Foliar Application of Nano, Chelated, and Conventional Iron Forms Enhanced Growth, Nutritional Status, Fruiting Aspects, and Fruit Quality of Washington Navel Orange Trees (Citrus sinensis L. Osbeck)
Abstract
:1. Introduction
2. Results
2.1. Vegetative Growth Measurements
2.2. Chlorophyll, Carotenoid, Macro and Micronutrient Content in Orange Tree Leaves
2.3. Fruit Aspect and Shelf Life
| Parameters Treatments | No. of New Shoots (NS) | Shoot Length, cm (SL) | Shoot Diameter, mm (SD) | No. of Leaves/Shoot (NOL) | Leaf Area, cm2 (LA) | Assimilation Area, m2/Shoot (TAA) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| T1 | 23.33 f | 23.33 d | 35.67 d | 36.33 e | 3.12 b | 3.14 e | 39.67 c | 40.67 c | 17.55 c | 17.59 c | 6.96 c | 7.15 c |
| T2 | 27.00 cd | 27.67 b | 41.33 b | 41.33 bc | 3.23 ab | 3.22 c | 43.33 b | 44.00 b | 17.70 ab | 17.71 a | 7.67 b | 7.79 b |
| T3 | 33.00 a | 32.67 a | 45.67 a | 44.33 a | 3.33 a | 3.29 a | 46.67 a | 46.33 a | 17.73 a | 17.71 a | 8.27 a | 8.20 a |
| T4 | 24.00 ef | 23.33 d | 37.00 cd | 37.33 e | 3.22 ab | 3.14 e | 39.67 c | 41.00 c | 17.58 c | 17.59 bc | 6.97 c | 7.21 c |
| T5 | 25.67 de | 25.33 c | 39.33 bcd | 39.00 d | 3.18 ab | 3.18 d | 43.33 b | 43.33 b | 17.66 b | 17.67 ab | 7.65 b | 7.66 b |
| T6 | 27.67 bc | 27.00 bc | 40.33 bc | 40.33 cd | 3.23 ab | 3.21 c | 44.33 b | 43.67 b | 17.71 a | 17.71 a | 7.85 b | 7.73 b |
| T7 | 29.33 b | 28.67 b | 43.33 ab | 42.33 b | 3.28 ab | 3.24 b | 46.33 a | 46.00 a | 17.71 a | 17.73 a | 8.21 a | 8.15 a |
| Parameters Treatments | Chlorophyll A, mg/g-1F.W. (CA) | Chlorophyll B, mg/g-1F.W. (CB) | Total Chlorophyll, mg/g-1F.W. (TC) | Carotenoids, mg/g-1F.W. (CAR) | ||||
|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| T1 | 6.45 c | 6.52 d | 3.18 d | 3.00 d | 9.63 d | 10.59 e | 3.04 d | 3.11 e |
| T2 | 7.56 ab | 7.31 bc | 4.16 b | 4.05 b | 11.72 b | 11.12 b | 3.78 b | 3.96 b |
| T3 | 8.04 a | 8.08 a | 4.53 a | 4.60 a | 12.57 a | 11.62 a | 4.04 a | 4.15 a |
| T4 | 7.15 b | 7.15 c | 3.70 c | 3.65 c | 10.8 c | 10.79 d | 3.16 d | 3.26 d |
| T5 | 7.94 a | 7.62 b | 3.81 c | 3.95 b | 11.75 b | 10.96 c | 3.48 c | 3.60 c |
| T6 | 7.96 a | 7.96 a | 4.11 b | 4.05 b | 12.07 ab | 10.99 c | 3.89 ab | 3.96 b |
| T7 | 8.00 a | 8.01 a | 4.24 ab | 4.18 b | 12.24 ab | 11.02 c | 3.98 a | 4.03 ab |
| Parameters Treatments | N (%) | P (%) | K (%) | Mg (%) | Ca (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| T1 | 2.73 e | 2.76 f | 0.163 a | 0.163 a | 1.66 d | 1.64 e | 0.565 c | 0.544 e | 4.50 e | 4.53 d |
| T2 | 2.95 c | 3.04 b | 0.162 ab | 0.158 b | 1.84 b | 1.87 b | 0.6095 b | 0.592 c | 4.73 b | 4.73 b |
| T3 | 3.04 a | 3.14 a | 0.160 abc | 0.158 b | 1.96 a | 1.99 a | 0.6355 ab | 0.633 a | 4.87 a | 4.83 a |
| T4 | 2.90 d | 2.83 e | 0.158 bcd | 0.151 c | 1.71 d | 1.73 d | 0.579 c | 0.572 d | 4.57 de | 4.56 d |
| T5 | 2.94 cd | 2.92 d | 0.157 cde | 0.155 bc | 1.78 c | 1.79 cd | 0.615 ab | 0.597 c | 4.64 c | 4.65 c |
| T6 | 2.98 bc | 2.99 c | 0.155 de | 0.158 b | 1.78 c | 1.83 bc | 0.624 ab | 0.613 b | 4.64 cd | 4.74 b |
| T7 | 3.01 ab | 3.04 b | 0.154 e | 0.163 a | 1.86 b | 1.87 b | 0.638 a | 0.627 a | 4.73 b | 4.75 b |
| Parameters Treatments | Fe (ppm) | Mn (ppm) | Zn (ppm) | |||
|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| T1 | 76.08 f | 75.17 e | 44.17 d | 43.94 d | 29.03 b | 27.60 g |
| T2 | 83.10 b | 83.03 b | 46.98 b | 46.42 b | 35.08 a | 31.62 b |
| T3 | 85.64 a | 85.11 a | 48.37 a | 47.51 a | 35.97 a | 32.38 a |
| T4 | 77.86 e | 76.37 de | 44.86 cd | 43.90 d | 32.23 ab | 28.36 f |
| T5 | 79.23 d | 77.27 d | 45.14 c | 44.48 cd | 30.25 ab | 29.14 e |
| T6 | 81.27 c | 80.49 c | 45.59 c | 45.31 c | 31.94 ab | 29.87 d |
| T7 | 82.52 b | 81.58 c | 47.31 b | 46.31 b | 33.52 ab | 30.28 c |
| Parameters Treatments | Fruit Set, % (FS) | Fruit Retention, % (FR) | Average Fruit Weight, g (AFW) | No. of Fruits/Tree (NOFT) | Yield (kg)/Tree (FWT) | Yield, t ha-1 (YPF) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| T1 | 17.91 e | 17.22 d | 12.44 e | 12.07 d | 261.0 b | 265.33 d | 155.00 f | 160.33 f | 40.45 f | 42.55 e | 25.16 e | 26.87 d |
| T2 | 19.68 b | 18.95 ab | 14.56 b | 13.91 b | 269.67 ab | 272.00 bc | 175.67 b | 178.00 b | 47.38 bc | 48.42 b | 30.42 bc | 31.37 b |
| T3 | 20.108 a | 19.287 a | 15.97 a | 14.67 a | 276.67 a | 281.00 a | 186.67 a | 184.33 a | 51.65 a | 51.82 a | 34.01 a | 34.68 a |
| T4 | 18.19 e | 17.15 d | 13.38 d | 12.39 d | 268.33 ab | 269.33 cd | 162.33 e | 165.33 e | 43.52 e | 44.53 d | 27.79 d | 28.56 cd |
| T5 | 18.64 d | 18.357 c | 13.98 c | 12.83 c | 266.33 ab | 273.67 bc | 171.67 cd | 169.33 d | 45.73 d | 46.34 c | 28.99 cd | 30.18 bc |
| T6 | 18.74 d | 18.59 bc | 14.87 b | 13.67 b | 273.33 ab | 271.33 bcd | 169.33 d | 173.33 c | 46.29 cd | 47.03 bc | 30.11 bc | 30.37 b |
| T7 | 19.17 c | 18.98 a | 15.62 a | 13.97 b | 276.33 a | 276.00 ab | 175.33 bc | 183.67 a | 48.46 b | 50.70 a | 31.87 ab | 33.32 a |
| Parameters Treatments | Peel Diameter, mm (PT) | Polar Diameter, cm (PD) | Equatorial Diameter, cm (ED) | Fruits Shape Index (FSI) | Juice Weight, g (JW) | Juice, % (J) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| T1 | 3.103 c | 3.113 d | 8.310 a | 8.293 d | 8.323 b | 8.327 e | 0.998 a | 0.996 b | 109.26 c | 109.57 e | 41.86 c | 41.29 d |
| T2 | 3.127 b | 3.130 c | 8.347 a | 8.327 b | 8.373 a | 8.350 bcd | 0.997 a | 0.997 ab | 113.87 abc | 113.98 bc | 42.22 bc | 41.90 bc |
| T3 | 3.167 a | 3.170 a | 8.350 a | 8.353 a | 8.367 ab | 8.367 ab | 0.998 a | 0.998 a | 118.92 a | 119.49 a | 42.98 a | 42.52 a |
| T4 | 3.107 c | 3.113 d | 8.330 a | 8.310 c | 8.360 ab | 8.333 de | 0.996 a | 0.997 ab | 112.32 bc | 111.08 de | 41.86 c | 41.24 d |
| T5 | 3.133 b | 3.130 | 8.347 a | 8.320 bc | 8.373 a | 8.340 cde | 0.997 a | 0.998 ab | 111.98 bc | 114.13 bc | 42.04 bc | 41.70 c |
| T6 | 3.160 a | 3.113 d | 8.340 a | 8.357 a | 8.370 a | 8.380 a | 0.996 a | 0.997 ab | 115.81 ab | 113.50 cd | 42.37 b | 41.83 c |
| T7 | 3.170 a | 3.157 b | 8.347 a | 8.333 b | 8.370 a | 8.357 bc | 0.997 a | 0.997 ab | 116.97 ab | 116.23 b | 42.33 b | 42.11 b |
| Parameters Treatments | T.S.S., % (TSS) | Total Acidity, % (TA) | TSS/Acid Ratio (TS/AC) | Total Sugars, % (TS) | V.C. (VC) | Shelf Life, Days (SLIF) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| T1 | 11.13 c | 11.60 d | 1.044 a | 1.062 a | 10.66 c | 10.92 e | 8.68 b | 9.59 c | 57.31 e | 59.12 e | 13.60 d | 16.33 e |
| T2 | 12.87 ab | 13.17 b | 0.988 b | 0.988 b | 13.03 b | 13.32 c | 9.07 ab | 10.11 bc | 62.66 bc | 63.36 b | 23.00 ab | 23.67 bc |
| T3 | 12.89 ab | 13.91 a | 0.907 d | 0.903 d | 14.22 a | 15.40 a | 9.62 a | 10.95 a | 63.79 a | 64.92 a | 25.33 a | 26.33 a |
| T4 | 12.34 b | 11.82 d | 0.9737 b | 1.000 b | 12.68 b | 11.82 d | 9.34 ab | 9.73 c | 60.64 d | 60.29 d | 15.67 cd | 17.33 |
| T5 | 12.59 ab | 12.27 c | 0.930 cd | 0.950 c | 13.54 ab | 12.92 c | 9.43 ab | 10.04 bc | 62.09 c | 61.00 d | 18.00 c | 20.33 d |
| T6 | 12.41 ab | 12.57 c | 0.938 c | 0.954 c | 13.23 b | 13.17 c | 9.34 ab | 10.07 bc | 62.19 c | 62.12 c | 20.67 b | 22.33 cd |
| T7 | 13.22 a | 13.24 b | 0.918 cd | 0.934 c | 14.40 a | 14.17 b | 9.70 a | 10.48 ab | 62.98 b | 63.36 b | 22.67 b | 25.33 ab |
3. Discussion
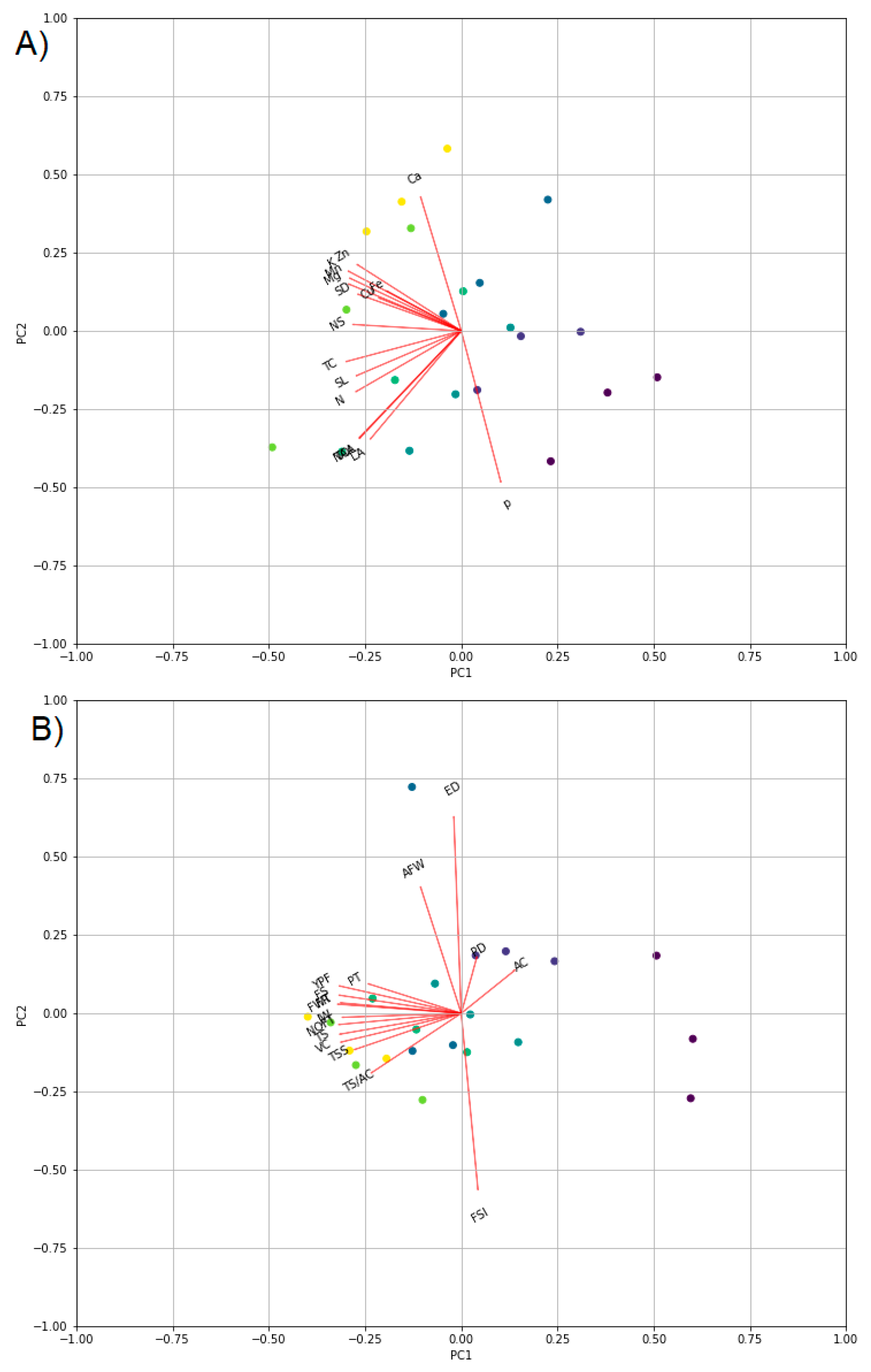
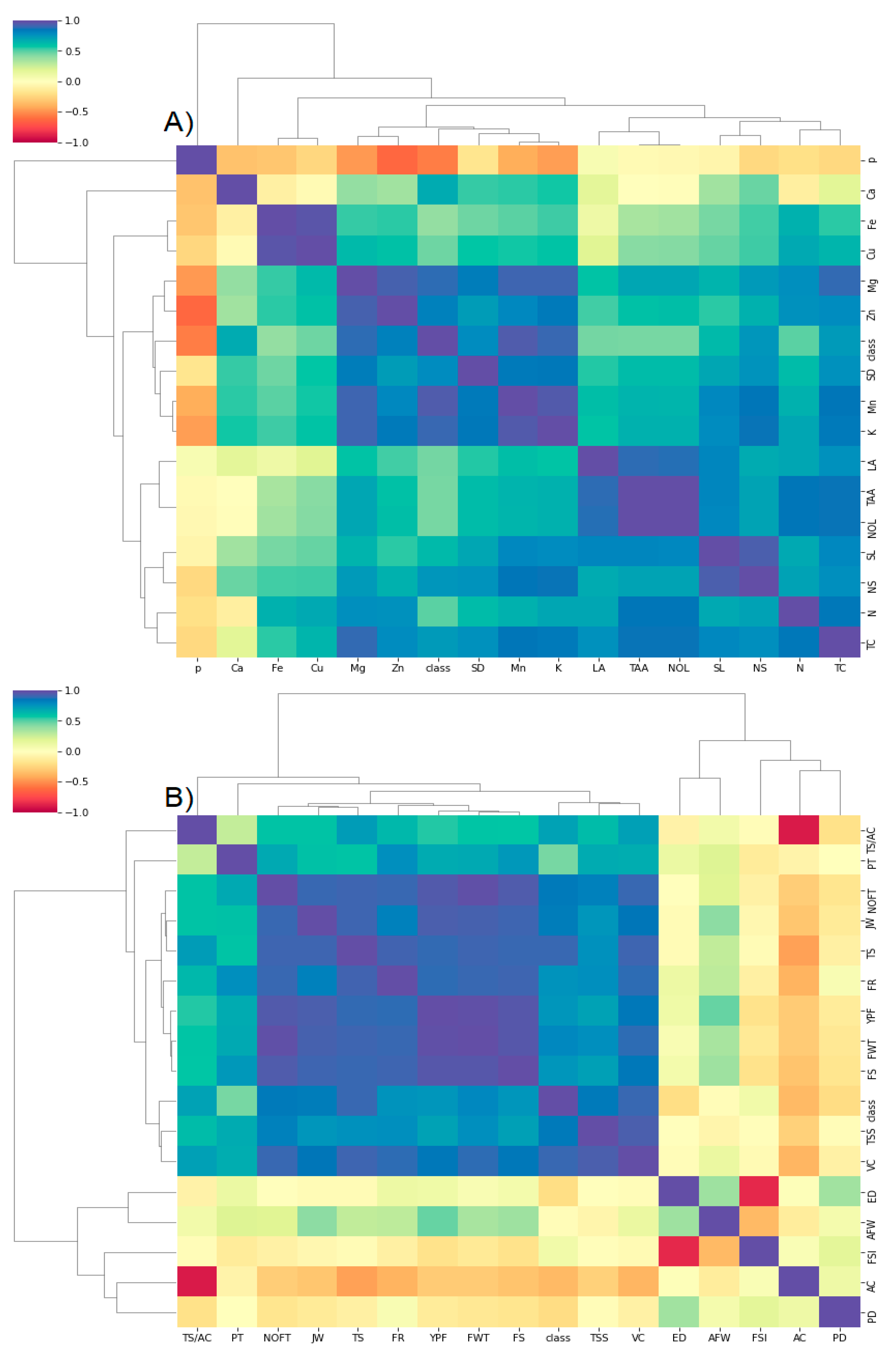
4. Materials and Methods
4.1. Study Location, Climate Data, and Soil Properties
4.2. Preparation of Green Fe-NPs
4.2.1. Guava Leaf Extract
4.2.2. Green Synthesis of Fe-NPs
4.2.3. Scanning Electron Microscopy (SEM)
4.2.4. Transition Electron Microscope (TEM)
4.2.5. Zeta Potential and Dynamic Light Scattering
4.3. Treatments and Experimental Layout
4.4. Measurements and Data Collection
4.4.1. Vegetative Growth Measurements
4.4.2. Nutritional Status Measurements and Leaf Mineral Composition
4.4.3. Productivity Measurements
4.4.4. Fruit Quality
4.5. Statistical Analysis
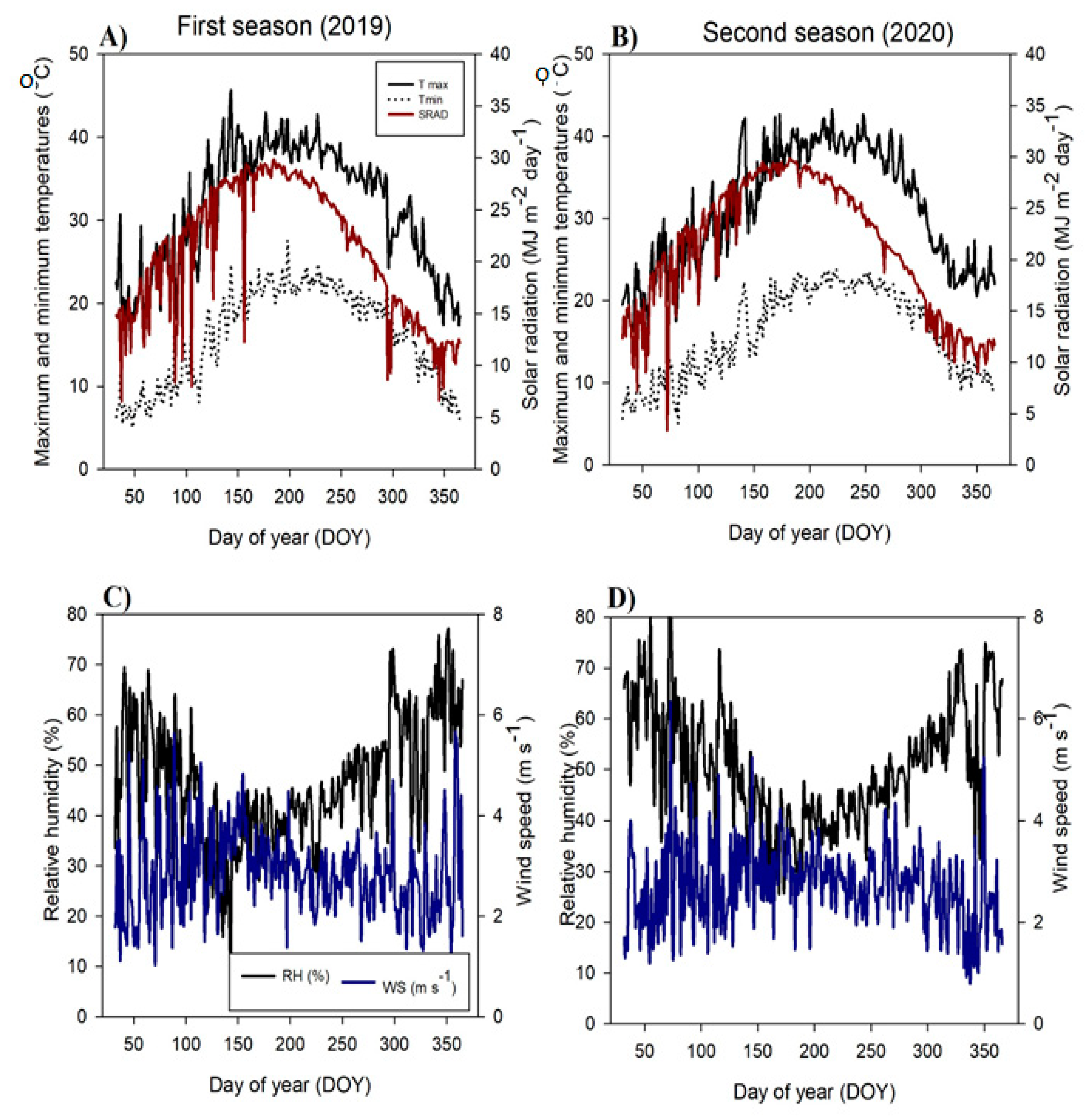
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inglese, P.; Sortino, G. Citrus History, Taxonomy, Breeding, and Fruit Quality. Oxford Research Encyclopedia of Environmental Science. 2019. Available online: https://doi.org/10.1093/acrefore/9780199389414.013.221 (accessed on 15 May 2020). [CrossRef]
- Aguado, A.; Frías, J.; García-Tejero, I.; Romero, F.; Muriel, J.L.; Capote, N. Towards the Improvement of Fruit-Quality Parameters in Citrus under Deficit Irrigation Strategies. ISRN Agron. 2012, 2012, 940896. [Google Scholar] [CrossRef]
- Wally, A.; Akingbe, O.; Attaché, A. Citrus Annual: Egypt Maintains its Position as the World Leading Orange Exporter. USDA, United States Departement of Agriculture 2020, Foriegn Agriculture Service. Available online: https://citrusbr.com/wp-content/uploads/2021/2003/Citrus-Annual_Cairo_Egypt_2012-2015-2020.pdf (accessed on 15 May 2020).
- Turner, T.; Burri, B.J. Potential Nutritional Benefits of Current Citrus Consumption. Agriculture 2013, 3, 165–168. [Google Scholar] [CrossRef]
- Kumrungsee, T.; Zhang, P.; Chartkul, M.; Yanaka, N.; Kato, N. Potential Role of Vitamin B6 in Ameliorating the Severity of COVID-19 and Its Complications. Front. Nutr. 2020, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P.; Donzelli, A. Hesperidin and SARS-CoV-2: New Light on the Healthy Function of Citrus Fruits. Antioxidants 2020, 9, 742. [Google Scholar] [CrossRef]
- Alberca, R.W.; Teixeira, F.M.E.; Beserra, D.R.; de Oliveira, E.A.; Andrade, M.M.d.S.; Pietrobon, A.J.; Sato, M.N. Perspective: The Potential Effects of Naringenin in COVID-19. Front. Immunol. 2020, 11, 2477. [Google Scholar] [CrossRef]
- Müller, C.; Kuki, K.N.; Pinheiro, D.T.; de Souza, L.R.; Siqueira Silva, A.I.; Loureiro, M.E.; Oliva, M.A.; Almeida, A.M. Differential physiological responses in rice upon exposure to excess distinct iron forms. Plant Soil 2015, 391, 123–138. [Google Scholar] [CrossRef]
- Bashir, K.; Nozoye, T.; Nagasaka, S.; Rasheed, S.; Miyauchi, N.; Seki, M.; Nakanishi, H.; Nishizawa, N.K. Paralogs and mutants show that one DMA synthase functions in iron homeostasis in rice. J. Exp. Bot. 2017, 68, 1785–1795. [Google Scholar] [CrossRef]
- Li, W.; Lan, P. The Understanding of the Plant Iron Deficiency Responses in Strategy I Plants and the Role of Ethylene in This Process by Omic Approaches. Front. Plant Sci. 2017, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Guerinot, M.L. Mining iron: Iron uptake and transport in plants. FEBS Lett. 2007, 581, 2273–2280. [Google Scholar] [CrossRef]
- Pereira, M.P.; Santos, C.; Gomes, A.; Vasconcelos, M.W. Cultivar variability of iron uptake mechanisms in rice (Oryzasativa L.). Plant Physiol. Biochem. 2014, 85, 21–30. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Vose, P.B. Iron nutrition in plants: A world overview. J. Plant Nutr. 1982, 5, 233–249. [Google Scholar] [CrossRef]
- Balk, J.; von Wirén, N.; Thomine, S. The iron will of the research community: Advances in iron nutrition and interactions in lockdown times. J. Exp. Bot. 2021, 72, 2011–2013. [Google Scholar] [CrossRef] [PubMed]
- Rout, G.R.; Sahoo, S. Role of Iron in Plant Growth and Metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Mori, S.; Nishizawa, N.; Hayashi, H.; Chino, M.; Yoshimura, E.; Ishihara, J. Why are young rice plants highly susceptible to iron deficiency? Plant Soil 1991, 130, 143–156. [Google Scholar] [CrossRef]
- Morrissey, J.; Guerinot, M.L. Iron Uptake and Transport in Plants: The Good, the Bad, and the Ionome. Chem. Rev. 2009, 109, 4553–4567. [Google Scholar] [CrossRef]
- Lynch, S.R. Why Nutritional Iron Deficiency Persists as a Worldwide Problem. J. Nutr. 2011, 141, 763S–768S. [Google Scholar] [CrossRef]
- Mwangi, M.N.; Phiri, K.S.; Abkari, A.; Gbané, M.; Bourdet-Sicard, R.; Braesco, V.A.; Zimmermann, M.B.; Prentice, A.M. Iron for Africa—Report of an Expert Workshop. Nutrients 2017, 9, 576. [Google Scholar] [CrossRef]
- Connorton, J.M.; Jones, E.R.; Rodríguez-Ramiro, I.; Fairweather-Tait, S.; Uauy, C.; Balk, J. Wheat Vacuolar Iron Transporter TaVIT2 Transports Fe and Mn and Is Effective for Biofortification. Plant Physiol. 2017, 174, 2434. [Google Scholar] [CrossRef]
- Nikolic, M.; Pavlovic, J. Chapter 3—Plant Responses to Iron Deficiency and Toxicity and Iron Use Efficiency in Plants. In Plant Micronutrient Use Efficiency; Hossain, M.A., Kamiya, T., Burritt, D.J., Phan Tran, L.-S., Fujiwara, T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 55–69. [Google Scholar]
- DeRosa, M.C.; Monreal, C.; Schnitzer, M.; Walsh, R.; Sultan, Y. Nanotechnology in fertilizers. Nat. Nanotechnol. 2010, 5, 91. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Almutairi, K.F.; Alotaibi, M.; Shami, A.; Alhammad, B.A.; Battaglia, M.L. Nano-Fertilization as an Emerging Fertilization Technique: Why Can Modern Agriculture Benefit from Its Use? Plants 2021, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Boutchuen, A.; Zimmerman, D.; Aich, N.; Masud, A.M.; Arabshahi, A.; Palchoudhury, S. Increased Plant Growth with Hematite Nanoparticle Fertilizer Drop and Determining Nanoparticle Uptake in Plants Using Multimodal Approach. J. Nanomater. 2019, 2019, 6890572. [Google Scholar] [CrossRef]
- Chand Mali, S.; Raj, S.; Trivedi, R. Nanotechnology a novel approach to enhance crop productivity. Biochem. Biophys. Rep. 2020, 24, 100821. [Google Scholar] [CrossRef] [PubMed]
- Kheir, A.M.S.; Ding, Z.; Gawish, M.S.; Abou El Ghit, H.M.; Hashim, T.A.; Ali, E.F.; Eissa, M.A.; Zhou, Z.; Al-Harbi, M.S.; El-Gioushy, S.F. The Exogenous Application of Micro-Nutrient Elements and Amino Acids Improved the Yield, Nutritional Status and Quality of Mango in Arid Regions. Plants 2021, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Nayan, R.; Negi, B.; Zaidi, M.G.H.; Arora, S. Physio-biochemical basis of iron-sulfide nanoparticle induced growth and seed yield enhancement in B. juncea. Plant Physiol. Biochem. 2017, 118, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Guha, T.; Ravikumar, K.V.G.; Mukherjee, A.; Mukherjee, A.; Kundu, R. Nanopriming with zero valent iron (nZVI) enhances germination and growth in aromatic rice cultivar (Oryzasativa cv. Gobindabhog L.). Plant Physiol. Biochem. 2018, 127, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Tavallali, V.; Kiani, M.; Hojati, S. Iron nano-complexes and iron chelate improve biological activities of sweet basil (Ocimumbasilicum L.). Plant Physiol. Biochem. 2019, 144, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Vigani, G.; Zocchi, G.; Bashir, K.; Philippar, K.; Briat, J.-F. Cellular iron homeostasis and metabolism in plant. Front. Plant Sci. 2013, 4, 490. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Gaur, S.; Singh, S.; Yadav, V.; Liu, S.; Singh, V.P.; Sharma, S.; Srivastava, P.; Prasad, S.M.; et al. Acquisition and Homeostasis of Iron in Higher Plants and Their Probable Role in Abiotic Stress Tolerance. Front. Environ. Sci. 2018, 5, 86. [Google Scholar] [CrossRef]
- Liu, J.; Chakraborty, S.; Hosseinzadeh, P.; Yu, Y.; Tian, S.; Petrik, I.; Bhagi, A.; Lu, Y. Metalloproteins containing cytochrome, iron-sulfur, or copper redox centers. Chem. Rev. 2014, 114, 4366–4469. [Google Scholar] [CrossRef]
- Mongon, J.; Chaiwong, N.; Bouain, N.; Prom-u-thai, C.; Secco, D.; Rouached, H. Phosphorus and Iron Deficiencies Influences Rice Shoot Growth in an Oxygen Dependent Manner: Insight from Upland and Lowland Rice. Int. J. Mol. Sci. 2017, 18, 607. [Google Scholar] [CrossRef]
- Schmidt, W.; Thomine, S.; Buckhout, T.J. Editorial: Iron Nutrition and Interactions in Plants. Front. Plant Sci. 2020, 10, 1670. [Google Scholar] [CrossRef]
- Jampílek, J.; Kráľová, K. Application Of Nanotechnology In Agriculture And Food Industry, Its Prospects And Risks. Ecol. Chem. Eng. S 2015, 22, 321–361. [Google Scholar] [CrossRef]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [PubMed]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef]
- Sabir, A.; Yazar, K.; Sabir, F.; Kara, Z.; Yazici, M.A.; Goksu, N. Vine growth, yield, berry quality attributes and leaf nutrient content of grapevines as influenced by seaweed extract (Ascophyllumnodosum) and nanosize fertilizer pulverizations. Sci. Hortic. 2014, 175, 1–8. [Google Scholar] [CrossRef]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Waters, B.M.; Amundsen, K.; Graef, G. Gene Expression Profiling of Iron Deficiency Chlorosis Sensitive and Tolerant Soybean Indicates Key Roles for Phenylpropanoids under Alkalinity Stress. Front. Plant Sci. 2018, 9, 10. [Google Scholar] [CrossRef]
- Wang, Z.; Yue, L.; Dhankher, O.P.; Xing, B. Nano-enabled improvements of growth and nutritional quality in food plants driven by rhizosphere processes. Environ. Int. 2020, 142, 105831. [Google Scholar] [CrossRef]
- Mohammadi, M.; Majnoun Hoseini, N.; Chaichi, M.R.; Alipour, H.; Dashtaki, M.; Safikhani, S. Influence of nano-iron oxide and zinc sulfate on physiological characteristics of peppermint. Commun. Soil Sci. Plant Anal. 2018, 49, 2315–2326. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Abdelaziz, S.M.; El-Mogy, M.M.; Abdeldaym, E.A. Effect of Foliar Zno and Feo Nanoparticles Application on Growth and Nutritional Quality of Red Radish and Assessment of Their Accumulation on Human Health. Agric. (Pol’nohospodárstvo) 2019, 65, 16–29. [Google Scholar] [CrossRef]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Fatima, Z.; Ahmed, M.; Hussain, M.; Abbas, G.; Ul-Allah, S.; Ahmad, S.; Ahmed, N.; Ali, M.A.; Sarwar, G.; Haque, E.u.; et al. The fingerprints of climate warming on cereal crops phenology and adaptation options. Sci. Rep. 2020, 10, 18013. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-Based Sustainable Agriculture and Food Science: Recent Advances and Future Outlook. Front. Nanotechnol. 2020, 2, 10. [Google Scholar] [CrossRef]
- Davarpanah, S.; Tehranifar, A.; Zarei, M.; Aran, M.; Davarynejad, G.; Abadía, J. Early Season Foliar Iron Fertilization Increases Fruit Yield and Quality in Pomegranate. Agronomy 2020, 10, 832. [Google Scholar] [CrossRef]
- El-Jendoubi, H.; Vázquez, S.; Calatayud, Á.; Vavpetič, P.; Vogel-Mikuš, K.; Pelicon, P.; Abadía, J.; Abadía, A.; Morales, F. The effects of foliar fertilization with iron sulfate in chlorotic leaves are limited to the treated area. A study with peach trees (Prunuspersica L. Batsch) grown in the field and sugar beet (Beta vulgaris L.) grown in hydroponics. Front. Plant Sci. 2014, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, A.a.; Havas, F.; Ghaderi, N. Application of iron nanoparticles and salicylic acid in in vitro culture of strawberries (Fragaria × ananassa Duch.) to cope with drought stress. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 132, 511–523. [Google Scholar] [CrossRef]
- Black, C.A.; Evans, D.O.; Ensminger, L.E.; White, J.L.; Clark, F.E.; Dinauer, R.C. Methods of soil analysis. Part 2. In Chemical and Microbiological Properties, 2nd ed.; Soil Science Society of America, Inc.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982. [Google Scholar]
- Patil, S.P.; Rane, P.M. Psidium guajava leaves assisted green synthesis of metallic nanoparticles: A review. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 60. [Google Scholar] [CrossRef]
- Gumustas, M.; Sengel-Turk, C.T.; Gumustas, A.; Ozkan, S.A.; Uslu, B. Chapter 5—Effect of Polymer-Based Nanoparticles on the Assay of Antimicrobial Drug Delivery Systems. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 67–108. [Google Scholar]
- Mahbubul, I.M. 3-Stability and Dispersion Characterization of Nanofluid. In Preparation, Characterization, Properties and Application of Nanofluid; Mahbubul, I.M., Ed.; William Andrew Publishing: Gazipur, Bangladesh, 2019; pp. 47–112. [Google Scholar]
- Saric, M.; Kastroi, R.; Curic, R.; Geric, L. Effect of salinity on some citrus rootstocks. Park Fiziol. Anjiga 1967, 215. Available online: www.curresweb.com/ije/ije/2017/128-138.pdf (accessed on 15 May 2020).
- Pregl, E. Quantitative Organic Micro Analysis, 4th ed.; Chundril: London, UK, 1945. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; Inter. Sci. Publishers: New York, NY, USA, 1958; pp. 213–217. [Google Scholar]
- Brown, J.D.; Lilliand, O. Rapid determination of potassium and sodium in plant material and soil extract by flame photometer. Proc. Amer. Soc. Hort. Sci. 1946, 48, 341–346. [Google Scholar]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soil, Plant and Waters, 6th ed.; University of California, Division of Agricultural Sciences: Berkley, CA, USA, 1961; pp. 56–64. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; Methods; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000; Available online: https://law.resource.org/pub/us/cfr/ibr/002/aoac.methods.1.1990.pdf (accessed on 11 January 2021).
- Smith, F.; Cilles, A.M.; Hamilton, K.J.; Gedes, A.P. Colorimetric methods for the determination of sugar and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980; 593p. [Google Scholar]
| No. | Treatment | Symbol |
|---|---|---|
| 1 | Control (spraying with tap water) | T1 |
| 2 | Fe-NPs1 (1/80 dilution of the Fe-NPs stock solution) | T2 |
| 3 | Fe-NPs2 (1/40 dilution of the Fe-NPs stock solution) | T3 |
| 4 | 0.1% ferrous sulphate (FeSO4·7H2O) | T4 |
| 5 | 0.2% ferrous sulphate (FeSO4·7H2O) | T5 |
| 6 | 0.05% Fe-chelated (EDTA) | T6 |
| 7 | 0.1% Fe-chelated (EDTA) | T7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Gioushy, S.F.; Ding, Z.; Bahloul, A.M.E.; Gawish, M.S.; Abou El Ghit, H.M.; Abdelaziz, A.M.R.A.; El-Desouky, H.S.; Sami, R.; Khojah, E.; Hashim, T.A.; et al. Foliar Application of Nano, Chelated, and Conventional Iron Forms Enhanced Growth, Nutritional Status, Fruiting Aspects, and Fruit Quality of Washington Navel Orange Trees (Citrus sinensis L. Osbeck). Plants 2021, 10, 2577. https://doi.org/10.3390/plants10122577
El-Gioushy SF, Ding Z, Bahloul AME, Gawish MS, Abou El Ghit HM, Abdelaziz AMRA, El-Desouky HS, Sami R, Khojah E, Hashim TA, et al. Foliar Application of Nano, Chelated, and Conventional Iron Forms Enhanced Growth, Nutritional Status, Fruiting Aspects, and Fruit Quality of Washington Navel Orange Trees (Citrus sinensis L. Osbeck). Plants. 2021; 10(12):2577. https://doi.org/10.3390/plants10122577
Chicago/Turabian StyleEl-Gioushy, Sherif F., Zheli Ding, Asmaa M. E. Bahloul, Mohamed S. Gawish, Hanan M. Abou El Ghit, Adel M. R. A. Abdelaziz, Heba S. El-Desouky, Rokayya Sami, Ebtihal Khojah, Taghred A. Hashim, and et al. 2021. "Foliar Application of Nano, Chelated, and Conventional Iron Forms Enhanced Growth, Nutritional Status, Fruiting Aspects, and Fruit Quality of Washington Navel Orange Trees (Citrus sinensis L. Osbeck)" Plants 10, no. 12: 2577. https://doi.org/10.3390/plants10122577







