Endosperm–Embryo Communications: Recent Advances and Perspectives
Abstract
:1. Endosperm and Embryo, a Tale of Two Developments
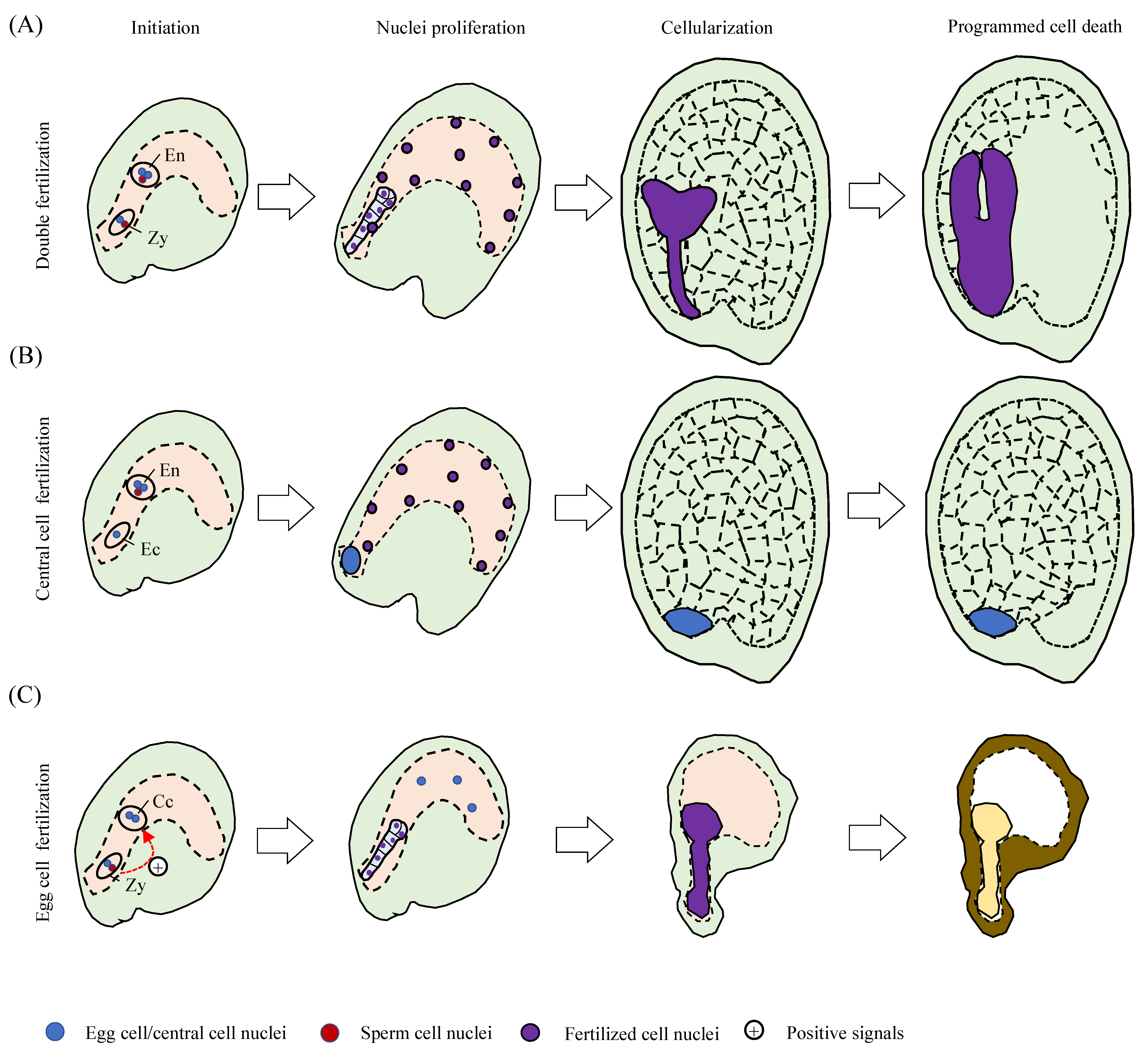
2. Initiation of Endosperm Development, Dependent or Independent on Egg Cell Fertilization?
3. Endosperm and Embryo Communications: Much More Than Just Nutrients
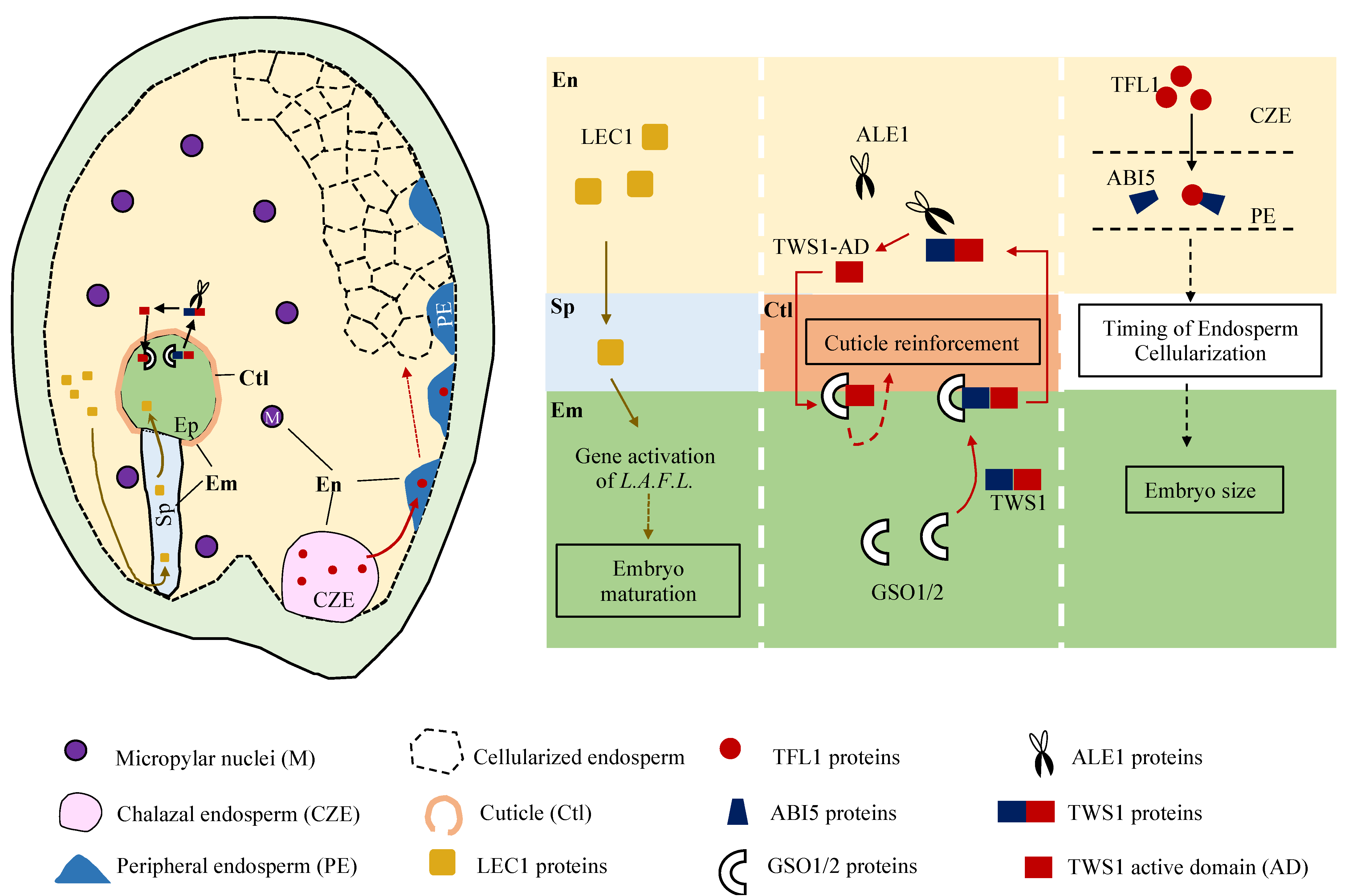
3.1. Endosperm-Synthesized LEC1, and Why It Matters
3.2. Timing the Endosperm Cellularization for Seed Sizes, When and How
3.3. Working Together to Build Extra Cuticular Sheath, Where and Why
4. Underlying the Communications, Route and Means
5. Endosperm and Embryo Dialogue, More to Discover
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Xiong, H.; Wang, W.; Sun, M. Endosperm development is an autonomously programmed process independent of embryogenesis. Plant Cell 2021, 33, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Berger, F. Endosperm: Food for humankind and fodder for scientific discoveries. New Phytol. 2012, 195, 290–305. [Google Scholar] [CrossRef] [PubMed]
- Lafon-Placette, C.; Köhler, C. Embryo and endosperm, partners in seed development. Curr. Opin. Plant Biol. 2014, 17, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.B.; De Paiva, G.; Yadegari, R. Plant embryogenesis: Zygote to seed. Science 1994, 266, 605–614. [Google Scholar] [CrossRef]
- Armenta-Medina, A.; Gillmor, C.S.; Gao, P.; Mora-Macias, J.; Kochian, L.V.; Xiang, D.; Dalta, R. Developmental and genomic architecture of plant embryogenesis: From model plant to crops. Plant Commun. 2021, 2, 100136. [Google Scholar] [CrossRef]
- Boisnard-Lorig, C.; Colon-Carmona, A.; Bauch, M.; Hodge, S.; Doerner, P.; Bancharel, E.; Dumas, C.; Haseloff, J.; Berger, F. Dynamic analyses of the expression of the histone::YFP fusion protein in Arabidopsis show that syncytial endosperm is divided in mitotic domains. Plant Cell 2001, 13, 495–509. [Google Scholar] [CrossRef] [Green Version]
- Olsen, O.A. Nuclear endosperm development in cereals and Arabidopsis thaliana. Plant Cell 2004, 16, 214–228. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Li, C.; Li, Y.; Yu, H. Mobile TERMINAL FLOWER1 determines seed size in Arabidopsis. Nat. Plants 2020, 6, 1146–1157. [Google Scholar] [CrossRef]
- Mori, T.; Igawa, T.; Tamiya, G.; Miyagishima, S.Y.; Berger, F. Gamete attachment requires GEX2 for successful fertilization in Arabidopsis. Curr. Biol. 2014, 24, 170–175. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Mori, T.; Ueda, K.; Yamada, L.; Nagahara, S.; Higashiyama, T.; Sawada, H.; Igawa, T. The male gamete membrane protein DMP9/DAU2 is required for double fertilization in flowering plants. Development 2018, 145, dev170076. [Google Scholar] [CrossRef] [Green Version]
- Cyprys, P.; Lindemeier, M.; Sprunck, S. Gamete fusion is facilitated by two sperm cell-expressed DUF679 membrane proteins. Nat. Plants 2019, 5, 253–257. [Google Scholar] [CrossRef]
- Nowack, M.K.; Grini, P.E.; Jakoby, M.J.; Lafos, M.; Koncz, C.; Schnittger, A. A positive signal from the fertilization of the egg cell sets off endosperm proliferation in angiosperm embryogenesis. Nat. Genet. 2006, 38, 63–67. [Google Scholar] [CrossRef]
- Chaudhury, A.M.; Ming, L.; Miller, C.; Craig, S.; Dennis, E.; Peacock, W.J. Fertilization-independent seed development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1997, 94, 4223–4228. [Google Scholar] [CrossRef] [Green Version]
- Nowack, M.K.; Shizadi, R.; Dissmeyer, N.; Dolf, A.; Endl, E.; Grini, P.E.; Schnittger, A. Bypassing genomic imprinting allows seed development. Nature 2007, 447, 312–316. [Google Scholar] [CrossRef]
- Song, J.; Xie, X.; Chen, C.; Shu, J.; Thapa, R.; Nguyen, V.; Bian, S.; Kohalmi, S.E.; Maraolais, F.; Zou, J.; et al. LEAFY COTYLEDON1 expression in the endosperm enables embryo maturation in Arabidopsis. Nat. Commun. 2021, 12, 3963. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.A.; Larkins, B.A. Endosperm origin, development, and function. Plant Cell 1993, 5, 1383–1399. [Google Scholar]
- Miyawaki, K.; Matsumoto-Kitano, M.; Kakimoto, T. Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: Tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J. 2004, 37, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T. Genome-wide demethylation of Arabidopsis endosperm. Science 2009, 324, 1451–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sroufe, L.A.; Erickson, M.; Dumont, K.; Czaja, S.; Hearn, E.F.; Do, D.; Rilling, J.K.; Herndon, J.G.; Fields, R.D.; Shick, H.E.; et al. Active DNA demethylation in plant companion cells reinforces transposon methylation in gametes. Science 2012, 337, 1360–1365. [Google Scholar]
- Köhler, C.; Hennig, L.; Bouveret, R.; Gheyselinck, J.; Grossniklaus, U.; Gruissem, W. Arabidopsis MSI1 is a component of the MEA/FIE Polycomb group complex and required for seed development. EMBO J. 2003, 22, 4804–4814. [Google Scholar] [CrossRef] [Green Version]
- Hehenberger, E.; Kradolfer, D.; Köhler, C. Endosperm cellularization defines an important developmental transition for embryo development. Development 2012, 139, 2031–2039. [Google Scholar] [CrossRef] [Green Version]
- Pignocchi, C.; Minns, G.E.; Nesi, N.; Koumproglou, R.; Kisios, G.; Benning, C.; Lloyd, C.W.; Doonan, J.H.; Hills, M.J. Endosperm Defective1 is a novel microtubule-associated protein essential for seed development in Arabidopsis. Plant Cell 2009, 21, 90–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, J.P.; Colon, K.; Jenik, P.D. The onset of embryo maturation in Arabidopsis is determined by its developmental stage and does not depend on endosperm cellularization. Plant J. 2019, 99, 286–301. [Google Scholar] [PubMed]
- Doll, N.M.; Royek, S.; Fujita, S.; Okuda, S.; Chamot, S.; Stintzi, A.; Widiez, T.; Hothorn, M.; Schaller, A.; Geldner, N.; et al. A two-way molecular dialogue between embryo and endosperm is required for seed development. Science 2020, 367, 431–435. [Google Scholar] [CrossRef] [PubMed]
- West, M.A.L.; Yee, K.M.; Danao, J.; Zimmerman, J.L.; Fischer, R.L.; Goldberg, R.B.; Harada, J. LEAFY COTYLEDON1 is an essential regulator of late embryogenesis and cotyledon identity in Arabidopsis. Plant Cell 1994, 6, 1731–1745. [Google Scholar] [CrossRef] [Green Version]
- Pelletier, J.M.; Kwong, R.W.; Park, S.; Le, B.H.; Baden, R.; Cagliari, A.; Hashimoto, M.; Munoz, M.D.; Fischer, R.L.; Goldberg, R.B.; et al. LEC1 sequentially regulates the transcription of genes involved in diverse developmental processes during seed development. Proc. Natl. Acad. Sci. USA 2017, 114, E6710–E6719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, L.; Pelletier, J.M.; Harada, J. Central role of the LEAFY COTYLEDON1 transcription factor in seed development. J. Integr. Plant Biol. 2019, 61, 564–580. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhang, X.; Kang, X.; Zhao, X.; Zhang, X.; Ni, M. Short Hypocotyl Under Blue1 associates with Miniseed3 and Haiku2 promoters in vivo to regulate Arabidopsis seed development. Plant Cell 2009, 21, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.J.; Zhao, X.Y.; Shao, X.X.; Wang, F.; Zhou, C.; Liu, Y.G.; Zhang, Y.; Zhang, X.S. Abscisic acid regulates early seed development in Arabidopsis by ABI5-Mediated transcription of Short Hypocotyl Under Blue1. Plant Cell 2014, 26, 1053–1068. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Johnston, N.; Taideh, E.; Mitchell, S.; Jeffree, C.; Goodrich, J.; Ingram, G. The endosperm-specific ZHOUPI gene of Arabidopsis thaliana regulates endosperm breakdown and embryonic epidermal development. Development 2008, 135, 3501–3509. [Google Scholar] [CrossRef] [Green Version]
- Moussu, S.; Doll, N.; Chamot, S.; Brocard, L.; Creff, A.; Fourquin, C.; Widiez, T.; Nimchuk, Z.; Ingram, G. ZHOUPI and KERBEROS mediate embryo/endosperm separation by promoting the formation of an extracuticular sheath at the embryo surface. Plant Cell 2017, 29, 1642–1656. [Google Scholar] [CrossRef] [Green Version]
- Kondou, Y.; Nakazawa, M.; Kawashima, M.; Ichikawa, T.; Yoshizumi, T.; Suzuki, K.; Ishikawa, A.; Koshi, T.; Matsui, R.; Muto, S.; et al. RETARDED GROWTH OF EMBRYO1, a new basic helix-loop-helix protein, expresses in endosperm to control embryo growth. Plant Physiol. 2008, 147, 1924–1935. [Google Scholar] [CrossRef] [Green Version]
- Fourquin, C.; Beauzamy, L.; Chamot, S.; Creff, A.; Goodrich, J.; Boudaoud, A.; Ingram, G. Mechanical stress mediated by both endosperm softening and embryo growth underlies endosperm elimination in Arabidopsis seeds. Development 2016, 143, 3300–3305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.M.; Jackson, D. Lights at the end of the tunnel: New views of plasmodesmal structure and function. Curr. Opin. Plant Biol. 2010, 13, 684–692. [Google Scholar] [CrossRef]
- Kim, I.; Kobayashi, K.; Cho, E.; Zambryski, P.C. Subdomains for transport via plasmodesmata corresponding to the apical-basal axis are established during Arabidopsis embryogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 11945–11950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadler, R.; Lauterbach, C.; Sauer, N. Cell-to-cell movement of green fluorescent protein reveals post-phloem transport in the outer integument and identifies symplastic domains in Arabidopsis seeds and embryos. Plant Physiol. 2005, 139, 701–712. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, T.; Goldberg, R.B. The suspensor: Not just suspending the embryo. Trends Plant Sci. 2010, 15, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Morley-Smith, E.R.; Pike, M.J.; Findlay, K.; Köckenberger, W.; Hill, L.M.; Smith, A.M.; Rawsthorne, S. The transport of sugars to developing embryos is not via the bulk endosperm in oilseed rape seeds. Plant Physiol. 2008, 147, 2121–2130. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.K.; Santos-González, J.; Köhler, C. INT-Hi-C reveals distinct chromatin architecture in endosperm and leaf tissues of Arabidopsis. Nucleic Acids Res. 2021, 49, 4371–4385. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, N.; Wobus, U. Seed-development programs: A systems biology-based comparison between dicots and monocots. Annu. Rev. Plant Biol. 2013, 64, 189–217. [Google Scholar] [CrossRef]
- Niu, B.; Zhang, Z.; Zhang, J.; Zhou, Y.; Chen, C. The rice LEC1-like transcription factor OsNF-YB9 interacts with SPK, an endosperm-specific sucrose synthase protein kinase, and functions in seed development. Plant J. 2021, 106, 1233–1246. [Google Scholar] [CrossRef]
- Schmid, M.; Simpson, D.; Gietl, C. Programmed cell death in castor bean endosperm is associated with the accumulation and release of a cysteine endopeptidase from ricinosomes. Proc. Natl. Acad. Sci. USA 1999, 96, 14159–14164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Romero, J.; Santos-González, J.; Hennig, L.; Köhler, C. Applying the INTACT method to purify endosperm nuclei and to generate parental-specific epigenome profiles. Nat. Protoc. 2017, 12, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Del Toro-De León, G.; Köhler, C. Endosperm-specific transcriptome analysis by applying the INTACT system. Plant Reprod. 2019, 32, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Palovaara, J.; Weijers, D. Adapting INTACT to analyse cell-type-specific transcriptomes and nucleocytoplasmic mRNA dynamics in the Arabidopsis embryo. Plant Reprod. 2019, 32, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picard, C.L.; Povilus, R.; Williams, B.; Gehring, M. Transcriptional and imprinting complexity in Arabidopsis seeds at single-nucleus resolution. Nat. Plants 2021, 7, 730–738. [Google Scholar] [CrossRef]
- Kao, P.; Schon, M.A.; Mosiolek, M.; Enugutti, B.; Nodine, M.D. Gene expression variation in Arabidopsis embryos at single-nucleus resolution. Development 2021, 148, dev199589. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Xie, X.; Cui, Y.; Zou, J. Endosperm–Embryo Communications: Recent Advances and Perspectives. Plants 2021, 10, 2511. https://doi.org/10.3390/plants10112511
Song J, Xie X, Cui Y, Zou J. Endosperm–Embryo Communications: Recent Advances and Perspectives. Plants. 2021; 10(11):2511. https://doi.org/10.3390/plants10112511
Chicago/Turabian StyleSong, Jingpu, Xin Xie, Yuhai Cui, and Jitao Zou. 2021. "Endosperm–Embryo Communications: Recent Advances and Perspectives" Plants 10, no. 11: 2511. https://doi.org/10.3390/plants10112511
APA StyleSong, J., Xie, X., Cui, Y., & Zou, J. (2021). Endosperm–Embryo Communications: Recent Advances and Perspectives. Plants, 10(11), 2511. https://doi.org/10.3390/plants10112511





