The Opposite Roles of White Light in Regulating Germination of Fresh and Aged Seed in Tobacco
Abstract
:1. Introduction
2. Results
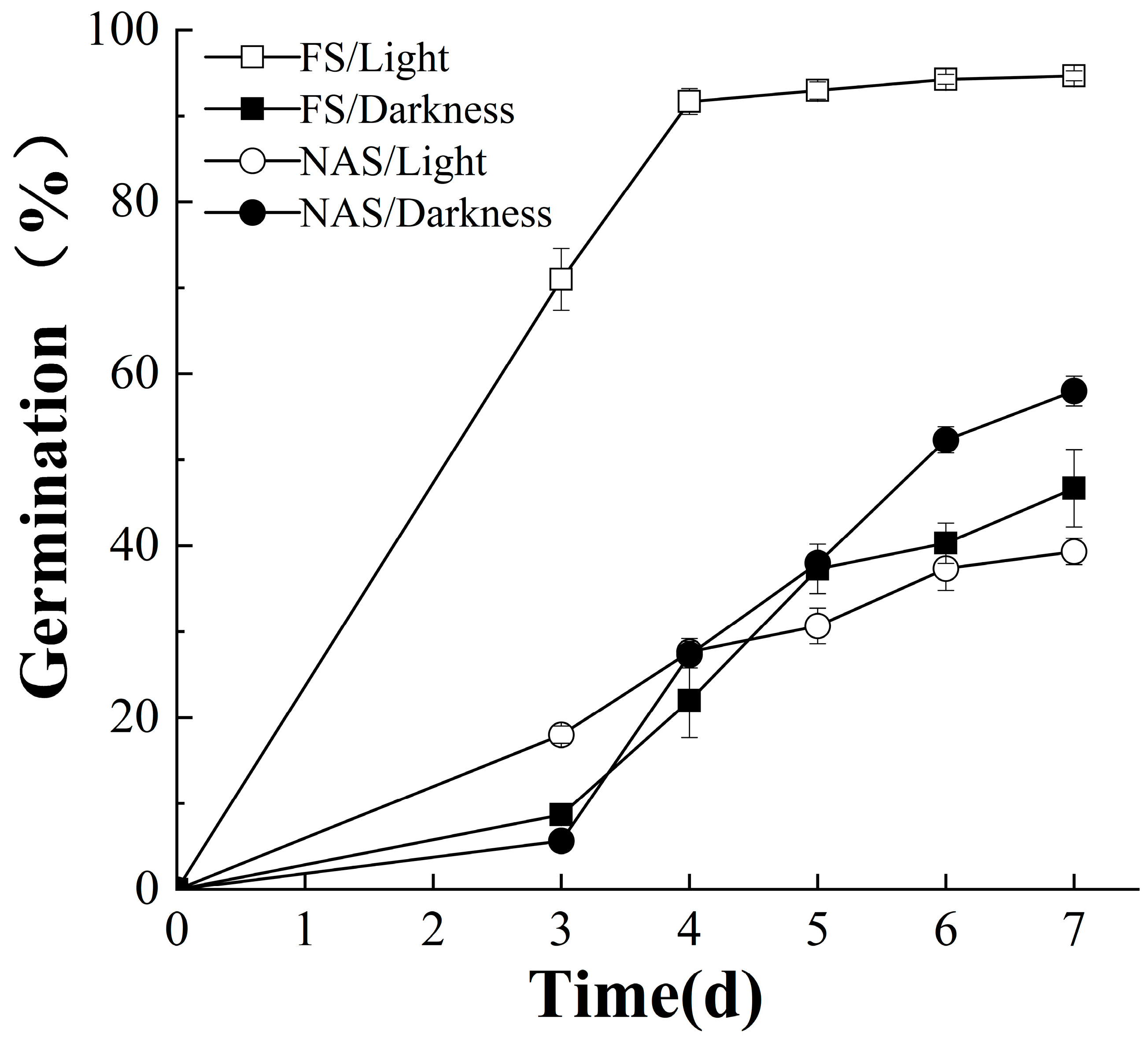
2.1. FS and NAS Germinate Better under Light and Dark Conditions
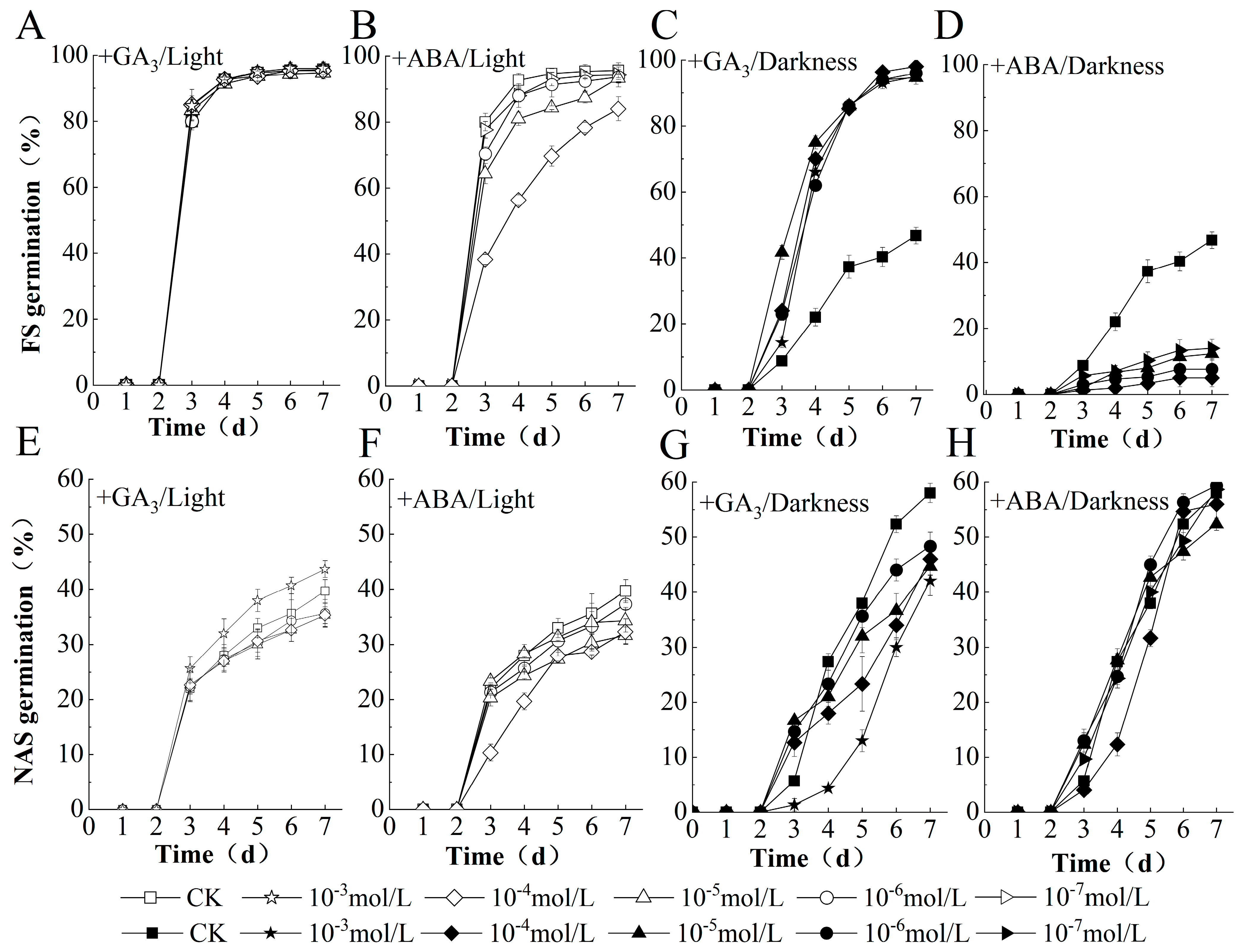
2.2. GA Replaced Light to Promote and Inhibit Germination of FS and NAS
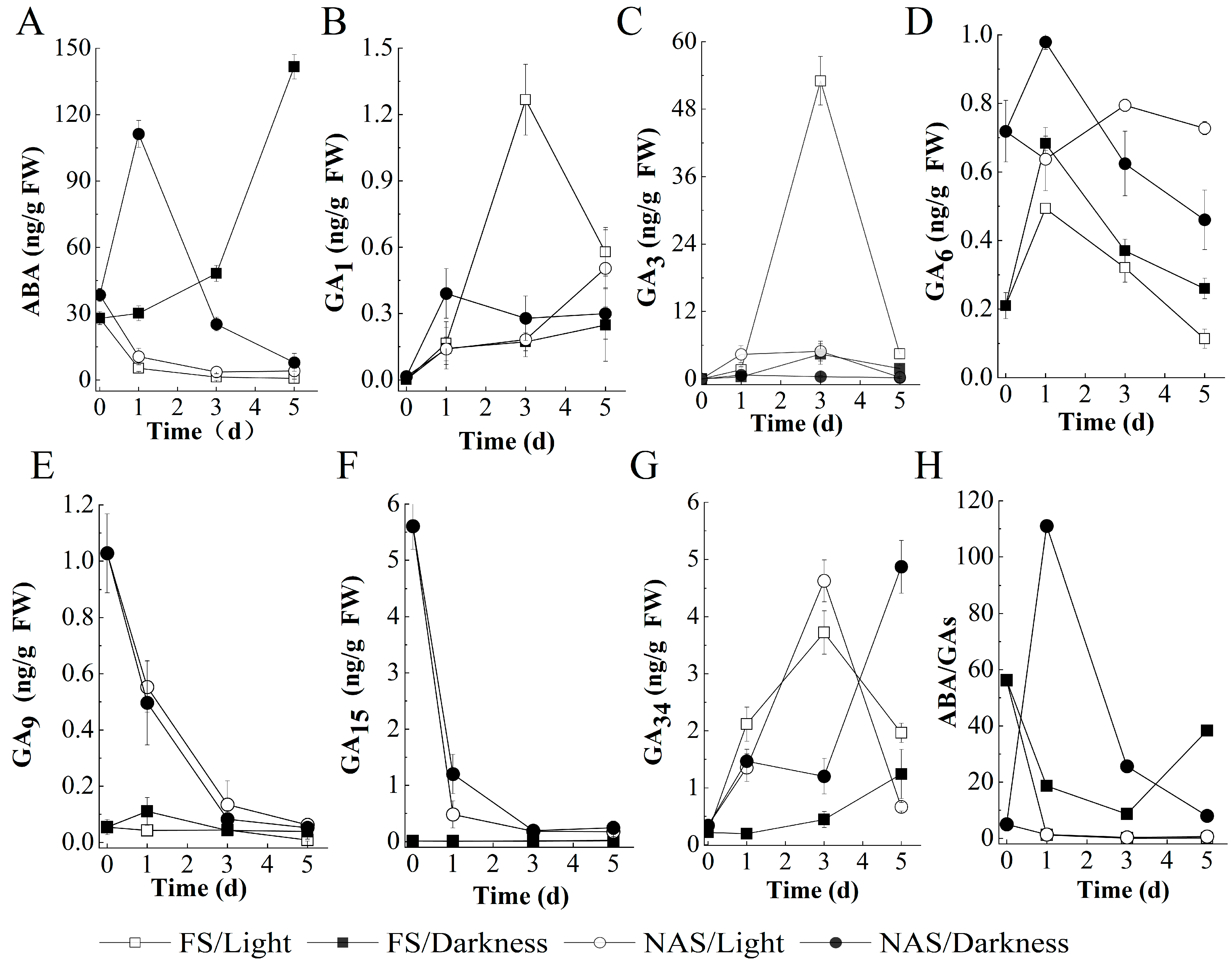
2.3. The Germination of FS Depending on Light to Promote GA Biosynthesis and ABA Reduction
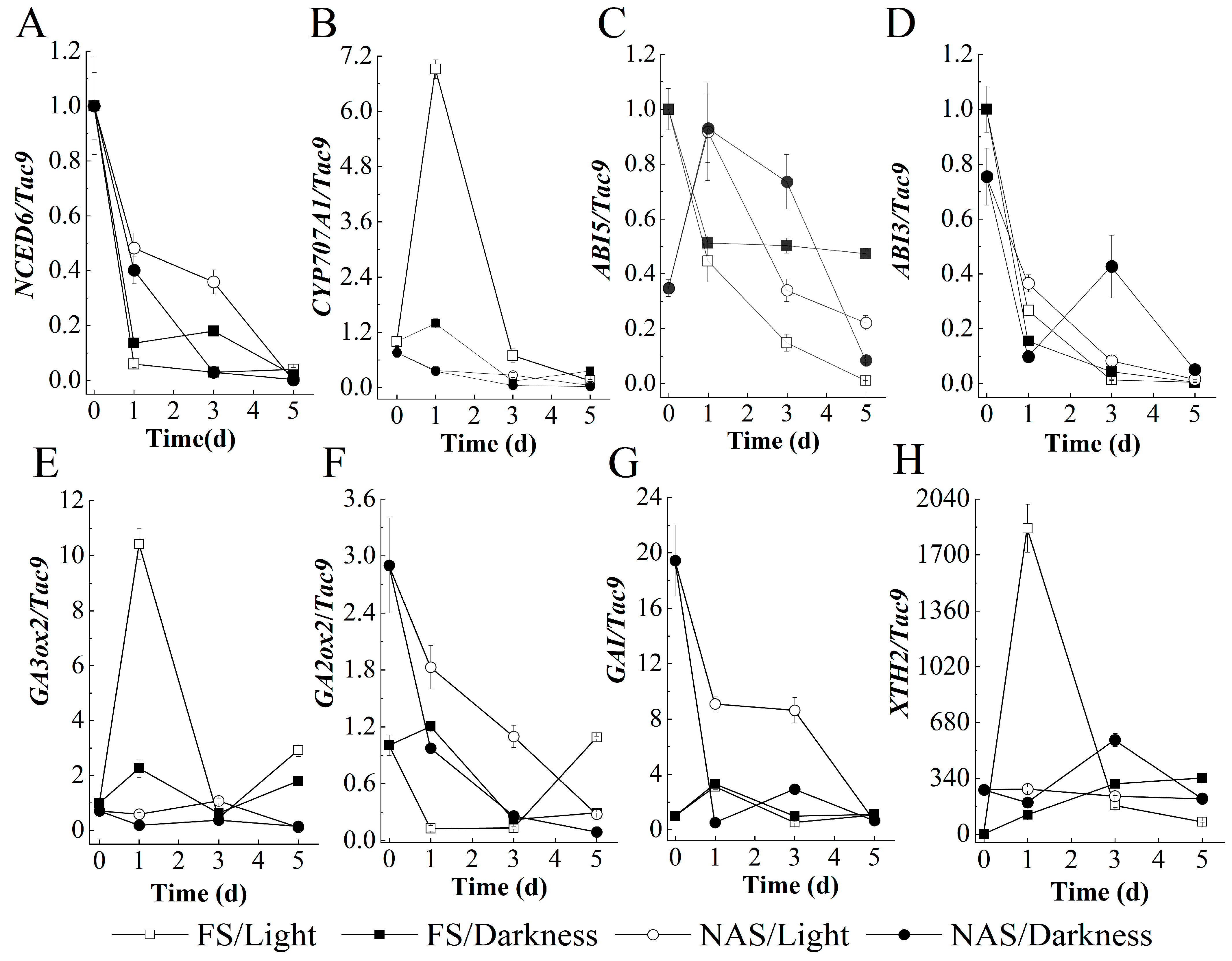
2.4. The Effect of ABA Catabolism on Germination of FS Promoted by Light
2.5. The Effect of GA Levels on Germination of FS Promoted by Light
2.6. The Effect of Cell Wall Hydrolysis on Light-Promoted and -Inhibited Germination of FS and NAS
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Seed Germination and Hormone Sensitivity Test
4.3. ABA and GAs Quantification
4.4. RNA Extraction and Quantitative RT-PCR
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bewley, J.D. Seed Germination and Dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [Green Version]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecher, T.; Leubner-Metzger, G. The biomechanics of seed germination. J. Exp. Bot. 2017, 68, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Debeaujon, I.; Koornneef, M. Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol. 2000, 122, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Piskurewicz, U.; Jikumaru, Y.; Kinoshita, N.; Nambara, E.; Kamiya, Y.; Lopez-Molina, L. The gibberellic acid signaling repressor RGL2 inhibits Arabidopsis seed germination by stimulating abscisic acid synthesis and ABI5 activity. Plant Cell 2008, 20, 2729–2745. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Ma, J.; Xu, Z.; Wang, X. Abscisic acid and ethephon regulation of cellulase in the endosperm cap and radicle during lettuce seed germination. J. Integr. Plant Biol. 2016, 58, 859–869. [Google Scholar] [CrossRef] [Green Version]
- Guo, G.; Liu, X.; Sun, F.; Cao, J.; Huo, N.; Wuda, B.; Xin, M.; Hu, Z.; Du, J.; Xia, R.; et al. Wheat miR9678 Affects Seed Germination by Generating Phased siRNAs and Modulating Abscisic Acid/Gibberellin Signaling. Plant Cell 2018, 30, 796–814. [Google Scholar] [CrossRef] [Green Version]
- Merai, Z.; Graeber, K.; Wilhelmsson, P.; Ullrich, K.K.; Arshad, W.; Grosche, C.; Tarkowska, D.; Tureckova, V.; Strnad, M.; Rensing, S.A.; et al. Aethionemaarabicum: A novel model plant to study the light control of seed germination. J. Exp. Bot. 2019, 70, 3313–3328. [Google Scholar] [CrossRef]
- Shu, K.; Liu, X.D.; Xie, Q.; He, Z.H. Two Faces of One Seed: Hormonal Regulation of Dormancy and Germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuan, P.A.; Kumar, R.; Rehal, P.K.; Toora, P.K.; Ayele, B.T. Molecular Mechanisms Underlying Abscisic Acid/Gibberellin Balance in the Control of Seed Dormancy and Germination in Cereals. Front. Plant Sci. 2018, 9, 668. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, P.; Kumar, P.P. Regulation of Seed Germination: The Involvement of Multiple Forces Exerted via Gibberellic Acid Signaling. Mol. Plant 2019, 12, 1416–1417. [Google Scholar] [CrossRef] [Green Version]
- Groot, S.P.; Karssen, C.M. Dormancy and Germination of Abscisic Acid-Deficient Tomato Seeds: Studies with the sitiens Mutant. Plant Physiol. 1992, 99, 952–958. [Google Scholar] [CrossRef] [Green Version]
- Nambara, E.; Hayama, R.; Tsuchiya, Y.; Nishimura, M.; Kawaide, H.; Kamiya, Y.; Naito, S. The role of ABI3 and FUS3 loci in Arabidopsis thaliana on phase transition from late embryo development to germination. Dev. Biol. 2000, 220, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassel, G.W.; Mullen, R.T.; Bewley, J.D. ABI3 expression ceases following, but not during, germination of tomato and Arabidopsis seeds. J. Exp. Bot. 2006, 57, 1291–1297. [Google Scholar] [CrossRef]
- Steber, C.M.; Cooney, S.E.; McCourt, P. Isolation of the GA-response mutant sly1 as a suppressor of ABI1-1 in Arabidopsis thaliana. Genetics 1998, 149, 509–521. [Google Scholar] [CrossRef]
- Iuchi, S.; Suzuki, H.; Kim, Y.C.; Iuchi, A.; Kuromori, T.; Ueguchi-Tanaka, M.; Asami, T.; Yamaguchi, I.; Matsuoka, M.; Kobayashi, M.; et al. Multiple loss-of-function of Arabidopsis gibberellin receptor AtGID1s completely shuts down a gibberellin signal. Plant J. 2007, 50, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Willige, B.C.; Ghosh, S.; Nill, C.; Zourelidou, M.; Dohmann, E.M.; Maier, A.; Schwechheimer, C. The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell 2007, 19, 1209–1220. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Liu, S.; Lin, R. The role of light in regulating seed dormancy and germination. J. Integr. Plant Biol. 2020, 62, 1310–1326. [Google Scholar] [CrossRef] [PubMed]
- Neff, M.M. Light-mediated seed germination: Connecting phytochrome B to gibberellic acid. Dev. Cell 2012, 22, 687–688. [Google Scholar] [CrossRef] [Green Version]
- Oh, E.; Yamaguchi, S.; Kamiya, Y.; Bae, G.; Chung, W.I.; Choi, G. Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant J. 2006, 47, 124–139. [Google Scholar] [CrossRef]
- Kim, D.H.; Yamaguchi, S.; Lim, S.; Oh, E.; Park, J.; Hanada, A.; Kamiya, Y.; Choi, G. SOMNUS, a CCCH-type zinc finger protein in Arabidopsis, negatively regulates light-dependent seed germination downstream of PIL5. Plant Cell 2008, 20, 1260–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriele, S.; Rizza, A.; Martone, J.; Circelli, P.; Costantino, P.; Vittorioso, P. The Dof protein DAG1 mediates PIL5 activity on seed germination by negatively regulating GA biosynthetic gene AtGA3ox1. Plant J. 2010, 61, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Nee, G.; Xiang, Y.; Soppe, W.J. The release of dormancy, a wake-up call for seeds to germinate. Curr. Opin. Plant Biol. 2017, 35, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, E.; Yamaguchi, S.; Hu, J.H.; Yusuke, J.; Jung, B.; Paik, I.; Lee, H.S.; Sun, T.P.; Kamiya, Y.; Choi, G. PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 2007, 19, 1192–1208. [Google Scholar] [CrossRef] [Green Version]
- Oh, E.; Kang, H.; Yamaguchi, S.; Park, J.; Lee, D.; Kamiya, Y.; Choi, G. Genome-Wide Analysis of Genes Targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during Seed Germination in Arabidopsis. Plant Cell 2009, 21, 403–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Lee, N.; Kim, W.; Lim, S.; Choi, G. ABI3 and PIL5 Collaboratively Activate the Expression of SOMNUS by Directly Binding to Its Promoter in Imbibed Arabidopsis Seeds. Plant Cell 2011, 23, 1404–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.P.; Piskurewicz, U.; Tureckova, V.; Carat, S.; Chappuis, R.; Strnad, M.; Fankhauser, C.; Lopez-Molina, L. Spatially and genetically distinct control of seed germination by phytochromes A and B. Genes Dev. 2012, 26, 1984–1996. [Google Scholar] [CrossRef] [Green Version]
- De Wit, M.; Galvao, V.C.; Fankhauser, C. Light-Mediated Hormonal Regulation of Plant Growth and Development. In Annual Review of Plant Biology; Merchant, S.S., Ed.; Annual Review of Plant Biology; Annual Reviews: Palo Alto, CA, USA, 2016; Volume 67, pp. 513–537. [Google Scholar]
- Sawada, Y.; Aoki, M.; Nakaminami, K.; Mitsuhashi, W.; Tatematsu, K.; Kushiro, T.; Koshiba, T.; Kamiya, Y.; Inoue, Y.; Nambara, E.; et al. Phytochrome- and gibberellin-mediated regulation of abscisic acid metabolism during germination of photoblastic lettuce seeds. Plant Physiol. 2008, 146, 1386–1396. [Google Scholar] [CrossRef] [Green Version]
- Toyomasu, T.; Kawaide, H.; Mitsuhashi, W.; Inoue, Y.; Kamiya, Y. Phytochrome regulates gibberellin biosynthesis during germination of photoblastic lettuce seeds. Plant Physiol. 1998, 118, 1517–1523. [Google Scholar] [CrossRef] [Green Version]
- Leubner-Metzger, G. Functions and regulation of ß-1,3-glucanases during seed germination, dormancy release and after-ripening. Seed Sci. Res. 2003, 13, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Leubner-Metzger, G.; Frundt, C.; Vogeli-Lange, R.; Meins, F., Jr. Class I [beta]-1,3-Glucanases in the Endosperm of Tobacco during Germination. Plant Physiol. 1995, 109, 751–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Leubner-Metzger, G. Brassinosteroids and gibberellins promote tobacco seed germination by distinct pathways. Planta 2001, 213, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Leubner-Metzger, G. Seed after-ripening and over-expression of class I beta-1,3-glucanase confer maternal effects on tobacco testa rupture and dormancy release. Planta 2002, 215, 959–968. [Google Scholar] [CrossRef]
- Flint, L.H. Light in Relation to Dormancy and Germination in Lettuce Seed. Science 1934, 80, 38–40. [Google Scholar] [CrossRef]
- Gupta, S.; Plackova, L.; Kulkarni, M.G.; Dolezal, K.; Van Staden, J. Role of Smoke Stimulatory and Inhibitory Biomolecules in Phytochrome-Regulated Seed Germination of Lactuca sativa. Plant Physiol. 2019, 181, 458–470. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Wang, X.; Mo, X.; Tang, C.; Zhong, S.; Deng, X.W. Arabidopsis DET1 degrades HFR1 but stabilizes PIF1 to precisely regulate seed germination. Proc. Natl. Acad. Sci. USA 2015, 112, 3817–3822. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Zhong, S.; Mo, X.; Liu, N.; Nezames, C.D.; Deng, X.W. HFR1 sequesters PIF1 to govern the transcriptional network underlying light-initiated seed germination in Arabidopsis. Plant Cell 2013, 25, 3770–3784. [Google Scholar] [CrossRef] [Green Version]
- Appenroth, K.J.; Lenk, G.; Goldau, L.; Sharma, R. Tomato seed germination: Regulation of different response modes by phytochrome B2 and phytochrome A. Plant Cell Environ. 2006, 29, 701–709. [Google Scholar] [CrossRef] [Green Version]
- Leubner-Metzger, G.; Frundt, C.; Meins, F., Jr. Effects of gibberellins, darkness and osmotica on endosperm rupture and class I β-1,3-glucanase induction in tobacco seed germination. Planta 1996, 199, 282–288. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Ogawa, M.; Kuwahara, A.; Hanada, A.; Kamiya, Y.; Yamaguchi, S. Activation of Gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell 2004, 16, 367–378. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, Y.; Takeda-Kamiya, N.; Hanada, A.; Ogawa, M.; Kuwahara, A.; Seo, M.; Kamiya, Y.; Yamaguchi, S. Contribution of gibberellin deactivation by AtGA2ox2 to the suppression of germination of dark-imbibed Arabidopsis thaliana seeds. Plant Cell Physiol. 2007, 48, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Oh, E.; Kim, J.; Park, E.; Kim, J.I.; Kang, C.; Choi, G. PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 2004, 16, 3045–3058. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.; Hanada, A.; Kuwahara, A.; Endo, A.; Okamoto, M.; Yamauchi, Y.; North, H.; Marion-Poll, A.; Sun, T.P.; Koshiba, T.; et al. Regulation of hormone metabolism in Arabidopsis seeds: Phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J. 2006, 48, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, H.; Park, J.; Kim, W.; Yoo, J.; Lee, N.; Kim, J.; Yoon, T.Y.; Choi, G. PIF1-Interacting Transcription Factors and Their Binding Sequence Elements Determine the in Vivo Targeting Sites of PIF1. Plant Cell 2016, 28, 1388–1405. [Google Scholar] [CrossRef] [Green Version]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manz, B.; Muller, K.; Kucera, B.; Volke, F.; Leubner-Metzger, G. Water uptake and distribution in germinating tobacco seeds investigated in vivo by nuclear magnetic resonance imaging. Plant Physiol. 2005, 138, 1538–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallardo, K.; Job, C.; Groot, S.P.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Proteomics of Arabidopsis seed germination. A comparative study of wild-type and gibberellin-deficient seeds. Plant Physiol. 2002, 129, 823–837. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, M.; Hanada, A.; Yamauchi, Y.; Kuwahara, A.; Kamiya, Y.; Yamaguchi, S. Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 2003, 15, 1591–1604. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Gene Name | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| NtGA3ox2 | TGGAAAAACTAGCCGGAAGA | GCCCATTTCATATCGTCCTTAC |
| NtGA2ox2 | TTGGAGGACCACCATTGAGT | CAAGCTGTCTTGATCCCCTTT |
| NtGAI | TCCACTAACAACAGATGCAACAACAAG | ACAGCTTCAGCACACGCCATT |
| NtNCED6 | AGTTTCGGGTTGGTGGATGCTAC | CTGTAATACGGACGCTATACGGAAGAT |
| NtCYP707A1 | GGTGATTCTGCTGGTGTTGTCTCT | GGGATATAGCTTAATGGGCAGA |
| NtABI3 | GAGTATCAGACCATGGAATCTGC | TTCCATCGCGGAGAATTG |
| NtABI5 | CGCAAAAGGCGACTAACAA | ACACATCAAGGGCAACTCAA |
| NtXTH2 | GGCTAGTCACCACATCAAGTACCTCA | CACCTGAAGACCTGTCAAGAACAAGAT |
| NtTOC1 | TGCTTCCACCACTGCTGCTCATA | TCCTGTCTGCCGTTCATTAGTTCCT |
| NtPHYB1 | GTGTGATACTGTGGTTGAGAGTGTGA | TTGAGGAATGTCGGTAGCAGGATAATG |
| Actin(Tac9) | CCTGAGGTCCTTTTCCAACCA | GGATTCCGGCAGCTTCCATT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, M.; Dong, S.; Liu, Y.-L.; Li, Z.-H. The Opposite Roles of White Light in Regulating Germination of Fresh and Aged Seed in Tobacco. Plants 2021, 10, 2457. https://doi.org/10.3390/plants10112457
Wang Y, Zhang M, Dong S, Liu Y-L, Li Z-H. The Opposite Roles of White Light in Regulating Germination of Fresh and Aged Seed in Tobacco. Plants. 2021; 10(11):2457. https://doi.org/10.3390/plants10112457
Chicago/Turabian StyleWang, Yao, Min Zhang, Shuai Dong, Yi-Ling Liu, and Zhen-Hua Li. 2021. "The Opposite Roles of White Light in Regulating Germination of Fresh and Aged Seed in Tobacco" Plants 10, no. 11: 2457. https://doi.org/10.3390/plants10112457
APA StyleWang, Y., Zhang, M., Dong, S., Liu, Y.-L., & Li, Z.-H. (2021). The Opposite Roles of White Light in Regulating Germination of Fresh and Aged Seed in Tobacco. Plants, 10(11), 2457. https://doi.org/10.3390/plants10112457






