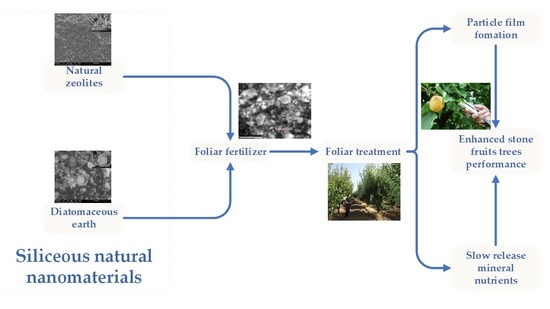
Effects of Siliceous Natural Nanomaterials Applied in Combination with Foliar Fertilizers on Physiology, Yield and Fruit Quality of the Apricot and Peach Trees
Abstract
:1. Introduction
2. Results
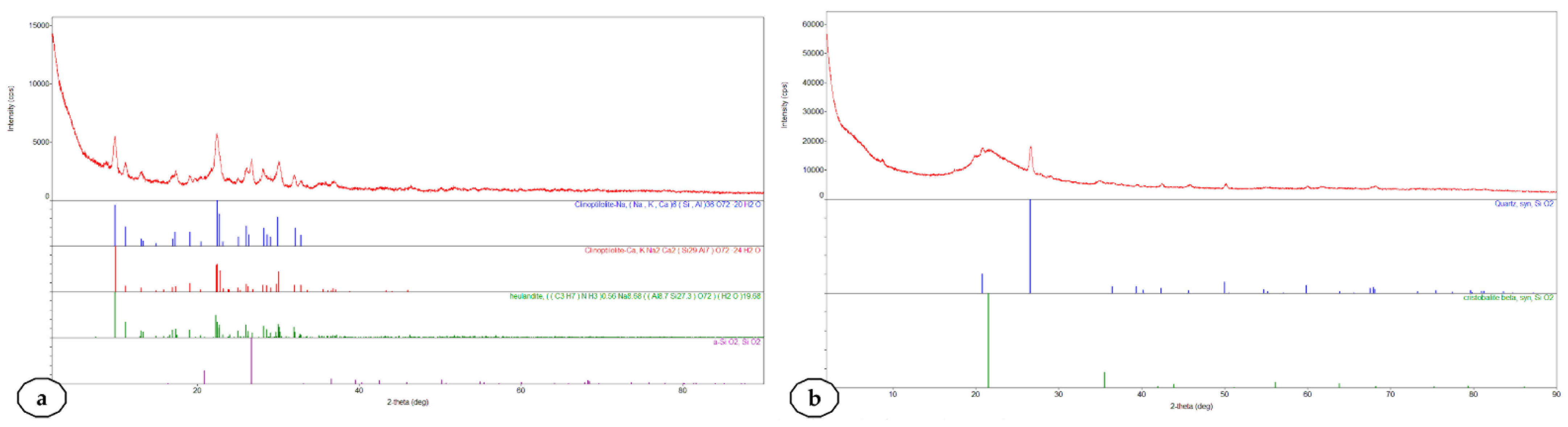
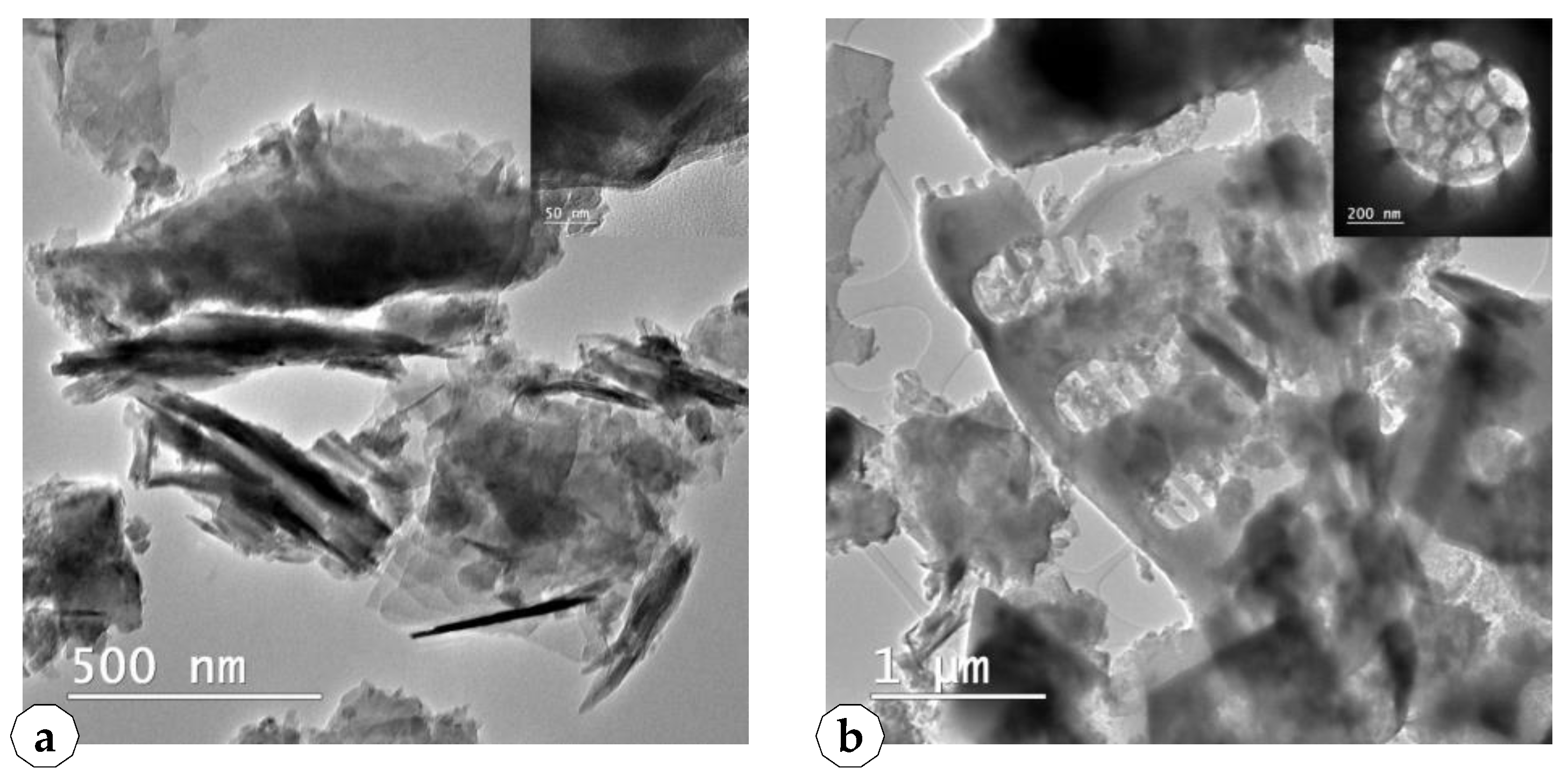
2.1. Characterization of the Used Siliceous Natural Nanomaterials
2.2. Analysis of the Foliar Fertilizer
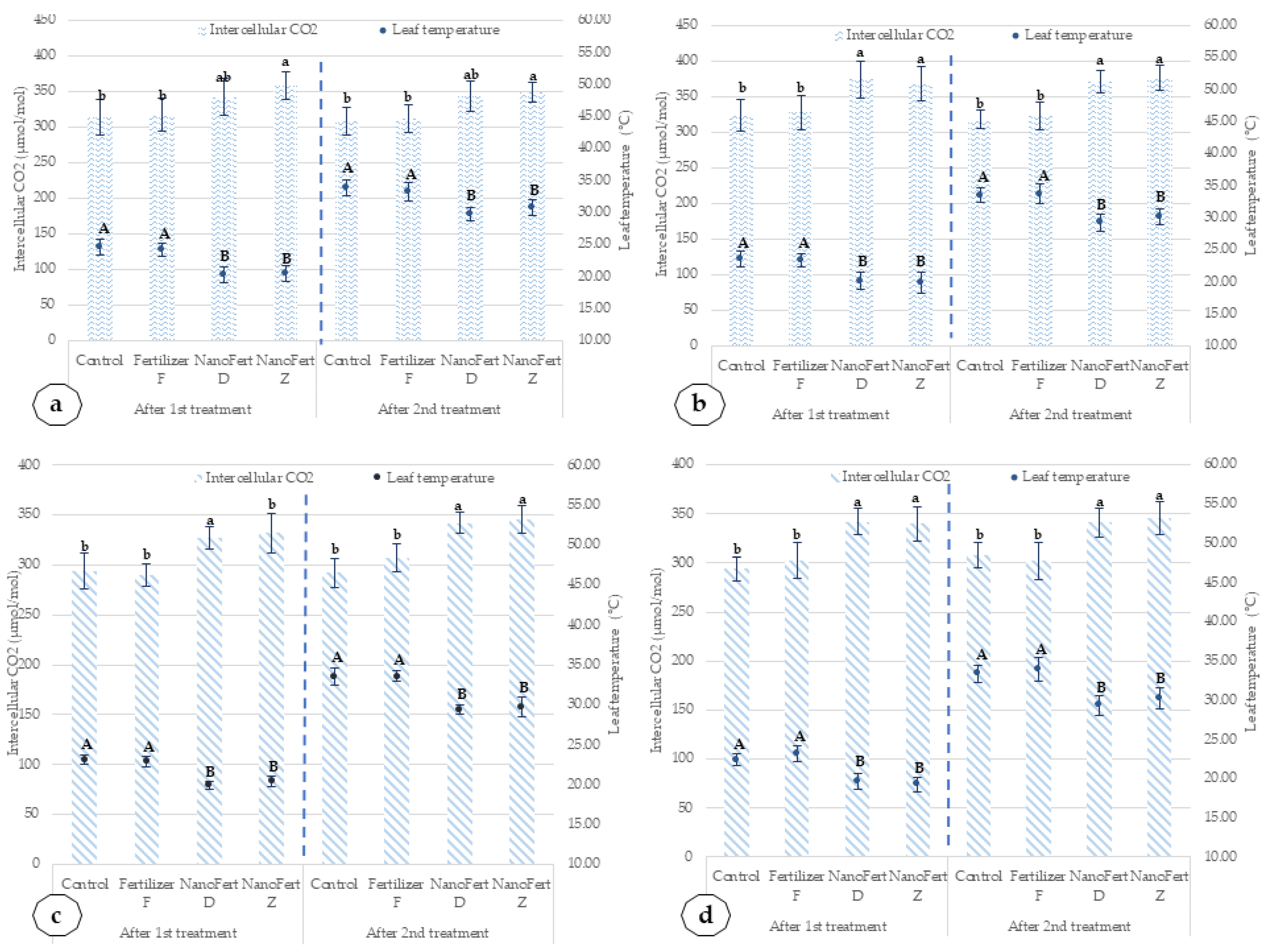
2.3. Effects of the SNNM Application on Physiological Characteristics of the Apricot and Peach Trees
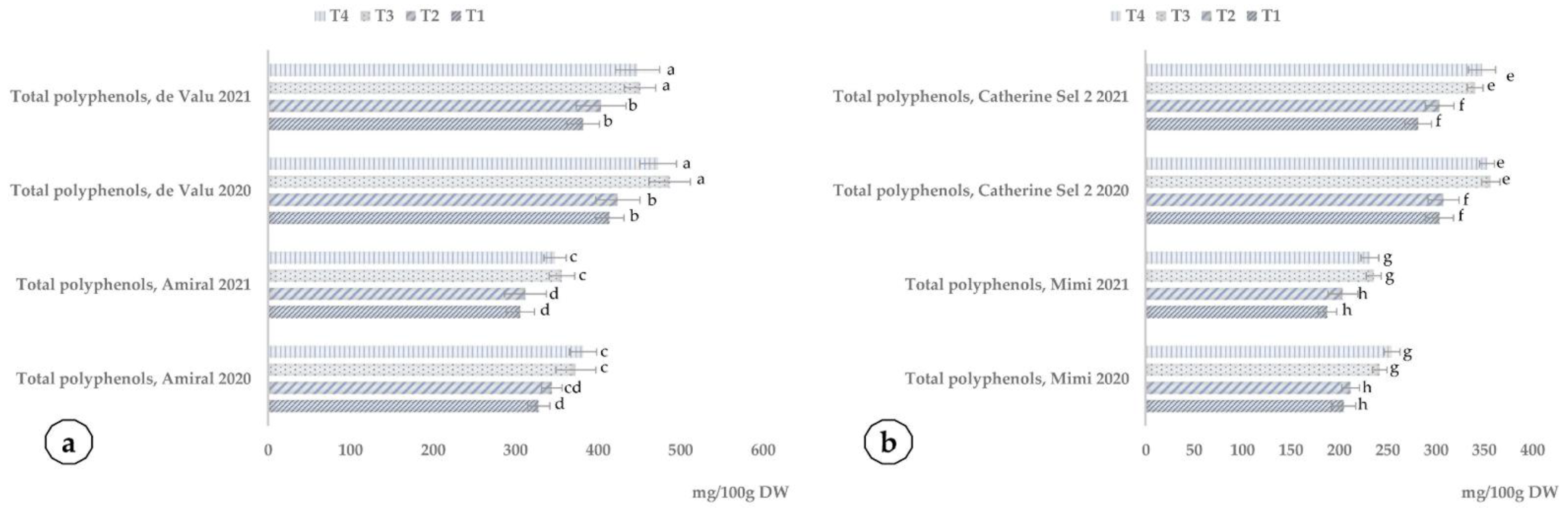
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Site
4.2. Siliceous Natural Nanomaterial Preparation and Characterization
4.3. Preparation and Analysis of Foliar Fertilizer with SNNMs
4.4. Application of Foliar Fertilizer with SNNMs
4.5. Determination of Physiological Characteristics of Apricot and Peach Plants
4.6. Determination of Yield and Fruit Quality
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Constantinescu-Aruxandei, D.; Lupu, C.; Oancea, F. Siliceous Natural Nanomaterials as Biorationals—Plant Protectants and Plant Health Strengtheners. Agronomy 2020, 10, 1791. [Google Scholar] [CrossRef]
- Eroglu, N.; Emekci, M.; Athanassiou, C.G. Applications of natural zeolites on agriculture and food production. J. Sci. Food Agric. 2017, 97, 3487–3499. [Google Scholar] [CrossRef]
- Korunic, Z. Diatomaceous earths, a group of natural insecticides. J. Stored Prod. Res. 1998, 34, 87–97. [Google Scholar] [CrossRef]
- McCoy, E.L. Commercial Amendments for Sand-based Root Zones: Review and Interpretation. Horttechnology 2013, 23, 803–813. [Google Scholar] [CrossRef]
- Sumper, M.; Kröger, N. Silica formation in diatoms: The function of long-chain polyamines and silaffins. J. Mater. Chem. 2004, 14, 2059–2065. [Google Scholar] [CrossRef]
- Scheffel, A.; Poulsen, N.; Shian, S.; Kröger, N. Nanopatterned protein microrings from a diatom that direct silica morphogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 3175–3180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Król, M. Natural vs. Synthetic Zeolites. Crystals 2020, 10, 622. [Google Scholar] [CrossRef]
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. 1999, 96, 3463–3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakhli, S.A.A.; Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Application of Zeolites for Sustainable Agriculture: A Review on Water and Nutrient Retention. Water Air Soil Pollut. 2017, 228, 34. [Google Scholar] [CrossRef]
- Balakhnina, T.I.; Bulak, P.; Matichenkov, V.V.; Kosobryukhov, A.A.; Wlodarczyk, T.M. The influence of Si-rich mineral zeolite on the growth processes and adaptive potential of barley plants under cadmium stress. Plant Growth Regul. 2015, 75, 557–565. [Google Scholar] [CrossRef]
- De Smedt, C.; Van Damme, V.; De Clercq, P.; Spanoghe, P. Insecticide effect of zeolites on the tomato leafminer Tuta absoluta (Lepidoptera: Gelechiidae). Insects 2016, 7, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyraz, D.; Nalbant, H. Comparison of zeolite (clinoptilolite) with diatomite and pumice as soil conditioners in agricultural soils. Pak. J. Agric. Sci. 2015, 52, 923–929. [Google Scholar]
- Jankauskiene, J.; Brazaityte, A.; Kairiene, V.V.; Zalatorius, V. Effects of peat and peat-zeolite substrates on quality, growth indices of cucumber seedlings and crop productivity. Acta Sci. Pol. -Hortorum Cultus 2019, 18, 161–170. [Google Scholar] [CrossRef]
- Li, D.Y.; Joo, Y.K.; Christians, N.E.; Minner, D.D. Inorganic soil amendment effects on sand-based sports turf media. Crop. Sci. 2000, 40, 1121–1125. [Google Scholar] [CrossRef]
- Sepaskhah, A.; Yousefi, F. Effects of zeolite application on nitrate and ammonium retention of a loamy soil under saturated conditions. Soil Res. 2007, 45, 368–373. [Google Scholar] [CrossRef]
- Li, Y.; Xia, G.; Wu, Q.; Chen, W.; Lin, W.; Zhang, Z.; Chen, Y.; Chen, T.; Siddique, K.H.; Chi, D. Zeolite increases grain yield and potassium balance in paddy fields. Geoderma 2022, 405, 115397. [Google Scholar] [CrossRef]
- Perumal, P.; Ahmed, O.H.; Omar, L.; Abdul Majid, N.M. Nitrogen, Phosphorus, and Potassium Adsorption and Desorption Improvement and Soil Buffering Capacity Using Clinoptilolite Zeolite. Agronomy 2021, 11, 379. [Google Scholar] [CrossRef]
- Najafi-Ghiri, M.; Rahimi, T. Zinc uptake by spinach (Spinacia oleracea L.) as affected by Zn application rate, zeolite, and vermicompost. Compost. Sci. Util. 2016, 24, 203–207. [Google Scholar] [CrossRef]
- Pasković, I.; Pecina, M.; Bronić, J.; Perica, S.; Ban, D.; Ban, S.G.; Pošćić, F.; Palčić, I.; Herak Ćustić, M. Synthetic zeolite a as zinc and manganese fertilizer in calcareous soil. Commun. Soil Sci. Plant Anal. 2018, 49, 1072–1082. [Google Scholar] [CrossRef]
- Nur Aainaa, H.; Haruna Ahmed, O.; Ab Majid, N.M. Effects of clinoptilolite zeolite on phosphorus dynamics and yield of Zea Mays L. cultivated on an acid soil. PLoS One 2018, 13, e0204401. [Google Scholar] [CrossRef]
- de Campos Bernardi, A.C.; de Oliveira, C.R. Improved Alfalfa Phosphate Utilization Using Zeolite Amendments in Low pH Soil. J. Soil Sci. Plant Nutr. 2021, 1–11. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.O.; Angle, S.; Rabbinge, R. Unlocking the multiple public good services from balanced fertilizers. Food Secur. 2018, 10, 273–285. [Google Scholar] [CrossRef]
- Fernandez, V.; Brown, P.H. From plant surface to plant metabolism: The uncertain fate of foliar-applied nutrients. Front. plant Sci. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Tanou, G.; Ziogas, V.; Molassiotis, A. Foliar Nutrition, Biostimulants and Prime-Like Dynamics in Fruit Tree Physiology: New Insights on an Old Topic. Front. plant Sci. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.; Liu, C.; Huang, M.; Liu, K.; Yan, D. Effects of Foliar Fertilization: A Review of Current Status and Future Perspectives. J. Soil Sci. Plant Nutr. 2021, 21, 104–118. [Google Scholar] [CrossRef]
- Tarantino, A.; Lops, F.; Disciglio, G.; Lopriore, G. Effects of plant biostimulants on fruit set, growth, yield and fruit quality attributes of ‘Orange rubis (R)’ apricot (Prunus armeniaca L.) cultivar in two consecutive years. Sci. Hortic. 2018, 239, 26–34. [Google Scholar] [CrossRef]
- Minas, I.S.; Tanou, G.; Molassiotis, A. Environmental and orchard bases of peach fruit quality. Sci. Hortic. 2018, 235, 307–322. [Google Scholar] [CrossRef]
- De Smedt, C.; Someus, E.; Spanoghe, P. Potential and actual uses of zeolites in crop protection. Pest Manag. Sci. 2015, 71, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Srivastava, R.; Koh, J. Utilization of zeolites as CO2 capturing agents: Advances and future perspectives. J. CO2 Util. 2020, 41, 101251. [Google Scholar] [CrossRef]
- Tang, X.; Houzé de l’Aulnoit, S.; Buelow, M.T.; Slack, J.; Singer, B.C.; Destaillats, H. Performance of a CO2 sorbent for indoor air cleaning applications: Effects of environmental conditions, sorbent aging, and adsorption of co-occurring formaldehyde. Indoor Air 2020. [Google Scholar] [CrossRef]
- De Smedt, C.; Steppe, K.; Spanoghe, P. Beneficial effects of zeolites on plant photosynthesis. Adv. Mater. Sci. 2017, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mucha-Pelzer, T.; Mewis, I.; Ulrichs, C. Response of glucosinolate and flavonoid contents and composition of Brassica rapa ssp. chinensis (L.) Hanelt to silica formulations used as insecticides. J. Agric. Food Chem. 2010, 58, 12473–12480. [Google Scholar] [CrossRef]
- Mphande, W.; Kettlewell, P.S.; Grove, I.G.; Farrell, A.D. The potential of antitranspirants in drought management of arable crops: A review. Agric. Water Manag. 2020, 236, 18. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Luzio, A.; Silva, E.; Goncalves, A.; Meijon, M.; Escandon, M.; Arrobas, M.; Rodrigues, M.A.; Moutinho-Pereira, J.; et al. Kaolin and salicylic acid alleviate summer stress in rainfed olive orchards by modulation of distinct physiological and biochemical responses. Sci. Hortic. 2019, 246, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Gullo, G.; Dattola, A.; Vonella, V.; Zappia, R. Effects of two reflective materials on gas exchange, yield, and fruit quality of sweet orange tree Citrus sinensis (L.) Osb. Eur. J. Agron. 2020, 118, 9. [Google Scholar] [CrossRef]
- Jifon, J.L.; Syvertsen, J.P. Kaolin particle film applications can increase photosynthesis and water use efficiency of ‘Ruby red’ grapefruit leaves. J. Am. Soc. Hortic. Sci. 2003, 128, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Glenn, D.M.; Cooley, N.; Walker, R.; Clingeleffer, P.; Shellie, K. Impact of Kaolin Particle Film and Water Deficit on Wine Grape Water Use Efficiency and Plant Water Relations. Hortscience 2010, 45, 1178–1187. [Google Scholar] [CrossRef]
- Glenn, D.M.; Erez, A.; Puterka, G.J.; Gundrum, P. Particle films affect carbon assimilation and yield in ‘Empire’ Apple. J. Am. Soc. Hortic. Sci. 2003, 128, 356–362. [Google Scholar] [CrossRef] [Green Version]
- Rosati, A.; Metcalf, S.G.; Buchner, R.P.; Fulton, A.E.; Lampinen, B.D. Physiological effects of kaolin applications in well-irrigated and water-stressed walnut and almond trees. Ann. Bot. 2006, 98, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotondi, A.; Morrone, L.; Facini, O.; Faccini, B.; Ferretti, G.; Coltorti, M. Distinct Particle Films Impacts on Olive Leaf Optical Properties and Plant Physiology. Foods 2021, 10, 1291. [Google Scholar] [CrossRef]
- Valentini, G.; Pastore, C.; Allegro, G.; Muzzi, E.; Seghetti, L.; Filippetti, I. Application of Kaolin and Italian Natural Chabasite-Rich Zeolitite to Mitigate the Effect of Global Warming in Vitis vinifera L. cv. Sangiovese. Agronomy 2021, 11, 1035. [Google Scholar] [CrossRef]
- Elaiopoulos, K.; Perraki, T.; Grigoropoulou, E. Monitoring the effect of hydrothermal treatments on the structure of a natural zeolite through a combined XRD, FTIR, XRF, SEM and N2-porosimetry analysis. Microporous Mesoporous Mater. 2010, 134, 29–43. [Google Scholar] [CrossRef]
- Yuan, P.; Wu, D.Q.; He, H.P.; Lin, Z.Y. The hydroxyl species and acid sites on diatomite surface: A combined IR and Raman study. Appl. Surf. Sci. 2004, 227, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Guatame-Garcia, A.; Buxton, M. The Use of Infrared Spectroscopy to Determine the Quality of Carbonate-Rich Diatomite Ores. Minerals 2018, 8, 120. [Google Scholar] [CrossRef] [Green Version]
- Benkacem, T.; Hamdi, B.; Chamayou, A.; Balard, H.; Calvet, R. Physicochemical characterization of a diatomaceous upon an acid treatment: A focus on surface properties by inverse gas chromatography. Powder Technol. 2016, 294, 498–507. [Google Scholar] [CrossRef] [Green Version]
- Di Benedetto, F.; Giaccherini, A.; Romanelli, M.; Montegrossi, G.; Belluso, E.; Capella, S.; Zoleo, A.; Arcangeli, G.; Marinaccio, A.; Gottardo, O. A study of radicals in industrial raw cristobalite powders. Phys. Chem. Miner. 2021, 48, 1–9. [Google Scholar] [CrossRef]
- Rice, F.; Park, R.; Stayner, L.; Smith, R.; Gilbert, S.; Checkoway, H. Crystalline silica exposure and lung cancer mortality in diatomaceous earth industry workers: A quantitative risk assessment. Occup. Environ. Med. 2001, 58, 38–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maletsika, P.; Nanos, G. Kaolin particle film on peach leaf physiology. Proceedings of VIII International Peach Symposium, International Society for Horticultural Science, Matera, Italy, 17–20 June 2013; pp. 327–334. [Google Scholar] [CrossRef]
- Sage, R.F.; Kubien, D.S. The temperature response of C3 and C4 photosynthesis. Plant cell Environ. 2007, 30, 1086–1106. [Google Scholar] [CrossRef]
- Fernández, V.; Gil-Pelegrín, E.; Eichert, T. Foliar water and solute absorption: An update. Plant J. 2021, 105, 870–883. [Google Scholar] [CrossRef]
- Neag, E.; Török, A.I.; Tanaselia, C.; Aschilean, I.; Senila, M. Kinetics and Equilibrium Studies for the Removal of Mn and Fe from Binary Metal Solution Systems Using a Romanian Thermally Activated Natural Zeolite. Water 2020, 12, 1614. [Google Scholar] [CrossRef]
- Lupu, C.; Delian, E.; Chira, L.; Chira, A. New insights into the multiple protective functions of diatomaceous earth during storage of agricultural products. Sci. Pap. Ser. A Agron. 2018, 61, 487–496. [Google Scholar]
- Popescu, M.; Oancea, F.; Desliu-Avram, M. Vegetable oil conversion into core-shell bioproducts for stored grain protection. Sci. Bulletin. Ser. F. Biotechnol. 2016, 20, 210–213. [Google Scholar]
- Calzarano, F.; Seghetti, L.; Pagnani, G.; Di Marco, S. Italian Zeolitites in the Control of Grey Mould and Sour Rot and Their Effect on Leaf Reflectance, Grape and Wine. Agriculture 2020, 10, 580. [Google Scholar] [CrossRef]
- Hamadneh, I.; Alatawi, A.; Zalloum, R.; Albuqain, R.; Alsotari, S.; Khalili, F.I.; Al-Dujaili, A.H. Comparison of Jordanian and standard diatomaceous earth as an adsorbent for removal of Sm (III) and Nd (III) from aqueous solution. Environ. Sci. Pollut. Res. 2019, 26, 20969–20980. [Google Scholar] [CrossRef]
- Sharma, S.; Sahu, B.K.; Cao, L.D.; Bindra, P.; Kaur, K.; Chandel, M.; Koratkar, N.; Huang, Q.L.; Shanmugam, V. Porous nanomaterials: Main vein of agricultural nanotechnology. Prog. Mater. Sci. 2021, 121. [Google Scholar] [CrossRef]
- Maletsika, P.A.; Nanos, G.D.; Stavroulakis, G.G. Peach leaf responses to soil and cement dust pollution. Environ. Sci. Pollut. Res. 2015, 22, 15952–15960. [Google Scholar] [CrossRef]
- Pornaroonthama, P.; Thouchprasitchai, N.; Pongstabodee, S. CO2 adsorption on diatomaceous earth modified with cetyltrimethylammonium bromide and functionalized with tetraethylenepentamine: Optimization and kinetics. J. Environ. Manag. 2015, 157, 194–204. [Google Scholar] [CrossRef]
- Lateef, A.; Nazir, R.; Jamil, N.; Alam, S.; Shah, R.; Khan, M.N.; Saleem, M. Synthesis and characterization of zeolite based nano–composite: An environment friendly slow release fertilizer. Microporous Mesoporous Mater. 2016, 232, 174–183. [Google Scholar] [CrossRef]
- Souza, I.M.S.; Gurgel, G.C.S.; Medeiros, A.M.; Zonta, E.; Ruiz, J.A.C.; Paskocimas, C.A.; Motta, F.V.; Bomio, M.R.D. The use of clinoptilolite as carrier of nitrogened fertilizer with controlled release. J. Environ. Chem. Eng. 2018, 6, 4171–4177. [Google Scholar] [CrossRef]
- Bernardi, A.C.D.; Werneck, C.G.; Haim, P.G.; Monte, M.B.D.; Barros, F.D.; Verruma-Bernardi, M.R. Nitrogen, Potassium, and Nitrate Concentrations of Lettuce Grown in a Substrate with KNO3-Enriched Zeolite. Commun. Soil Sci. Plant Anal. 2015, 46, 819–826. [Google Scholar] [CrossRef]
- Nur Aainaa, H.; Osumanu, A.; Ab Majid, H.; Muhamad, N. Effects of Amending Phosphatic Fertilizers with Clinoptilolite Zeolite on Phosphorus Availability and Its Fractionation in an Acid Soil. Appl. Sci. 2020, 10, 3162. [Google Scholar]
- Pickering, H.W.; Menzies, N.W.; Hunter, M.N. Zeolite/rock phosphate—a novel slow release phosphorus fertiliser for potted plant production. Sci. Hortic. 2002, 94, 333–343. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Masini, C.M.; Mattii, G.B. Application of Zeolites in Agriculture and Other Potential Uses: A Review. Agronomy-Basel 2021, 11, 1547. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Beezhold, D.H.; Shvedova, A.A. Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risks. Toxicol. Appl. Pharmacol. 2017, 329, 96–111. [Google Scholar] [CrossRef]
- Pena-Rodriguez, G.; Rodriguez, M.H.; Becerra, E.; Caballero, X.; Dulce-Moreno, J. Use of recycled diatomaceous earth from breweries as a means of transporting of macronutrients. Uis Ing. 2019, 18, 139–145. [Google Scholar] [CrossRef]
- Higashi, T.; Oshio, T.; Kawakami, T.; Bio, C. In situ evaluation of enbedded carrier in soil to reduce nitrate leaching from cropland. Proceedings of 6th International In Situ and On-Site Bioremediation Symposium, San Diego, CA, USA, 4−7 June 2001; pp. 187–194. [Google Scholar]
- La Torre, A.; Righi, L.; Iovino, V.; Battaglia, V. Evaluation of copper alternative products to control grape downy mildew in organic farming. J. Plant Pathol. 2019, 101, 1005–1012. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G. Biostimulant activity of silicon in horticulture. Sci. Hortic. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.; Schmitzer, V.; Jakopic, J.; Stampar, F. First fruit in season: Seaweed extract and silicon advance organic strawberry (Fragaria×ananassa Duch.) fruit formation and yield. Sci. Hortic. 2018, 242, 103–109. [Google Scholar] [CrossRef]
- Spinde, K.; Pachis, K.; Antonakaki, I.; Paasch, S.; Brunner, E.; Demadis, K.D. Influence of polyamines and related macromolecules on silicic acid polycondensation: Relevance to “Soluble Silicon Pools”? Chem. Mater. 2011, 23, 4676–4687. [Google Scholar] [CrossRef]
- Lobel, K.; West, J.; Hench, L. Computational model for protein-mediated biomineralization of the diatom frustule. Mar. Biol. 1996, 126, 353–360. [Google Scholar] [CrossRef]
- Sandhya, K.; Prakash, N.B.; Meunier, J.D. Diatomaceous earth as source of silicon on the growth and yield of rice in contrasted soils of Southern India. J. Soil Sci. Plant Nutr. 2018, 18, 344–360. [Google Scholar] [CrossRef] [Green Version]
- Gokavi, N.; Jayakumar, M.; Mote, K.; Surendran, U. Diatomaceous Earth as a Source of Silicon and its Impact on Soil Physical and Chemical Properties, Yield and Quality, Pests and Disease Incidence of Arabica Coffee cv. Chandragiri. Silicon 2020, 12, 1–18. [Google Scholar]
- Mills-Ibibofori, T.; Dunn, B.; Maness, N.; Payton, M. Use of Diatomaceous Earth as a Silica Supplement on Potted Ornamentals. Horticulturae 2019, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Hellal, F.; Abdelhameid, M.; Abo-BAsha, D.M.; Zewainy, R. Alleviation of the adverse effects of soil salinity stress by foliar application of silicon on faba bean (Vica faba L.). J. Appl. Sci. Res. 2012, 8, 4428–4433. [Google Scholar]
- do Nascimento, C.W.A.; de Souza Nunes, G.H.; Preston, H.A.F.; da Silva, F.B.V.; Preston, W.; Loureiro, F.L.C. Influence of Silicon Fertilization on Nutrient Accumulation, Yield and Fruit Quality of Melon Grown in Northeastern Brazil. Silicon 2020, 12, 937–943. [Google Scholar] [CrossRef]
- Prentice, P. Efficacy of silica in increasing fields in Marocco. In Proceedings of Proceedings of the 7th International Conference on Silicon in Agriculture, Bengaluru, India, 24−28 October 2017; pp. 24–28. [Google Scholar]
- Encina Oliva, K.M.; Araújo do Nascimento, C.W.; Vieira da Silva, F.B.; Muniz Araújo, P.R.; Almeida de Oliveira, E.C.; Feitosa, M.M.; Vieira Lima, L.H. Biomass and concentration of nutrients and silicon in sugarcane grown on soil fertilized with diatomite. Braz. J. Agric. Sci. /Rev. Bras. de Ciências Agrárias 2020, 15, e8755. [Google Scholar] [CrossRef]
- Dumitraş, D.G.; Marincea, Ş.; C, L. A chemical and mineralogical survey of some diatomaceous deposits in Romania. Proceedings of 12th International Congress for Applied Mineralogy, Istambul, Turkey, 10−12 August 2015. [Google Scholar]
- Cerri, G.; Farina, M.; Brundu, A.; Daković, A.; Giunchedi, P.; Gavini, E.; Rassu, G. Natural zeolites for pharmaceutical formulations: Preparation and evaluation of a clinoptilolite-based material. Microporous Mesoporous Mater. 2016, 223, 58–67. [Google Scholar] [CrossRef]
- Pérez-Pastor, A.; Ruiz-Sánchez, M.; Domingo, R.; Torrecillas, A. Growth and phenological stages of Búlida apricot trees in south-east Spain. Agronomie 2004, 24, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Meier, U.; Graf, H.; Hack, H.; Hess, M.; Kennel, W.; Klose, R.; Mappes, D.; Seipp, D.; Stauss, R.; Streif, J. Phanologische Entwicklungsstadien des Kernobstes (Malus domestica Borkh. und Pyrus communis L.), des Steinobstes (Prunus-Arten), der Johannisbeere Ribes-Arten) und der Erdbeere (Fragaria x ananassa.). Nachr. des Dtsch. Pflanzenschutzd. 1994, 46, 141–153. [Google Scholar]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, O.; Constantin, D.; Gaspar, A.; Toma, L.; Utoiu, E.; Moldovan, L. Evaluation of antioxidant and cytoprotective activities of Arnica montana L. and Artemisia absinthiumL. ethanolic extracts. Chem. Cent. J. 2012, 6, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Sample | Total Volume of the Pores (cm3 g−1) | The Average Diameter of the Pores (nm) |
|---|---|---|
| Natural zeolites, before activation | 0.0578 ± 0.0027 | 5.339 ± 0.347 |
| Natural zeolites, after activation | 0.0608 ± 0.0032 | 5.517 ± 0.383 |
| Diatomaceous earth, before activation | 0.0692 ± 0.0042 | 8.919 ± 0.0483 |
| Diatomaceous earth, after activation | 0.0754 ± 0.0038 | 9.127 ± 0.417 |
| Analyte | Unit | Estimated Value | Determined Value | Uncertainty |
|---|---|---|---|---|
| Nitrogen (N total) | % | 3.5 | 3.57 | ±0.12 |
| Phosphorus (as P2O5) | % | 15 | 15.62 | ±1.14 |
| Potassium (as K2O) | % | 2 | 2.41 | ±0.20 |
| Copper (Cu) | % | 0.002 | 0.0032 | ±0.0003 |
| Zinc (Zn) | % | 0.0025 | 0.0024 | ±0.0004 |
| Iron (Fe) | % | 0.017 | 0.0159 | ±0.0016 |
| Manganese (Mn) | % | 0.008 | 0.0076 | ±0.0006 |
| Boron (B) | % | 0.003 | 0.0035 | ±0.0004 |
| Analyte | Unit | Estimated Value | Determined Value | Uncertainty |
|---|---|---|---|---|
| Nitrogen (N total) | % | 3.5 | 3.78 | ±0.11 |
| Phosphorus (as P2O5) | % | 15 | 15.38 | ±1.31 |
| Potassium (as K2O) | % | 2 | 2.23 | ±0.25 |
| Copper (Cu) | % | 0.002 | 0.0028 | ±0.0003 |
| Zinc (Zn) | % | 0.0025 | 0.0028 | ±0.0004 |
| Iron (Fe) | % | 0.017 | 0.0172 | ±0.0016 |
| Manganese (Mn) | % | 0.008 | 0.0084 | ±0.0006 |
| Boron (B) | % | 0.003 | 0.0029 | ±0.0004 |
| Analyte | Unit | Estimated Value | Determined Value | Uncertainty |
|---|---|---|---|---|
| Nitrogen (N total) | % | 3.5 | 3.54 | ±0.13 |
| Phosphorus (as P2O5) | % | 15 | 15.87 | ±1.20 |
| Potassium (as K2O) | % | 2 | 1.89 | ±0.17 |
| Copper (Cu) | % | 0.002 | 0.0024 | ±0.0003 |
| Zinc (Zn) | % | 0.0025 | 0.0032 | ±0.0004 |
| Iron (Fe) | % | 0.017 | 0.0182 | ±0.0013 |
| Manganese (Mn) | % | 0.008 | 0.0090 | ±0.0010 |
| Boron (B) | % | 0.003 | 0.0033 | ±0.0004 |
| Cultivar | Treatment | 2020 | 2021 | ||||
|---|---|---|---|---|---|---|---|
| E | Pn | WUE | E | Pn | WUE | ||
| Amiral | T1, control, 1st treatment | 4.52 a | 12.57 b | 2.78 c | 5.46 a | 13.43 b | 2.46 c |
| T2, fertilizer F, 1st treatment | 4.41 a | 12.83 b | 2.91 c | 5.02 a | 14.88 a | 2.96 c | |
| T3, NanoFert D, 1st treatment | 3.84 b | 15.28 a | 3.98 a | 4.92 a | 15.37 a | 3.12 bc | |
| T4, NanoFert Z, 1st treatment | 3.92 b | 14.74 a | 3.76 a | 4.81 a | 15.14 a | 3.15 bc | |
| Amiral | T1, control, 2nd treatment | 4.29 a | 11.82 b | 2.75 c | 5.19 a | 12.76 bc | 2.48 c |
| T2, fertilizer F, 2nd treatment | 4.23 a | 11.93 b | 2.82 c | 4.67 a | 13.91 b | 2.98 c | |
| T3, NanoFert D, 2nd treatment | 3.53 b | 14.06 a | 3.94 a | 4.53 ab | 14.32 a | 3.16 bc | |
| T4, NanoFert Z, 2nd treatment | 3.68 b | 13.71 a | 3.72 a | 4.69 a | 14.19 ab | 3.08 bc | |
| de Valu | T1, control, 1st treatment | 2.74 c | 8.33 e | 3.04 c | 3.81 c | 10.16 d | 2.67 c |
| T2, fertilizer F, 1st treatment | 2.71 c | 8.25 e | 3.05 c | 3.28 d | 10.71 d | 3.26 bc | |
| T3, NanoFert D, 1st treatment | 2.23 d | 8.62 e | 3.87 a | 3.32 d | 11.73 c | 3.53 b | |
| T4, NanoFert Z, 1st treatment | 2.32 d | 8.76 e | 3.78 a | 3.05 d | 11.64 c | 3.83 b | |
| de Valu | T1, control, 2nd treatment | 2.94 c | 8.71 e | 2.96 c | 3.74 c | 10.27 d | 2.75 c |
| T2, fertilizer F, 2nd treatment | 2.88 c | 8.64 e | 3.00 c | 3.53 cd | 11.42 c | 3.24 bc | |
| T3, NanoFert D, 2nd treatment | 2.37 d | 9.12 de | 3.85 a | 3.42 cd | 12.83 b | 3.75 a | |
| T4, NanoFert Z, 2nd treatment | 2.44 d | 8.82 e | 3.61 a | 3.35 cd | 12.76 b | 3.81 a | |
| Mimi | T1, control, 1st treatment | 3.82 b | 11.15 c | 2.90 c | 4.68 a | 12.72 bc | 2.72 c |
| T2, fertilizer F, 1st treatment | 3.66 b | 12.27 b | 3.35 b | 4.79 a | 13.24 b | 2.76 c | |
| T3, NanoFert D, 1st treatment | 3.14 c | 12.62 b | 4.02 a | 4.16 bc | 14.12 ab | 3.39 b | |
| T4, NanoFert Z, 1st treatment | 3.07 c | 12.54 b | 4.08 a | 4.24 b | 14.27 b | 3.37 b | |
| Mimi | T1, control, 2nd treatment | 3.53 b | 10.50 c | 2.97 c | 4.40 b | 11.95 c | 2.72 c |
| T2, fertilizer F, 2nd treatment | 3.37 bc | 11.41 b | 3.39 b | 4.45 b | 12.62 bc | 2.83 c | |
| T3, NanoFert D, 2nd treatment | 2.86 c | 11.61 b | 4.06 a | 3.83 c | 13.44 b | 3.51 b | |
| T4, NanoFert Z, 2nd treatment | 2.82 c | 11.54 b | 4.08 a | 4.07 bc | 13.70 b | 3.37 bc | |
| Catherine Sel. 1 | T1, control, 1st treatment | 3.30 bc | 9.03 de | 2.74 c | 4.34 b | 11.33 c | 2.61 c |
| T2, fertilizer F, 1st treatment | 3.09 c | 9.73 d | 3.15 b | 4.14 bc | 10.99 cd | 2.64 c | |
| T3, NanoFert D, 1st treatment | 2.81 c | 10.01 d | 3.57 b | 3.78 c | 11.49 c | 3.04 c | |
| T4, NanoFert Z, 1st treatment | 2.80 c | 9.88 d | 3.53 b | 3.72 c | 11.75 c | 3.16 bc | |
| Catherine Sel. 1 | T1, control, 2nd treatment | 3.47 b | 9.82 d | 2.83 c | 4.57 b | 11.68 c | 2.56 c |
| T2, fertilizer F, 2nd treatment | 3.32 bc | 10.24 cd | 3.08 c | 4.43 b | 11.82 c | 2.67 c | |
| T3, NanoFert D, 2nd treatment | 3.05 c | 10.48 c | 3.44 b | 4.06 bc | 12.42 bc | 3.06 bc | |
| T4, NanoFert Z, 2nd treatment | 2.98 c | 10.62 c | 3.56 b | 3.96 c | 12.58 bc | 3.18 bc | |
| Cultivar | Treatment | 2020 | 2021 | ||||
|---|---|---|---|---|---|---|---|
| Yield | TSS | A | Yield | TSS | A | ||
| Amiral | T1, control | 10235 i | 12.92 c | 1.24 b | 11125 i | 13.23 c | 1.42 b |
| T2, fertilizer F | 10806 h | 13.40 bc | 1.35 b | 11375 i | 13.14 c | 1.52 a | |
| T3, NanoFert D | 11671 g | 14.05 b | 1.46 b | 12086 h | 14.27 b | 1.38 b | |
| T4, NanoFert Z | 11530 g | 13.82 bc | 1.41 b | 12194 h | 13.82 bc | 1.58 a | |
| de Valu | T1, control | 12321 f | 13.30 c | 1.50 a | 13006 g | 13.38 bc | 1.62 a |
| T2, fertilizer F | 12652 ef | 14.10 b | 1.58 a | 13813 fg | 13.26 c | 1.48 b | |
| T3, NanoFert D | 13615 e | 14.40 b | 1.64 a | 14676 e | 14.03 b | 1.75 q | |
| T4, NanoFert Z | 13513 e | 14.22 b | 1.72 a | 14485 e | 13.52 bc | 1.64 a | |
| Mimi | T1, control | 15085 e | 12.08 d | 0.51 d | 15750 d | 12.68 cd | 0.55 d |
| T2, fertilizer F | 15752 d | 12.35 d | 0.53 d | 16884 c | 13.12 c | 0.55 d | |
| T3, NanoFert D | 17222 c | 13.28 c | 0.48 d | 18326 b | 13.45 bc | 0.58 d | |
| T4, NanoFert Z | 17020 c | 13.07 c | 0.55 d | 18301 b | 13.17 c | 0.57 d | |
| Catherine Sel. 1 | T1, control | 16125 b | 14.46 b | 0.62 c | 16938 c | 15.24 a | 0.65 c |
| T2, fertilizer F | 16803 ab | 14.37 b | 0.62 c | 18250 b | 15.45 a | 0.69 c | |
| T3, NanoFert D | 18188 a | 14.69 ab | 0.65 c | 19345 a | 15.87 a | 0.72 c | |
| T4, NanoFert Z | 17831 a | 15.18 a | 0.69 c | 19126 a | 15.72 a | 0.73 c | |
| Parameter, Year | March | April | May | June | July | August |
|---|---|---|---|---|---|---|
| Temperature, 2020 | 8.1 | 10.3 | 16 | 21.2 | 23.8 | 23.6 |
| Temperature, 2021 | 4.7 | 9.2 | 16.2 | 20 | 24.2 | 23.5 |
| Temperature, multi-annual average | 5.8 | 10.7 | 16/4 | 20.8 | 23.7 | 22.8 |
| Precipitation, 2020 | 19 | 7.2 | 19.2 | 39.8 | 9.6 | 2.2 |
| Precipitation, 2021 | 65.8 | 66.8 | 87.8 | 124.5 | 39.4 | 17.6 |
| Precipitation, multi-annual average | 34.1 | 34.1 | 43.7 | 42.7 | 69.3 | 43.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moale, C.; Ghiurea, M.; Sîrbu, C.E.; Somoghi, R.; Cioroianu, T.M.; Faraon, V.A.; Lupu, C.; Trică, B.; Constantinescu-Aruxandei, D.; Oancea, F. Effects of Siliceous Natural Nanomaterials Applied in Combination with Foliar Fertilizers on Physiology, Yield and Fruit Quality of the Apricot and Peach Trees. Plants 2021, 10, 2395. https://doi.org/10.3390/plants10112395
Moale C, Ghiurea M, Sîrbu CE, Somoghi R, Cioroianu TM, Faraon VA, Lupu C, Trică B, Constantinescu-Aruxandei D, Oancea F. Effects of Siliceous Natural Nanomaterials Applied in Combination with Foliar Fertilizers on Physiology, Yield and Fruit Quality of the Apricot and Peach Trees. Plants. 2021; 10(11):2395. https://doi.org/10.3390/plants10112395
Chicago/Turabian StyleMoale, Cristina, Marius Ghiurea, Carmen Eugenia Sîrbu, Raluca Somoghi, Traian Mihai Cioroianu, Victor Alexandru Faraon, Carmen Lupu, Bogdan Trică, Diana Constantinescu-Aruxandei, and Florin Oancea. 2021. "Effects of Siliceous Natural Nanomaterials Applied in Combination with Foliar Fertilizers on Physiology, Yield and Fruit Quality of the Apricot and Peach Trees" Plants 10, no. 11: 2395. https://doi.org/10.3390/plants10112395