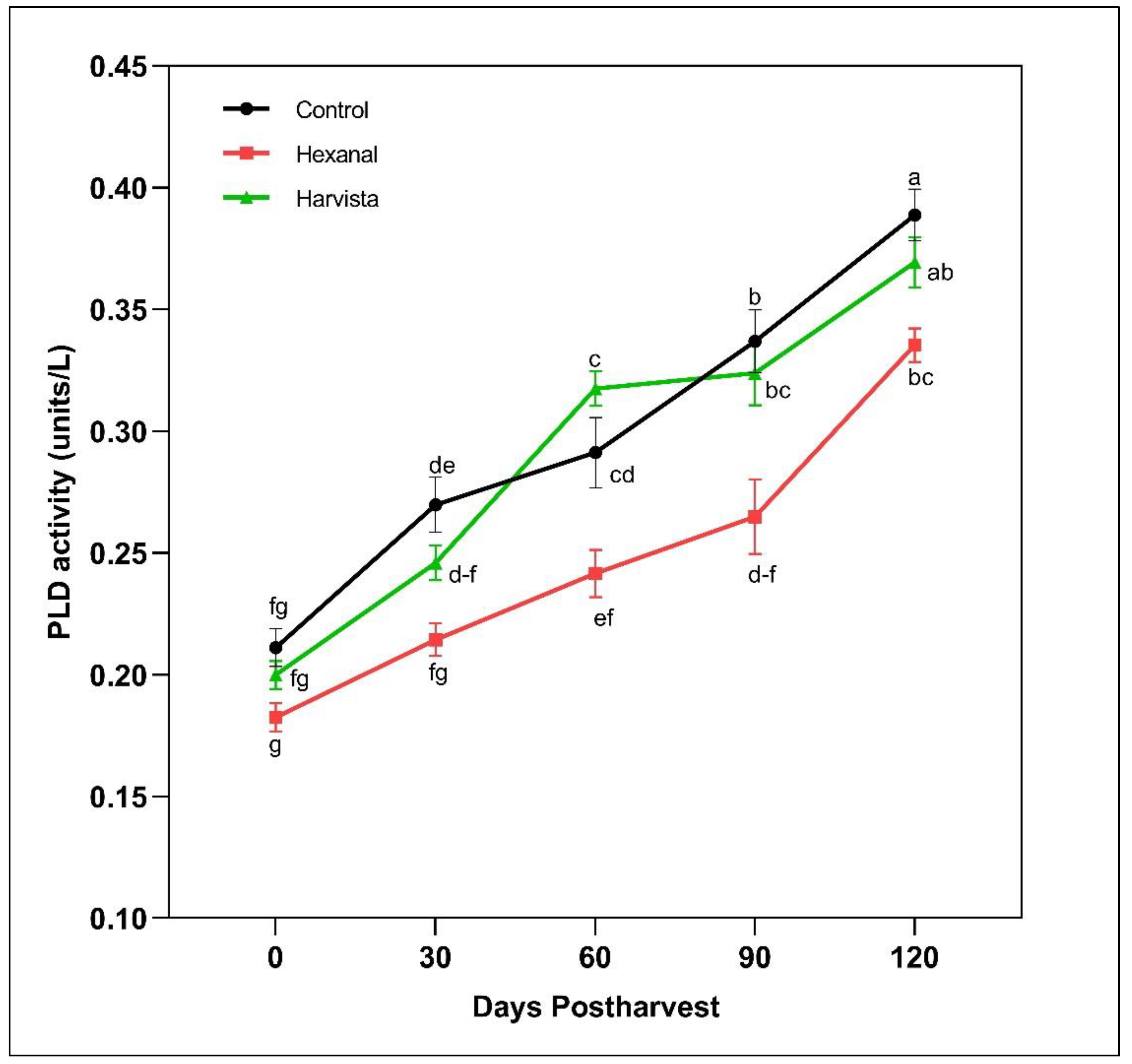
2.2.2. Phospholpase D (PLD) Enzyme Activity
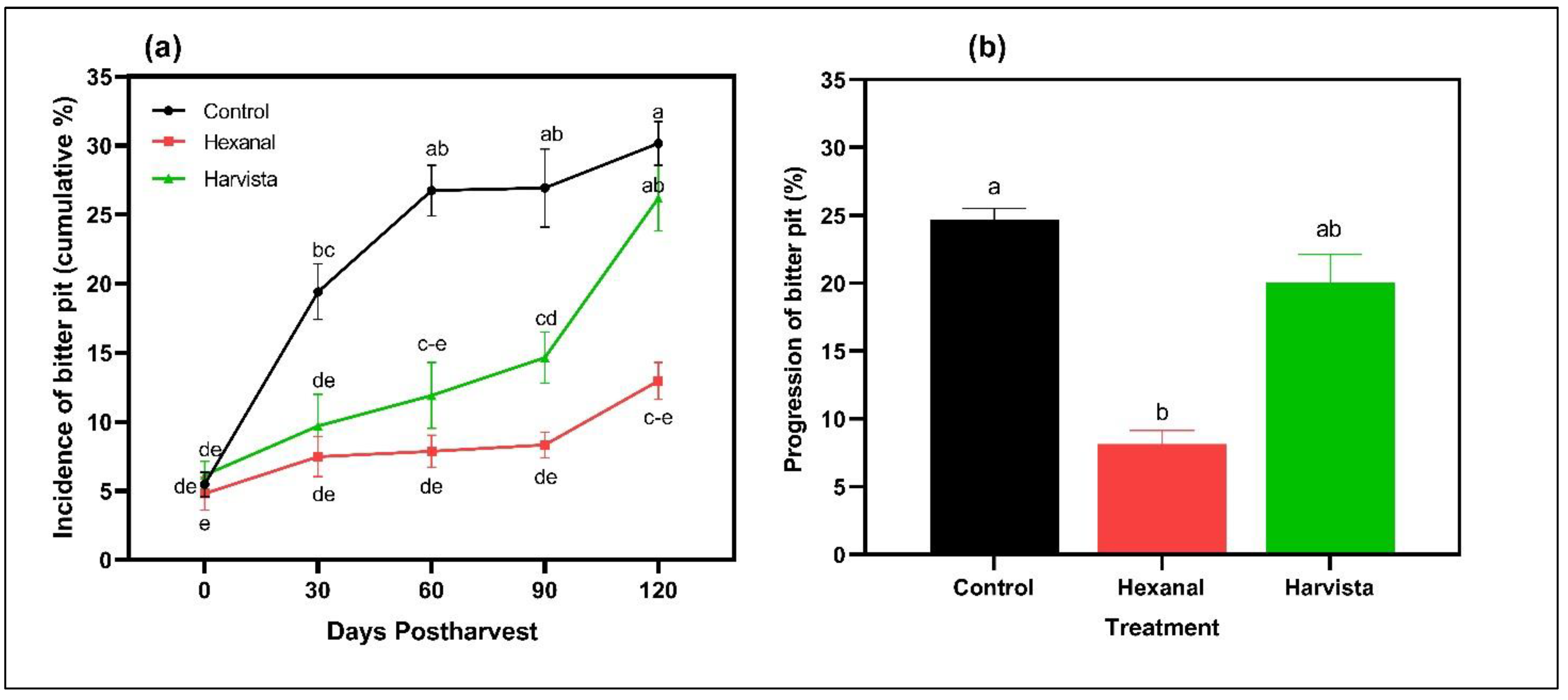
2.3. Effects of Preharvest Spray on Bitter Pit (BP) Development
2.4. Effect of Preharvest Sprays on Fruit Quality Traits during Cold Storage
2.5. Expression Profiles of Genes Encoding PLD and Calcium Sensor Proteins
2.6. Effects of Hexanal and HarvistaTM on Fruit Quality Traits at Room Temperature Storage
3. Discussion
4. Materials and Methods
4.1. Experimental Location and Treatments
4.2. Storage Studies
4.3. Standard Quality Assessment during Storage
4.4. Measurement of Plant Hormones
4.4.1. Ethylene
4.4.2. Phytohormones and Metabolites
4.5. Phospholipase-D Assay
4.6. Bitter Pit (BP) Assessment
4.7. Gene Expression Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cline, J.A. Gala, Honeycrisp, and Ambrosia®—Strong Contenders in the Ontario Apple Market. 2014. Available online: http://www.omafra.gov.on.ca/english/crops/hort/news/orchnews/2014/on-1214a1.htm (accessed on 3 June 2021).
- Mailvaganam, S. Apple Crop Estimate for Ontario, as of November 2014. 2015. Available online: http://www.omafra.gov.on.ca/english/stats/hort/applecropestimate.htm (accessed on 3 June 2021).
- Tong, C.; Krueger, D.; Vickers, Z.; Bedford, D.; Luby, J.; El-Shiekh, A.; Shackel, K.; Ahmadi, H. Comparison of Softening-related Changes during Storage of Honeycrisp apple, its Parents, and Delicious. J. Am. Soc. Hortic. Sci. 1999, 124, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Cline, J.A. Commercial Production of Honeycrisp Apples in Ontario. 2009. Available online: http://www.omafra.gov.on.ca/english/crops/facts/05-047.htm (accessed on 20 August 2021).
- Watkins, C.B.; Nock, J.F.; Weis, S.A.; Jayanty, S.; Beaudry, R.M. Storage temperature, diphenylamine, and pre-storage delay effects on soft scald, soggy breakdown and bitter pit of ‘Honeycrisp’ apples. Postharvest Biol. Technol. 2004, 32, 213–221. [Google Scholar] [CrossRef]
- Chiu, G.Z.; Shelp, B.J.; Bowley, S.R.; DeEll, J.R.; Bozzo, G.G. Controlled atmosphere-related injury in ‘Honeycrisp’ apples is associated with γ-aminobutyrate accumulation. Can. J. Plant Sci. 2015, 95, 879–886. [Google Scholar] [CrossRef]
- DeEll, J.R.; Lum, G.B.; Ehsani-Moghaddam, B. Effects of delayed controlled atmosphere storage on disorder development in ‘Honeycrisp’ apples. Can. J. Plant Sci. 2016, 96, 621–629. [Google Scholar]
- Delong, J.M.; Prange, R.K.; Schotsmans, W.C.; Nichols, D.S.; Harrison, P. Determination of the optimal pre-storage delayed cooling regime to control disorders and maintain quality in ‘Honeycrisp’TM apples. J. Hortic. Sci. Biotechnol. 2009, 84, 410–414. [Google Scholar] [CrossRef]
- De Freitas, S.T.; do Amarante, C.V.; Labavitch, J.M.; Mitcham, E.J. Cellular approach to understand bitter pit development in apple fruit. Postharvest Biol. Technol. 2010, 57, 6–13. [Google Scholar] [CrossRef]
- Saure, M.C. Reassessment of the role of calcium in development of bitter pit in apple. Funct. Plant Biol. 1996, 23, 237–243. [Google Scholar] [CrossRef]
- Saure, M.C. Calcium translocation to fleshy fruit: Its mechanism and endogenous control. Sci. Hortic. 2005, 105, 65–89. [Google Scholar] [CrossRef]
- Paliyath, G.; Tiwari, K.; Yuan, H.; Whitaker, B.D. Structural deterioration in produce: Phospholipase D, membrane deterioration, and senescence. In Post-Harvest Biology and Technology of Fruit, Vegetables, and Flowers, 1st ed.; Paliyath, G., Murr, D.P., Handa, A.K., Lurie, S., Gill, K.S., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; Chapter 9; pp. 195–239. [Google Scholar]
- Tiwari, K.; Paliyath, G. Microarray analysis of ripening-regulated gene expression and its modulation by 1-MCP and hexanal. Plant Physiol. Biochem. 2011, 49, 329–340. [Google Scholar] [CrossRef]
- Brown, J.H.; Paliyath, G.; Thompson, J.E. Influence of acyl chain composition on the degradation of phosphatidylcholine by phospholipase D in carnation microsomal membranes. J. Exp. Bot. 1990, 41, 979–986. [Google Scholar] [CrossRef]
- Barry, C.S.; Giovannoni, J.J. Ethylene and fruit ripening. J. Plant Growth Regul. 2007, 26, 143–159. [Google Scholar] [CrossRef]
- Harb, J.; Gapper, N.E.; Giovannoni, J.J.; Watkins, C.B. Molecular analysis of softening and ethylene synthesis and signaling pathways in a non-softening apple cultivar, ‘Honeycrisp’ and a rapidly softening cultivar, ‘McIntosh’. Postharvest Biol. Technol. 2012, 64, 94–103. [Google Scholar] [CrossRef]
- Johnston, J.W.; Hewett, E.W.; Hertog, M.L. Postharvest softening of apple (Malus domestica) fruit: A review. N. Z. J. Crop Hortic. Sci. 2002, 30, 145–160. [Google Scholar] [CrossRef]
- DeEll, J.R.; Ayres, J.T.; Murr, D.P. 1-Methylcyclopropene influences ‘Empire’ and ‘Delicious’ apple quality during long-term commercial storage. Horttechnology 2007, 17, 46–51. [Google Scholar] [CrossRef] [Green Version]
- DeEll, J.R.; Ayres, J.T.; Murr, D.P. 1-Methylcyclopropene concentration and timing of postharvest application alters the ripening of ‘McIntosh’ apples during storage. Horttechnology 2008, 18, 624–630. [Google Scholar] [CrossRef]
- Fan, X.; Mattheis, J.P.; Blankenship, S. Development of apple superficial scald, soft scald, core flush, and greasiness is reduced by MCP. J. Agric. Food Chem. 1999, 47, 3063–3068. [Google Scholar] [CrossRef]
- DeEll, J.R.; Ehsani-Moghaddam, B. Preharvest 1-methylcyclopropene treatment reduces soft scald in ‘Honeycrisp’ apples during storage. HortScience 2010, 45, 414–417. [Google Scholar] [CrossRef] [Green Version]
- Dek, M.S.; Padmanabhan, P.; Subramanian, J.; Paliyath, G. Inhibition of tomato fruit ripening by 1-MCP, wortmannin and hexanal is associated with a decrease in transcript levels of phospholipase D and other ripening related genes. Postharvest Biol. Technol. 2018, 140, 50–59. [Google Scholar] [CrossRef]
- Sakaldas, M.; Gundogdu, M.A. The effects of preharvest 1-methylcyclopropene (Harvista) treatments on harvest maturity of ‘Golden Delicious’ apple cultivar. In Proceedings of the III Balkan Symposium on Fruit Growing, Belgrade, Serbia, 16–18 September 2015; Volume 1139, pp. 601–608. [Google Scholar]
- Nock, J.F.; Watkins, C.B.; James, H.; Reed, N.; Oakes, R.L. Preharvest application of 1-methylcyclopropene (1-MCP) to control fruit drop of apples, and its effects on postharvest quality. In Proceedings of the VI International Postharvest Symposium, Antalya, Turkey, 8–12 April 2009; Volume 877, pp. 365–374. [Google Scholar]
- Doerflinger, F.C.; Sutanto, G.; Nock, J.F.; Shoffe, Y.A.; Zhang, Y.; Watkins, C.B. Stem-end flesh browning of ‘Gala’ apples is decreased by preharvest 1-MCP (Harvista) and conditioning treatments. Fruit Q. 2017, 25, 9–14. [Google Scholar]
- Watkins, C.B.; Nock, J.F.; Kang, I.K.; Ma, Y.; Cheng, Y.; Fargione, M.F. ReTain and Harvista Effects on Maturity and Interactions with SmartFresh on Storage Quality of ‘Honeycrisp’ Apples from Three New York Growing Regions. In Hortscience; American Society for Horticultural Science: Alexandria, VA, USA, 2012; Volume 47, p. S233. [Google Scholar]
- Watkins, C.; Al Shoffe, Y.; Nock, J.F.; Zhang, Y. Harvista Treatment Effects on Quality and Storage Disorders of ‘Honeycrisp’ Apples. In Proceedings of the 2019 ASHS Annual Conference, Las Vegas, NV, USA, 21–25 July 2019. [Google Scholar]
- Vendrell, M.; Buesa, C. Relationship between abscisic acid content and ripening of apples. In Proceedings of the InInternational Symposium on Postharvest Handling of Fruit and Vegetables, Leuven, Belgium, 29 August–2 September 1988; Volume 258, pp. 389–396. [Google Scholar]
- Lara, I.; Vendrell, M. Development of ethylene-synthesizing capacity in preclimacteric apples: Interaction between abscisic acid and ethylene. J. Am. Soc. Hortic. Sci. 2000, 125, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Rudell, D.R.; Fellman, J.K.; Mattheis, J.P. Preharvest application of methyl jasmonate to ‘Fuji’ apples enhances red coloration and affects fruit size, splitting, and bitter pit incidence. HortScience 2005, 40, 1760–1762. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in flowering, fruit set and fruit ripening. Plant Reprod. 2020, 33, 77–87. [Google Scholar] [CrossRef]
- Greene, D.W. The development and use of plant bioregulators in tree fruit production. In Proceedings of the XI International Symposium on Plant Bioregulators in Fruit Production, Bologna, Italy, 20–24 September 2009; Volume 884, pp. 31–40. [Google Scholar]
- Paliyath, G.; Lynch, D.V.; Thompson, J.E. Regulation of membrane phospholipid catabolism in senescing carnation flowers. Physiol. Plant. 1987, 71, 503–511. [Google Scholar] [CrossRef]
- Paliyath, G.; Thompson, J.E. Calcium-and calmodulin-regulated breakdown of phospholipid by microsomal membranes from bean cotyledons. Plant Physiol. 1987, 83, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Poovaiah, B.W. Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci. 2003, 8, 505–512. [Google Scholar] [CrossRef]
- Poovaiah, B.W.; Du, L.; Wang, H.; Yang, T. Recent advances in calcium/calmodulin-mediated signaling with an emphasis on plant-microbe interactions. Plant Physiol. 2013, 163, 531–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.; Xiong, T.; Li, X.; Chen, W.; Zhu, X. Calcium and calcium sensors in fruit development and ripening. Sci. Hortic. 2019, 253, 412–421. [Google Scholar] [CrossRef]
- Li, C.; Meng, D.; Zhang, J.; Cheng, L. Genome-wide identification and expression analysis of calmodulin and calmodulin-like genes in apple (Malus× domestica). Plant Physiol. Biochem. 2019, 139, 600–612. [Google Scholar] [CrossRef]
- Jincy, M.; Djanaguiraman, M.; Jeyakumar, P.; Subramanian, K.S.; Jayasankar, S.; Paliyath, G. Inhibition of phospholipase D enzyme activity through hexanal leads to delayed mango (Mangifera indica L.) fruit ripening through changes in oxidants and antioxidant enzymes activity. Sci. Hortic. 2017, 218, 316–325. [Google Scholar] [CrossRef]
- Yumbya, P.M.; Hutchinson, M.J.; Ambuko, J.; Owino, W.O.; Sullivan, A.; Paliyath, G.; Subramanian, J. Efficacy of hexanal application on the postharvest shelf life and quality of banana fruit (Musa acuminata) in Kenya. J. Trop. Agric. 2018, 95, 14–35. [Google Scholar]
- Sriskantharajah, K.; El Kayal, W.; Torkamaneh, D.; Ayyanath, M.M.; Saxena, P.K.; Sullivan, A.J.; Paliyath, G.; Subramanian, J. Transcriptomics of improved fruit retention by hexanal in ‘Honeycrisp’ reveals hormonal crosstalk and reduced cell wall degradation in the fruit abscission zone. Int. J. Mol. Sci. 2021, 22, 8830. [Google Scholar] [CrossRef]
- Sharma, M.; Jacob, J.K.; Subramanian, J.; Paliyath, G. Hexanal and 1-MCP treatments for enhancing the shelf life and quality of sweet cherry (Prunus avium L.). Sci. Hortic. 2010, 125, 239–247. [Google Scholar] [CrossRef]
- Cheema, A.; Padmanabhan, P.; Amer, A.; Parry, M.J.; Lim, L.T.; Subramanian, J.; Paliyath, G. Postharvest hexanal vapor treatment delays ripening and enhances shelf life of greenhouse grown sweet bell pepper (Capsicum annum L.). Postharvest Biol. Technol. 2018, 136, 80–89. [Google Scholar] [CrossRef]
- DeBrouwer, E.J.; Sriskantharajah, K.; El Kayal, W.; Sullivan, J.A.; Paliyath, G.; Subramanian, J. Pre-harvest hexanal spray reduces bitter pit and enhances postharvest quality in ‘Honeycrisp’ apples (Malus domestica Borkh.). Sci. Hortic. 2020, 273, 109610. [Google Scholar] [CrossRef]
- Paliyath, G.; Murr, D.P. Compositions for the Preservation of Fruit and Vegetables. U.S. Patent 6,514,914, 3 April 2007. [Google Scholar]
- El Kayal, W.; Paliyath, G.; Sullivan, J.A.; Subramanian, J. Phospholipase D inhibition by hexanal is associated with calcium signal transduction events in raspberry. Hortic. Res. 2017, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Johnston, J.W.; Hewett, E.W.; Hertog, M.L.; Harker, F.R. Harvest date and fruit size affect postharvest softening of apple fruit. J. Hortic. Sci. 2002, 77, 355–360. [Google Scholar] [CrossRef]
- Palego, L.; Betti, L.; Rossi, A.; Giannaccini, G. Tryptophan biochemistry: Structural, nutritional, metabolic, and medical aspects in humans. J. Amino Acids 2016, 2016, 8952520. [Google Scholar] [CrossRef] [Green Version]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Ludford, P.M. Hormonal changes during postharvest. In Postharvest Physiology and Pathology of Vegetables, 2nd ed.; Bartz, J.A., Brecht, K.B., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 57–107. ISBN 9780824706876. [Google Scholar]
- Ranty, B.; Aldon, D.; Galaud, J.P. Plant calmodulins and calmodulin-related proteins: Multifaceted relays to decode calcium signals. Plant Signal. Behav. 2006, 1, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Peng, H.; Bauchan, G.R. Functional analysis of tomato calmodulin gene family during fruit development and ripening. Hortic. Res. 2016, 1, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.; Zhang, L.; Hao, Y.; Xiao, S.; Wu, Z.; Chen, W.; Li, X.; Zhu, X. Genome-wide identification and expression analyses of the calmodulin and calmodulin-like proteins reveal their involvement in stress response and fruit ripening in papaya. Postharvest Biol. Technol. 2018, 143, 13–27. [Google Scholar] [CrossRef]
- Lara, I.; Heredia, A.; Domínguez, E. Shelf life potential and the fruit cuticle: The unexpected player. Front. Plant. Sci. 2019, 10, 770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukumoto, M.; Nagai, K.; Yoshioka, H.; Aoba, K. Mechanism of the development of a calcium-related disorder (bitter pit) in apple. JARQ 1987, 20, 248–252. [Google Scholar]
- Jemrić, T.; Fruk, I.; Fruk, M.; Radman, S.; Sinkovič, L.; Fruk, G. Bitter pit in apples: Pre-and postharvest factors: A review. Span. J. Agric. Res. 2016, 14, 15. [Google Scholar] [CrossRef]
- Kumar, S.K.; El Kayal, W.; Sullivan, J.A.; Paliyath, G.; Jayasankar, S. Pre-harvest application of hexanal formulation enhances shelf life and quality of ‘Fantasia’ nectarines by regulating membrane and cell wall catabolism-associated genes. Sci. Hortic. 2018, 229, 117–124. [Google Scholar] [CrossRef]
- Taheri-Garavand, A.; Mumivand, H.; Fanourakis, D.; Fatahi, S.; Taghipour, S. An artificial neural network approach for non-invasive estimation of essential oil content and composition through considering drying processing factors: A case study in Mentha aquatica. Ind. Crop. Prod. 2021, 171, 113985. [Google Scholar] [CrossRef]
- Erland, L.A.; Shukla, M.R.; Glover, W.B.; Saxena, P.K. A simple and efficient method for analysis of plant growth regulators: A new tool in the chest to combat recalcitrance in plant tissue culture. Plant Cell Tissue Organ Cult. 2017, 131, 459–470. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| Parameter | Treatments | |||
|---|---|---|---|---|
| Control | Hexanal | HarvistaTM | ||
| Firmness (N) | 57.07 ± 1.54 a | 60.05 ± 1.67 a | 59.09 ± 1.24 a | |
| TSS (°Brix) | 13.23 ± 0.08 a | 13.51 ± 0.0.1 a | 13.57 ± 0.08 a | |
| Blush Color | a * | 32.72 ± 0.24 a | 31.58 ± 0.48 a | 30.44 ± 0.35 a |
| b * | 15.23 ± 0.08 a | 13.11 ± 0.20 a | 13.35 ± 0.27 a | |
| Lightness (L) | 32.01 ± 1.69 a | 35.41 ± 1.68 a | 37.95 ± 1.68 a | |
| Chroma (C) | 36.13 ± 1.86 a | 34.28 ± 1.85 a | 33.28 ± 1.86 a | |
| Hue Angle (H) | 24.98 ± 1.88 a | 23.11 ± 1.88 a | 23.76 ± 1.88 a | |
| Background Color | a * | −2.84 ± 0. 50 b | 08.33 ± 0.62 ab | 12.80 ± 0.80 a |
| B * | 25.53 ± 0.21 a | 20.63 ± 0.21 a | 20.64 ± 0.39 a | |
| Lightness (L) | 59.79 ± 2.76 a | 55.05 ± 4.34 a | 52.25 ± 2.78 a | |
| Chroma (C) | 24.37 ± 1.80 a | 23.78 ± 1.73 a | 28.54 ± 1.34 a | |
| Hue Angle (H) | 95.22 ± 10.52 a | 69.12 ± 10.52 a | 62.96 ± 8.95 a | |
| Phytohormonesand metabolites | Ethylene (nL/kg/hr) | 48.83 ± 1.38 a | 39.97 ± 2.29 ab | 36.00 ± 2.05 b |
| ABA (ng/g, DW) | 737.73 ± 10.8 b | 603.47 ± 12.03 c | 968.41 ± 11.71 a | |
| Zeatin (ng/g, DW) | 423.49 ± 8.81 b | 650.91 ± 8.77 a | 735.02 ± 9.61 a | |
| Melatonin (ng/g, DW) | 164.19 ± 7.05 a | 128.87 ± 5.4 a | 135.50 ± 4.31 a | |
| Tryptophan (ng/g, DW) | 4496.46 ± 117 c | 12,964.0 ± 161 a | 9220.23 ± 90 b | |
| Parameter | Treatment | Storage Time (Days) | ||||
|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | ||
| Firmness (N) | Control | 57.07 (1.54) a–c | 54.87 (2.46) a–d | 52.58 (1.01) a–d | 51.71 (1.01) cd | 47.38 (1.84) b-d |
| Hexanal | 60.05 (1.67) ab | 57.15 (2.36) a–c | 54.93 (1.53) a–d | 52.73 (1.33) a–d | 52.05 (2.18) a–d | |
| Harvista | 59.09 (1.24) ab | 57.94 (1.65) a–c | 54.01 (1.82) a–d | 52.88 (1.33) a–d | 49.79 (1.01) cd | |
| TSS (°Brix) | Control | 13.23 (0.08) a–c | 13.55 (0.16) ab | 12.89 (0.18) c | 12.93 (0.11) bc | 13.03 (0.15) a–c |
| Hexanal | 13.51 (0.10) ab | 13.65 (0.11) a | 13.56 (0.19) ab | 13.60 (0.05) a | 13.40 (0.17) a–c | |
| Harvista | 13.57 (0.08) a | 13.44 (0.17) ab | 13.58 (0.19) a | 13.44 (0.09) ab | 13.45 (0.18) ab | |
| Removal after 30 d | Removal after 60 d | Removal after 90 d | |||||
|---|---|---|---|---|---|---|---|
| Parameter | Treatment | 7 Days | 14 Days | 7 Days | 14 Days | 7 Days | 14 Days |
| Weight (g) | Control | 261 ± 17.6 bc | 263 ± 17.6 bc | 259 ± 14.2 b | 254 ± 17.5 b | 343 ± 22.1 a | 334 ± 21.0 a |
| Hexanal | 284 ± 17.8 a | 285 ± 8.0 a | 282 ± 18.0 a | 278 ± 18.0 a | 339 ± 28.79 a | 314 ± 55.45 ab | |
| Harvista | 258 ± 3.1 c | 261 ± 2.7 c | 257 ± 3.2 b | 252 ± 4.1 b | 279 ± 28.49 b | 266 ± 28.29 b | |
| Firmness (N) | Control | 54.31 ± 2.55 c | 54.78 ± 3.87 b | 55.73 ± 3.83 b | 52.82 ± 4.91 a | 50.72 ± 4.04 a | 52.69 ± 4.51 a |
| Hexanal | 59.8 ± 3.99 b | 60.82 ± 5.02 a | 58.79 ± 5.81 ab | 54.99 ± 2.09 a | 53.93 ± 2.96 a | 51.52 ± 3.87 a | |
| Harvista | 64.07 ± 2.8 a | 64.48 ± 1.38 a | 60.76 ± 2.42 a | 52.08 ± 7.4 a | 52.19 ± 3.32 a | 49.27 ± 4.99 a | |
| TSS (°Brix) | Control | 12.15 ± 0.42 b | 12.03 ± 0.82 b | 12.30 ± 0.07 b | 12.48 ± 0.33 a | 12.35 ± 0.07 c | 12.82 ± 0.43 b |
| Hexanal | 13.06 ± 0.13 a | 12.98 ± 0.07 a | 13.00 ± 0.11 a | 12.86 ± 0.09 a | 13.77 ± 0.24 a | 13.50 ± 0.5 a | |
| Harvista | 12.92 ± 0.18 a | 12.98 ± 0.26 a | 12.85 ± 0.21 a | 12.87 ± 1.56 a | 13.02 ± 0.49 b | 13.72 ± 0.76 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sriskantharajah, K.; El Kayal, W.; Ayyanath, M.M.; Saxena, P.K.; Sullivan, A.J.; Paliyath, G.; Subramanian, J. Preharvest Spray Hexanal Formulation Enhances Postharvest Quality in ‘Honeycrisp’ Apples by Regulating Phospholipase D and Calcium Sensor Proteins Genes. Plants 2021, 10, 2332. https://doi.org/10.3390/plants10112332
Sriskantharajah K, El Kayal W, Ayyanath MM, Saxena PK, Sullivan AJ, Paliyath G, Subramanian J. Preharvest Spray Hexanal Formulation Enhances Postharvest Quality in ‘Honeycrisp’ Apples by Regulating Phospholipase D and Calcium Sensor Proteins Genes. Plants. 2021; 10(11):2332. https://doi.org/10.3390/plants10112332
Chicago/Turabian StyleSriskantharajah, Karthika, Walid El Kayal, Murali Mohan Ayyanath, Praveen K. Saxena, Alan J. Sullivan, Gopinadhan Paliyath, and Jayasankar Subramanian. 2021. "Preharvest Spray Hexanal Formulation Enhances Postharvest Quality in ‘Honeycrisp’ Apples by Regulating Phospholipase D and Calcium Sensor Proteins Genes" Plants 10, no. 11: 2332. https://doi.org/10.3390/plants10112332
APA StyleSriskantharajah, K., El Kayal, W., Ayyanath, M. M., Saxena, P. K., Sullivan, A. J., Paliyath, G., & Subramanian, J. (2021). Preharvest Spray Hexanal Formulation Enhances Postharvest Quality in ‘Honeycrisp’ Apples by Regulating Phospholipase D and Calcium Sensor Proteins Genes. Plants, 10(11), 2332. https://doi.org/10.3390/plants10112332







