Green Synthesis of Silver Nanoparticles Using Pomegranate and Orange Peel Extracts and Their Antifungal Activity against Alternaria solani, the Causal Agent of Early Blight Disease of Tomato
Abstract
:1. Introduction
2. Results
2.1. Identification of the Fungal Pathogen
2.1.1. Morphological Identification of Alternaria Isolates
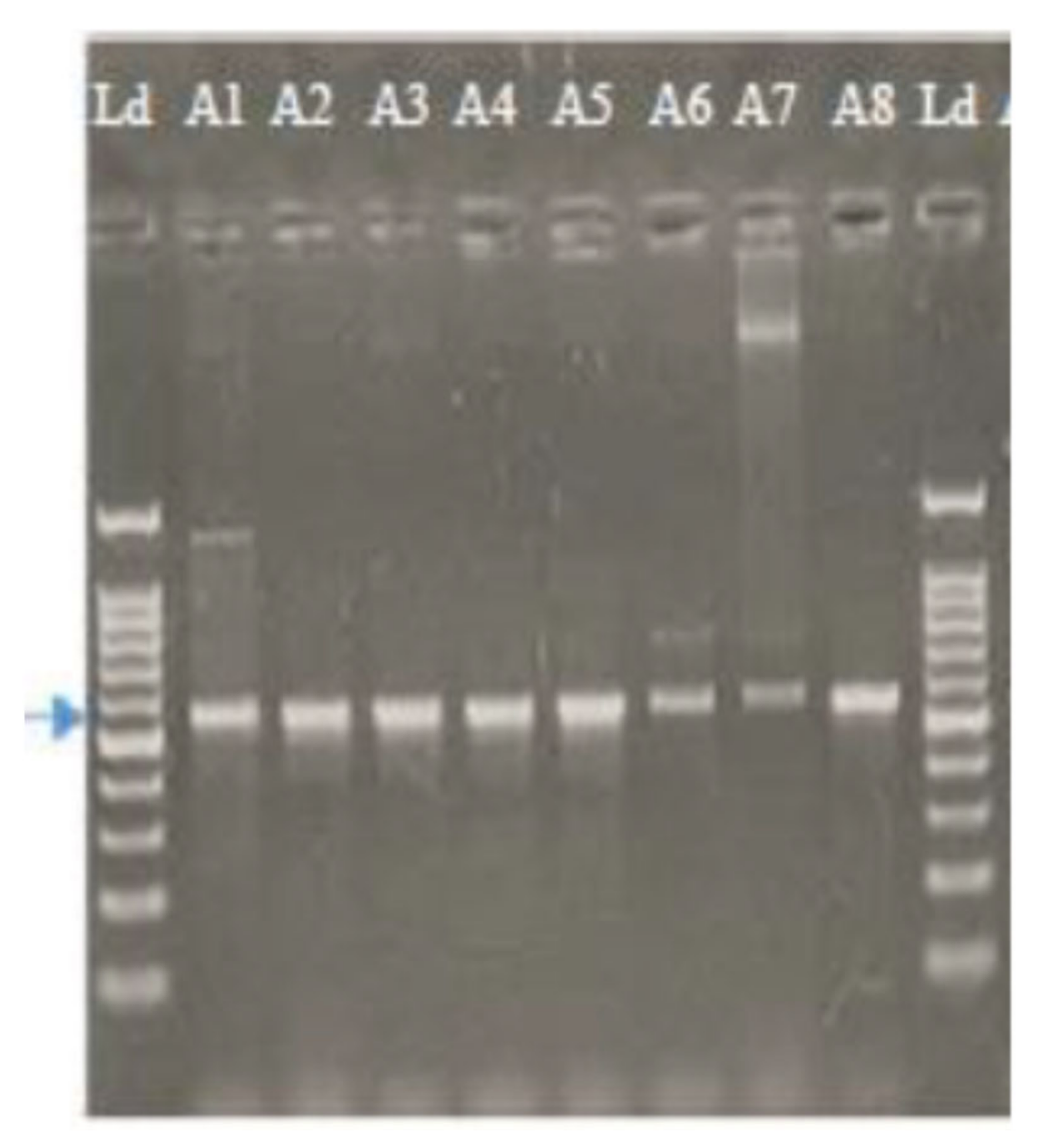
2.1.2. Molecular Characterization of Alternaria Isolates Using Sequence Analysis of the ITS Region
2.2. Pathogenicity Tests
2.3. Total Phenolic Content
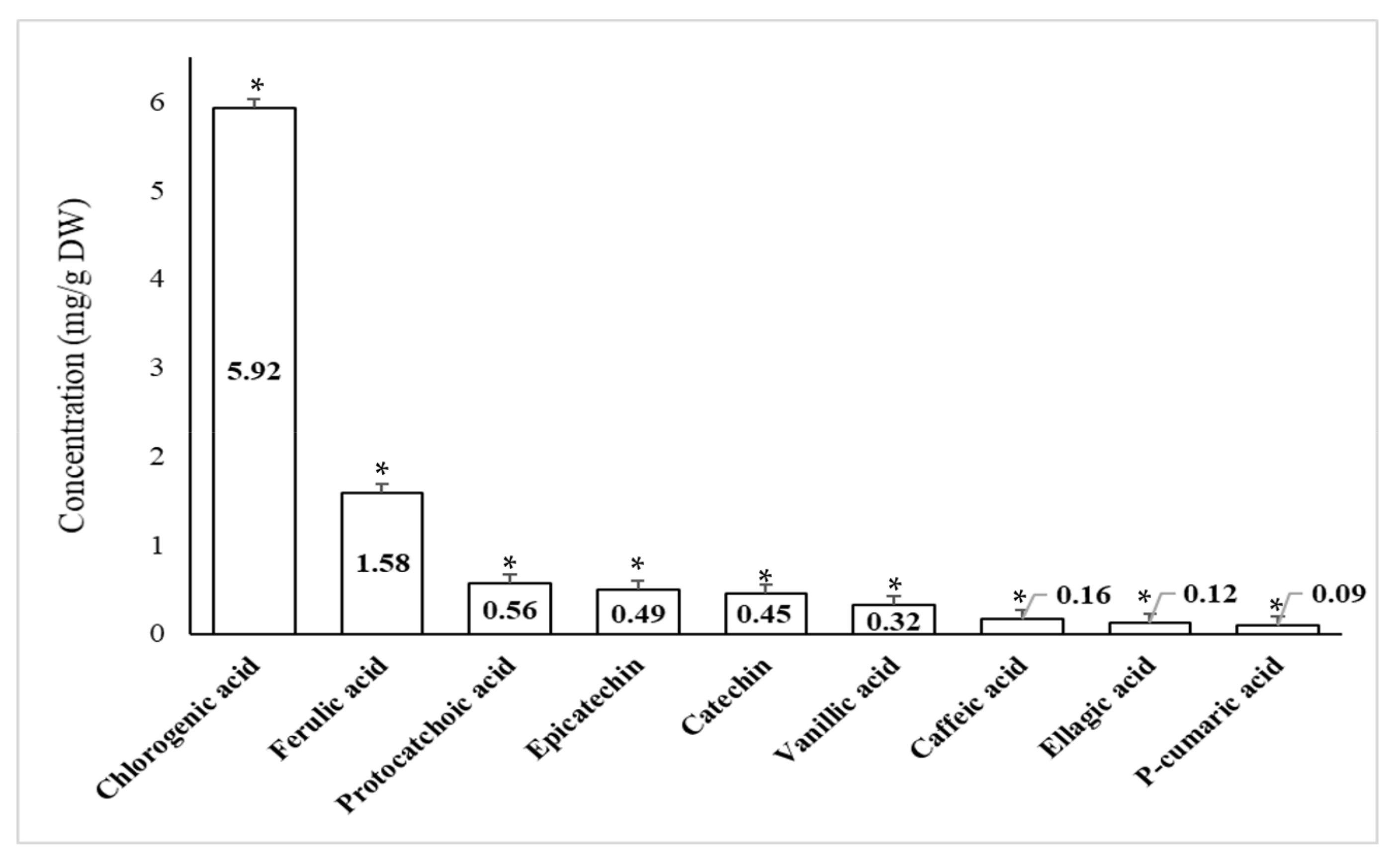
2.4. Quantitative Phenolic Compounds Present in Peel Extracts of Pomegranate and Orange Using High Performance Liquid Chromatography (HPLC)
2.5. Characteriuzation of AgPNs
2.5.1. Ultraviolet–Visible Spectroscopy (UV-Vis Spectroscopy)
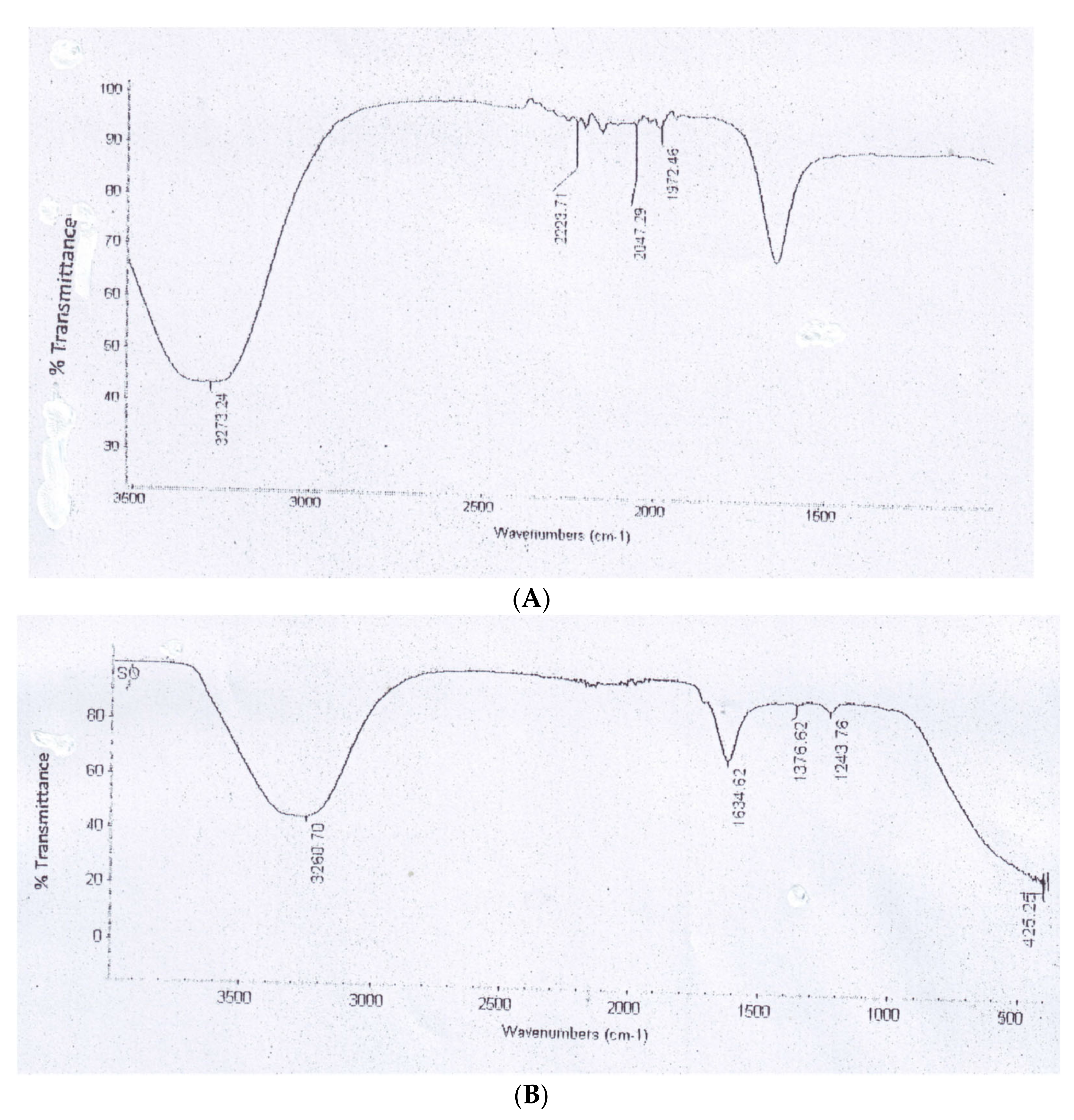
2.5.2. Fourier Transform Infrared Spectroscopy (FTIR)
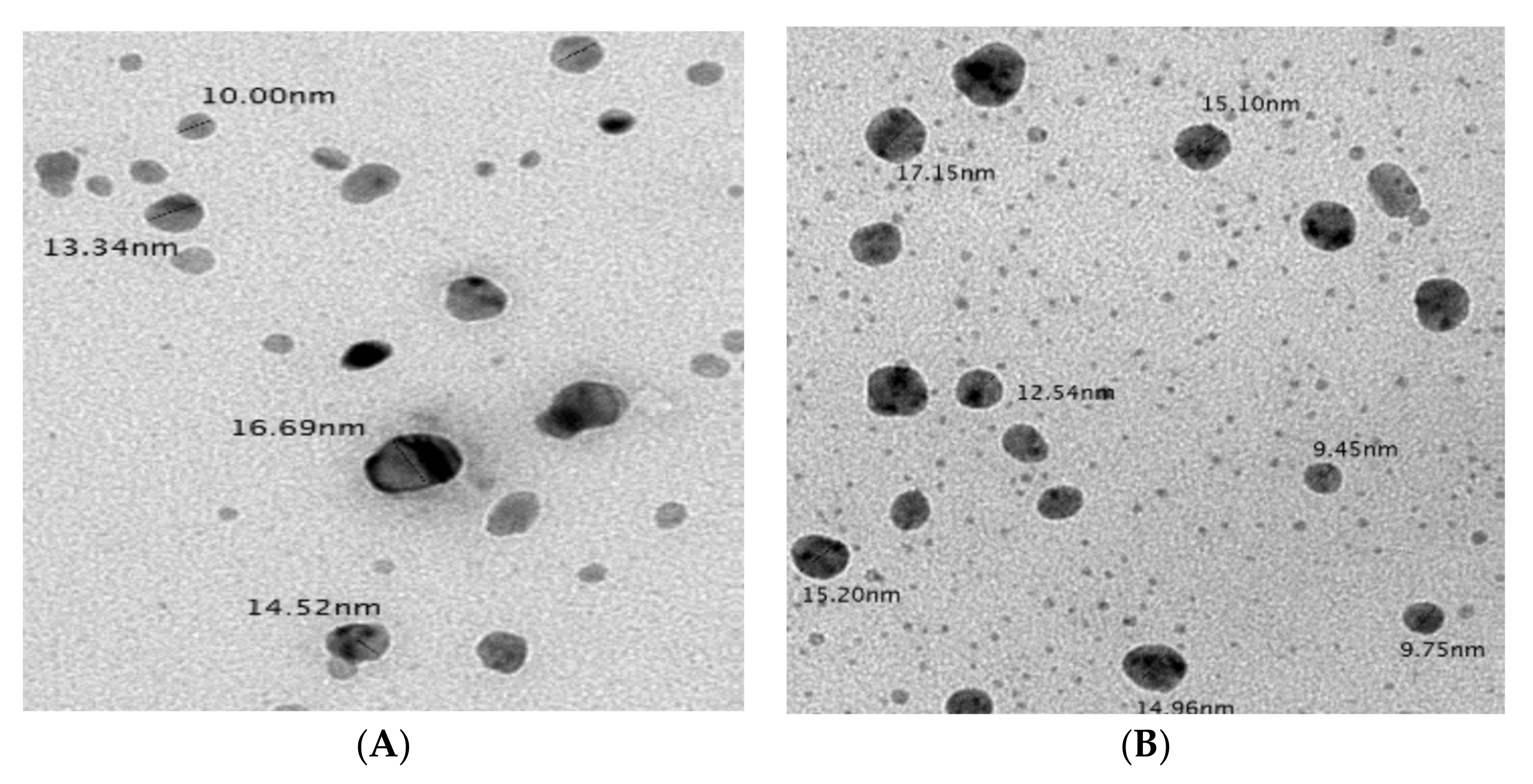
2.5.3. Transmission Electron Microscopy
2.5.4. Zeta Potential and Particle Size
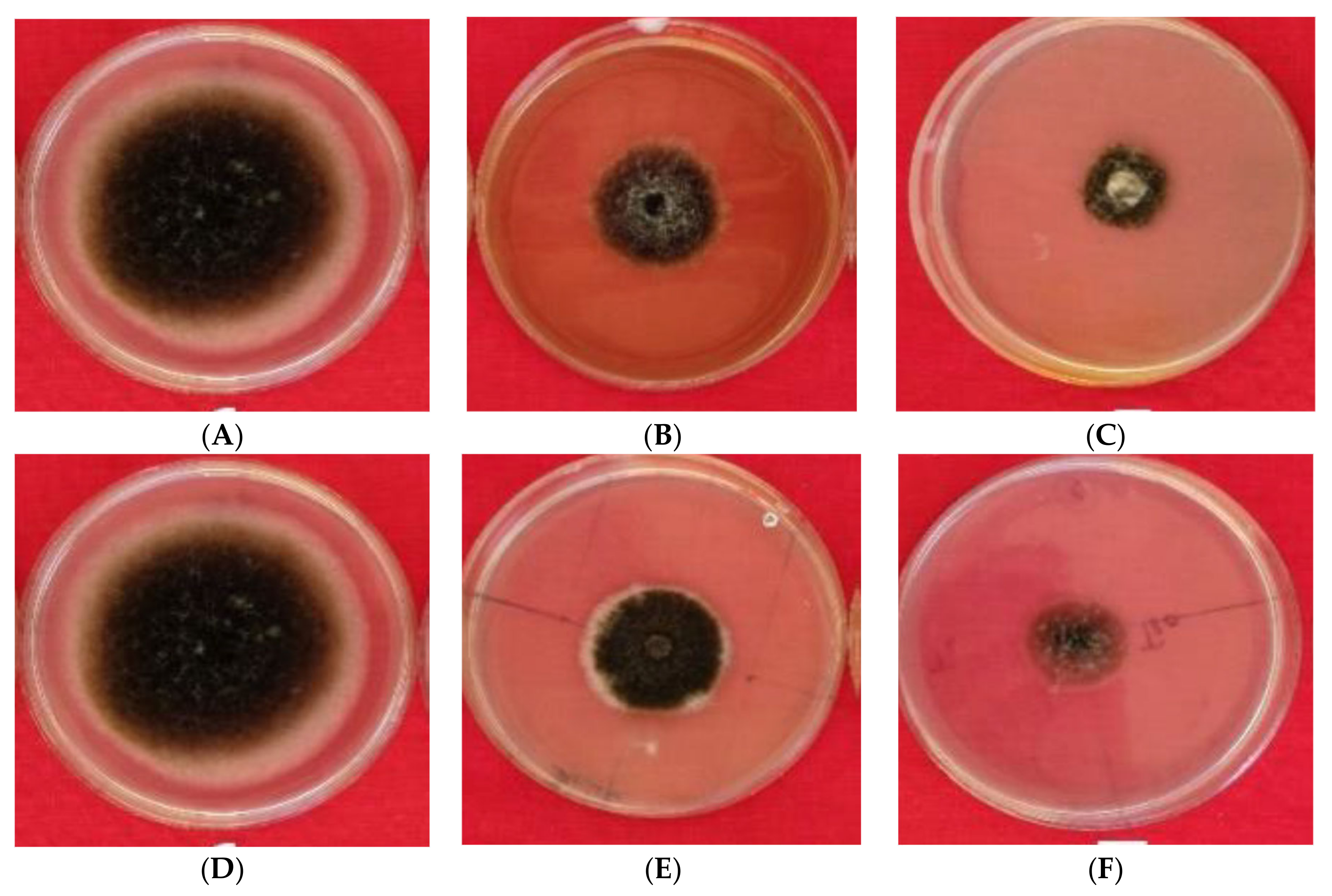
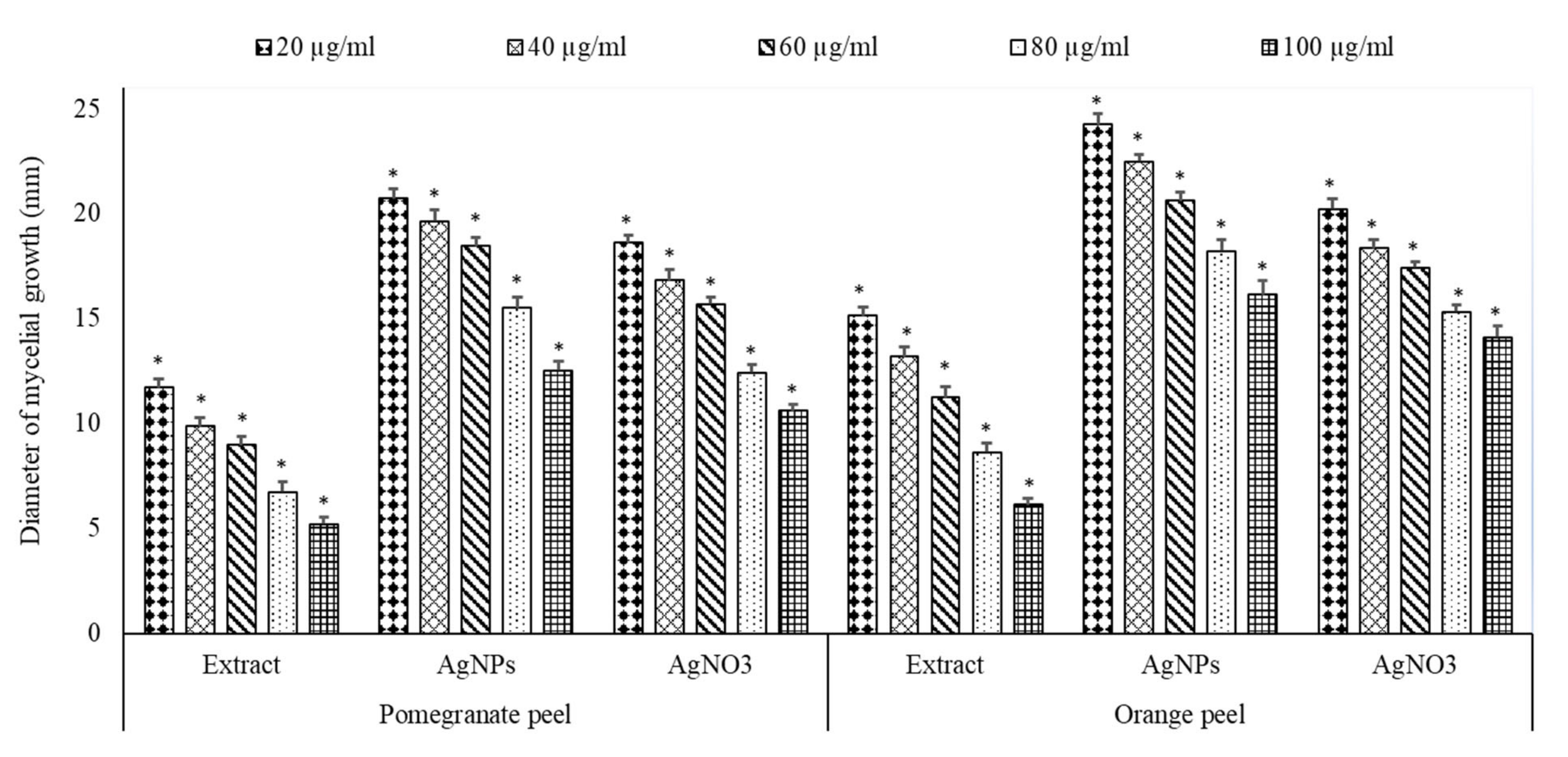
2.6. Antifungal Activity
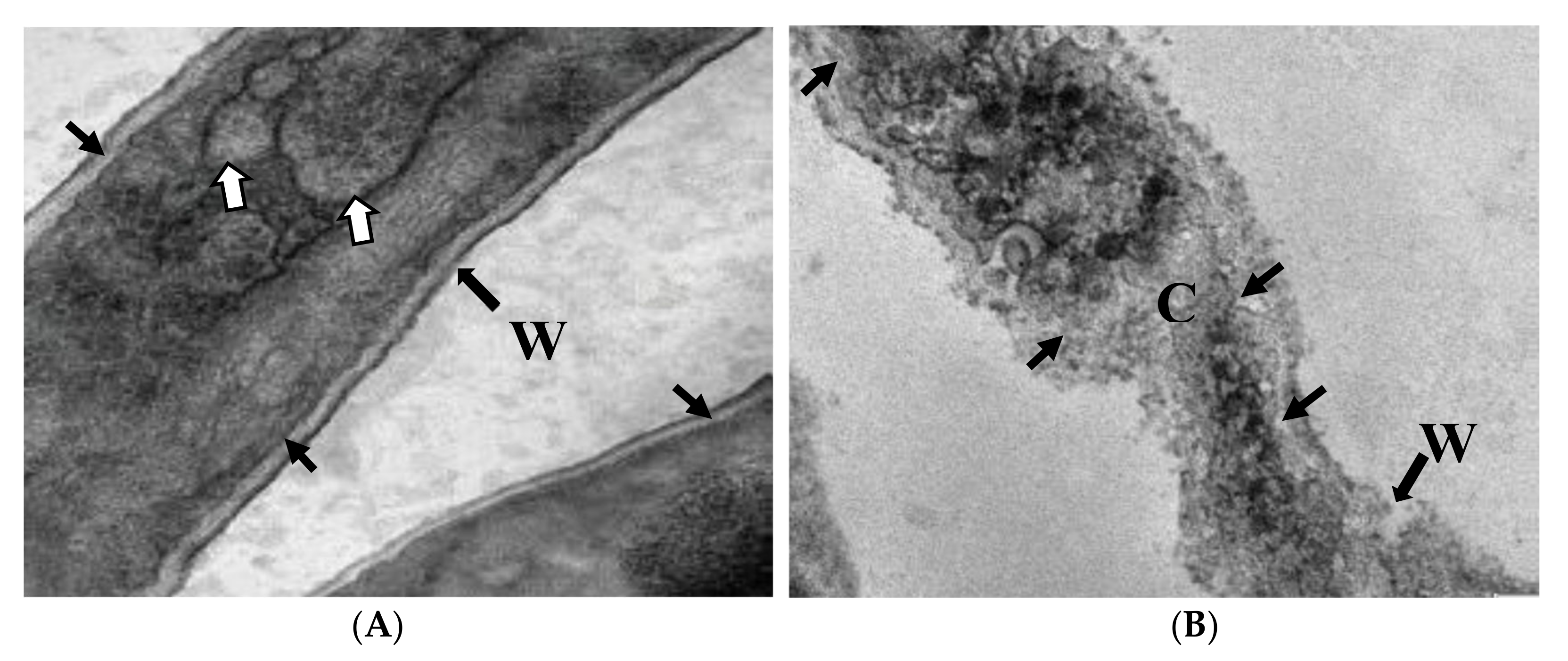
2.7. Ultrastructural Studies
3. Discussion
4. Materials and Methods
4.1. Isolation of the Fungal Pathogen
4.2. Pathogenicity Test
4.3. Identification of the Fungal Pathogen
4.3.1. Morphological Features
4.3.2. Molecular Identification of Alternaria isolates
Extraction of DNA
Polymerase Chain Reaction
4.4. Collection and Drying of Selected Fruit Peels
4.5. Preparation of Peel Extracts
4.6. Determination of Total Phenolic Compounds
4.7. Determination of Phenolic Compounds by HPLC
4.8. Biosynthesis of Silver Nanoparticles (AgNPs)
4.9. Characterization of Silver Nanoparticles
4.10. Antifungal Activity of Crud Peel Extracts and Their Biosynthesized AgNPs
4.11. Ultrastructural Studies
4.12. Statistical Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Ganainy, S.M.; El-Abeid, S.E.; Ahmed, Y.; Iqbal, Z. Morphological and molecular characterization of large-spored Alternaria species associated with potato and tomato early blight in Egypt. Int. J. Agric. Biol. 2021, 25, 1101–1110. [Google Scholar] [CrossRef]
- Sharma, O.P.; Pruthi, S.; Mohan, G.; Kaur, M.; Kumari, M. Assessment of fungicides against early blight of tomato induced by Alternaria solani (Ellis & Martin) under field conditions. Int. J. Chem. Stud. 2020, 8, 693–696. [Google Scholar] [CrossRef]
- Baka, Z. Antifungal activity of six Saudi medicinal plant extracts against five phyopathogenic fungi. Arch. Phytopathol. Plant Prot. 2010, 43, 736–743. [Google Scholar] [CrossRef]
- Baka, Z. Antifungal activity of extracts from five Egyptian wild medicinal plants against late blight disease of tomato. Arch. Phytopathol. Plant Prot. 2014, 47, 1988–2002. [Google Scholar] [CrossRef]
- Baka, Z. Plant extract control of the fungi associated with different Egyptian wheat cultivars grains. J. Plant Prot. Res. 2014, 54, 231–237. [Google Scholar] [CrossRef]
- Baka, Z. Efficacy of wild medicinal plant extracts against predominant seed-borne fungi of broad bean cultivars. Acta Phytopathol. Entomol. Hung. 2015, 50, 45–66. [Google Scholar] [CrossRef] [Green Version]
- Baka, Z.A.M.; Rashad, Y.M. Alternative control of early blight of tomato using plant extracts from Acacia nilotica, Achillea fragrantissima and Calotropis procera. Phytopathol. Mediterr. 2016, 55, 121–129. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Amin, B.H.; Baka, Z. Efficiency of pomegranate (Punica granatum L.) peels extract as a high potential natural tool towards Fusarium dry rot on potato tubers. Postharvest Biol. Technol. 2016, 111, 256–263. [Google Scholar] [CrossRef]
- Ahmad, F.; Raziq, F.; Ullah, N.; Khan, H.; Din, N. In vitro and in vivo bioassay of phytobiocidal effect of plant extracts on Alternaria solani causing agent of early blight disease in tomato. Arch. Phytopathol. Plant Prot. 2017, 50, 568–583. [Google Scholar] [CrossRef]
- Khaledi, N.; Hassani, F. Antifungal activity of Bunium persicum essential oil and its constituents on growth and pathogenesis of Colletotrichum lindemuthianum. J. Plan. Prot. Res. 2018, 58, 431–441. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Behiry, S.I.; Salem, M.Z.M.; Ali, H.M.; Siddiqui, M.H.; Salem, A. Antifungal, antibacterial, and antioxidanta of Acacia saligna (Labill.) H. L. Wendl. flower extract: HPLC analysis of phenolic and flavonoid compounds. Molecules 2019, 24, 700. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Bhatnagar, S.K.; Tomar, A. Study on fungicidal effect of plant extracts on plant pathogenic fungi and the economy of extract preparation and efficacy in comparison to synthetic/chemical fungicides. J. Appl. Nat. Sci. 2019, 11, 333–337. [Google Scholar] [CrossRef]
- Baka, Z.A.M.; Mousa, M.A. In vitro and in vivo, biocontrol activity of extracts prepared from Egyptian indigenous medicinal plants for the management of anthracnose of mango fruits. Arch. Phytopathol. Plant Prot. 2020, 53, 715–730. [Google Scholar] [CrossRef]
- Hernández-Ceja, A.; Loeza-Lara, P.D.; Espinosa-García, F.J.; García-Rodríguez, Y.M.; José Medina-Medrano, R.M.; Gutiérrez-Hernández, G.F.; Ceja-Torres, L.F. In vitro antifungal activity of plant extracts on pathogenic fungi of blueberry (Vaccinium sp.). Plants 2021, 10, 852. [Google Scholar] [CrossRef]
- Belgacem, I.; Maria, G.; Nicosia, L.D.; Pangallo, S.; Abdelfattah, A.; Benuzzi, M.; Agosteo, G.A.; Schena, L. Pomegranate peel extracts as safe natural treatments to control plant siseases and increase the shelf-life and safety of fresh fruits and vegetables. Plants 2021, 10, 453. [Google Scholar] [CrossRef]
- Tenore, G.C.; Novellino, E.; Basile, A. Nutraceutical potential and antioxidant benefits of red pitaya (Hylocereus polyrhizus) extracts. J. Funct. Foods 2012, 4, 129–136. [Google Scholar] [CrossRef]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; ElSohly, M.A.; Khan, I.A. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative dtudy. Evid. Based Complement. Alternat. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef]
- Wadhwa, M.; Bakshi, M.P.S.; Makkar, H.P.S. Wastes to worth: Value added products from fruit and vegetable wastes. CAB Rev. Perspect. Agric. Vet. 2015, 10, 43–2015. [Google Scholar] [CrossRef]
- Kafshgari, M.H.; Voelcker, N.H.; Harding, F.J. Applications of zero valent silicon nanostructures in biomedicine. Nanomedicine 2015, 10, 2553–2571. [Google Scholar] [CrossRef]
- Sharma, R.K.; Gulati, S.; Mehta, S. Preparation of gold nanoparticles using tea: A green chemistry experiment. J. Chem. Educ. 2012, 89, 1316–1318. [Google Scholar] [CrossRef]
- Khatami, M.; Pourseyedi, S.; Khatami, M.; Hamidi, H.; Zaeifi, M.; Soltani, L. Synthesis of silver nanoparticles using seed exudates of Sinapis arvensis as a novel bioresource, and evaluation of their antifungal activity. Bioresour. Bioprocess. 2015, 2, 19–20. [Google Scholar] [CrossRef] [Green Version]
- El-Dein, M.M.N.; Baka, Z.A.M.; Abou-Dobara, M.I.; El-Sayed, A.K.A.; El-Zahed, M.M. Extracellular biosynthesis, optimization, characterization and antimicrobial potential of Escherichia coli D8 silver nanoparticles. JMBFS 2021, 10, 648–656. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Karimadom, B.R.; Kornweitz, H. Mechanism of producing metallic nanoparticles, with an emphasis on silver and gold nanoparticles, using bottom-up methods. Molecules 2021, 26, 2968. [Google Scholar] [CrossRef] [PubMed]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostruct. Chem. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Singh, B.R.; Singh, A.; Keswani, C.; Naqvi, A.H.; Singh, H.B. Biofabricated silver nanoparticles act as a strong fungicide against Bipolaris sorokiniana causing spot blotch disease in wheat. PLoS ONE 2014, 9, e97881. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Pandey, S.; Bhattacharya, A.; Mishra, A.; Nautiyal, C.S. Protective role of biosynthesized silver nanoparticles against early blight disease in Solanum lycopersicum. Plant Physiol. Biochem. 2017, 121, 216–225. [Google Scholar] [CrossRef]
- Ahmed, R.H.; Mustafa, D.E. Green synthesis of silver nanoparticles mediated by traditionally used medicinal plants in Sudan. Int. Nano Lett. 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Naik, M.K.; Prasad, Y.; Bhat, K.V.; Rani, G.S.D. Morphological, physiological, pathogenic and molecular variability among isolates of Alternaria solani from tomato. Indian Phytopthol. 2010, 63, 168–173. [Google Scholar]
- Singh, A.; Singh, V.; Yadav, S.M. Cultural, morphological and pathogenic variability of Alternaria solani causing early blight in tomato. Plant Pathol. J. 2014, 13, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Nikam, P.S.; Suryawanshi, A.P.; Chavan, A.A. Pathogenic, cultural, morphological and molecular variability among eight isolates of Alternaria solani, causing early blight of tomato. Afr. J. Biotechnol. 2015, 14, 872–877. [Google Scholar] [CrossRef] [Green Version]
- Rahmatzai, N.; Zaitoun, A.A.; Madkour, M.H.; Abdullah Ahmady, A.; Zainullah Hazim, Z.; Magdi, A.A.; Mousa, M.A.A. Morphologicaql, pathogenic, cultural and physiological variability of the isolates of Alternaria solani causing eaqrly blight of tomato. Int. J. Adv. Res. 2016, 4, 808–817. [Google Scholar] [CrossRef] [Green Version]
- Mugao, L.G.; Muturi, P.W.; Gichimu, B.M.; Kamir, A.K. Morphological and molecular characterization of Alternaria solani and Phytophthora infestans isolates from tomato farms in Kenya. Plant Pathol. J. 2021, 20, 29–40. [Google Scholar] [CrossRef]
- Okayo, R.O.; Andika, D.O.; Dida, M.M.; K’Otuto, G.O.; Gichimu, B.M. Morphological and molecular characterization of toxigenic Aspergillus flavus from groundnut kernels in Kenya. Int. J. Microbiol. 2020, 2020, 8854718. [Google Scholar] [CrossRef]
- Simmons, E.G. Alternaria: An Identification Manual; CBS Fungal Biodiversity Centre: Utrecht, The Netherlands, 2007; p. 775. ISBN 13978-9070351687. [Google Scholar]
- Leiminger, J.H.; Auinger, H.J.; Wenig, M.; Bahnweg, G.; Hausladen, H. Genetic variability among Alternaria solani isolates from potatoes in Southern Germany based on rapd-profiles. J. Plant Dis. Prot. 2013, 120, 164–172. [Google Scholar] [CrossRef]
- Meng, J.W.; Zhu, W.; He, M.H.; Wu, E.J.; Yang, L.N.; Shang, L.P.; Zhan, J. High genotype diversity and lack of isolation by distance in the Alternaria solani populations from China. Plant Pathol. 2015, 64, 434–441. [Google Scholar] [CrossRef]
- Chaerani, R.; Voorrips, R.E. Tomato early blight (Alternaria solani): The pathogen, genetics and breeding for resistance. J. Gen. Plant Pathol. 2006, 72, 335–347. [Google Scholar] [CrossRef]
- Craven, K.D.; Velez, H.; Cho, Y.; Lawrence, C.B.; Mitchell, T.K. Anastomosis is required for virulence of the fungal necrotroph Alternaria brassicicola. Eukaryot. Cell 2008, 7, 675–683. [Google Scholar] [CrossRef] [Green Version]
- McDonald, B.A.; Linde, C. Pathogen population genetics evolutionary potential and durable resistance. Annu. Rev. Phytopathol. 2002, 40, 349–379. [Google Scholar] [CrossRef] [Green Version]
- Van der Waals, J.E.; Korsten, L.; Slippers, B. Genetic diversity among Alternaria solani isolates from potatoes in South Africa. Plant Dis. 2004, 88, 959–964. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol. Plant Pathol. 2010, 11, 705–719. [Google Scholar] [CrossRef]
- Farkas, G.L.; Kiraaly, Z. Role of Phenolic Compounds in the Physiology of Plant Diseases and Disease Resistance. J. Phytopathol. 2008, 44, 105–150. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry Advances in Research; Imperato, F., Ed.; Research Signpost: Kerala, India, 2006; pp. 23–67. [Google Scholar]
- Langcake, P.; Irvine, J.A.; Jeger, M.J. Alternative chemical agents for controlling plant disease. Phil. Trans. R. Soc. Lond. B Biol. Sci. 1981, 295, 83–101. [Google Scholar]
- Karou, D.; Dicko, M.H.; Simpore, J.; Traore, A.S. Antioxidant and antibacterial activities of polyphenols from ethnomedicinal plants of Burkina Faso. Afr. J. Biotechnol. 2005, 4, 823–828. [Google Scholar]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Kaviya, S.; Santhanalakshmi, J.; Viswanathan, B.; Muthumar, J.; Srinivasan, K. Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Usha, S.; Ramappa, K.T.; Hiregoudar, S.; Vasanthkumar, G.D.; Aswathanarayana, D.S. Biosynthesis and characterization of copper nanoparticles from tulasi (Ocimum sanctum L.) leaves. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2219–2228. [Google Scholar] [CrossRef]
- Faraji, M.; Yamini, Y.; Rezaee, M. Magnetic nanoparticles: Synthesis, stabilization, functionalization, characterization, and applications. J. Iran. Chem. Soc. 2010, 1, 1–37. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Nimesh, S., Chandra, R., Gupta, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 43–58. [Google Scholar] [CrossRef]
- Gumustas, M.; Sengel-Turk, C.T.; Gumustas, A.; Ozkan, S.A.; Uslu, B. Effect of polymer-based nanoparticles on the assay of antimicrobial drug delivery systems. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 67–108. [Google Scholar] [CrossRef]
- El-Batal, A.I.; El-Sayed, M.H.; Refaat, B.M.; Askar, A.A. Marine Streptomyces cyaneus strain alex-sk121 mediated eco-friendly synthesis of silver nanoparticles using gamma radiation. Brit. J. Pharma. Res. 2014, 4, 2525–2547. [Google Scholar] [CrossRef]
- Arshad, H.; Sami, M.A.; Saima Sadaf, S.; Hassan, U. Salvadora persica mediated synthesis of silver nanoparticles and their antimicrobial efficacy. Sci. Rep. 2021, 11, 5996. [Google Scholar] [CrossRef]
- Srivastava, A.A.; Kulkarni, A.P.; Harpale, P.M.; Zunjarrao, R.S. Plant mediated synthesis of silver nanoparticles using a bryophyte: Fissidens minutus and its anti-microbial activity. Int. J. Eng. Sci. Technol. 2011, 3, 8342–8347. [Google Scholar]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 8–662. [Google Scholar] [CrossRef]
- Gajbhiye, M.; Kesharwani, J.; Ingle, A.; Gade, A.; Rai, M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed. Nanotech. Biol. Med. 2009, 5, 382–386. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Guilger-Casagrande, M.; de Lima, R. Synthesis of silver nanoparticles mediated by fungi: A review. Front. Bioeng. Biotechnol. 2019, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Lamsal, K.; Kim, S.W.; Jung, J.H.; Kim, Y.S.; Kim, K.S.; Lee, Y.S. Application of silver nanoparticles for the control of Colletotrichum species in vitro and pepper anthracnose disease in field. Mycobiol 2011, 39, 94–199. [Google Scholar] [CrossRef] [Green Version]
- Christoforidis, G.A.; Frank, J.A.; Lindau, M.; Lockman, D.H.; Geho, C.D.; Jones, E.F.; Petricoin, L.A.; Liotta, S.P.; Manninger, Y.; Qiang, A.M.S.; et al. Nanoparticles: Potential biomarker harvesters. Curr. Opinion Chem. Biol. 2006, 10, 56–61. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis cinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; de Lampasona, M.P.; Catalan, C.A.N. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem. Toxicol. 2007, 45, 1650–1661. [Google Scholar] [CrossRef]






| Groups | Isolate Code | Colony Color | Substrate Color | Margin Color | Margin Growth | Transverse Conidial Septa | Longitudinal Conidial Septa | Beak Elongation | Beak Branshing |
|---|---|---|---|---|---|---|---|---|---|
| 1 | A1 | Dark brown | Dark grey | Brownish white | Circular | Three | One | Slender short | Branched |
| 2 | A2, A3, A8 | Greenish brown | Dark brown | Grey | Irregular | Three | None | Elongated | Unbranched |
| 3 | A4, A6 | Dark grey | Greyish brown | White | Circular | Two | One | Elongated | Unbranched |
| 4 | A5, A7 | Grey | Brown | Greyish white | Circular | Four | One | Selender short | Unbranched |
| Groups | Isolate Code | Accession Number | Closest Match | Similarity to Genebank Accessions | Frequency (%) |
|---|---|---|---|---|---|
| 1 | A1 | MN871620 | Clone 107 | 100 | 12.25 |
| 2 | A2, A3, A8 | MN871620 | Clone 107 | 100 | 38.75 |
| 3 | A4, A6 | MN871615 | Clone 180 | 99.50 | 24.50 |
| 4 | A5, A7 | MN871610 | Clone 42 | 99.85 | 24.50 |
| Peels | Total Phenolic Content (mg/g DW) |
|---|---|
| Pomegranate | 105.4 ± 1.98 |
| Orange | 86.32 ± 1.78 |
| Fruit Peel | Size (nm) | Zeta Potential (mV) |
|---|---|---|
| Pomegranate | 8 | −16.9 |
| Orange | 14 | −19.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa, Y.S.; Alamri, S.A.; Alrumman, S.A.; Hashem, M.; Baka, Z.A. Green Synthesis of Silver Nanoparticles Using Pomegranate and Orange Peel Extracts and Their Antifungal Activity against Alternaria solani, the Causal Agent of Early Blight Disease of Tomato. Plants 2021, 10, 2363. https://doi.org/10.3390/plants10112363
Mostafa YS, Alamri SA, Alrumman SA, Hashem M, Baka ZA. Green Synthesis of Silver Nanoparticles Using Pomegranate and Orange Peel Extracts and Their Antifungal Activity against Alternaria solani, the Causal Agent of Early Blight Disease of Tomato. Plants. 2021; 10(11):2363. https://doi.org/10.3390/plants10112363
Chicago/Turabian StyleMostafa, Yasser S., Saad A. Alamri, Sulaiman A. Alrumman, Mohamed Hashem, and Zakaria A. Baka. 2021. "Green Synthesis of Silver Nanoparticles Using Pomegranate and Orange Peel Extracts and Their Antifungal Activity against Alternaria solani, the Causal Agent of Early Blight Disease of Tomato" Plants 10, no. 11: 2363. https://doi.org/10.3390/plants10112363






