By-Products of the Black Soybean Sauce Manufacturing Process as Potential Antioxidant and Anti-Inflammatory Materials for Use as Functional Foods
Abstract
:1. Introduction
2. Results
2.1. pH Value, Flavonoids, Total Phenol, and Protein Contents in BBSL and BBSLP
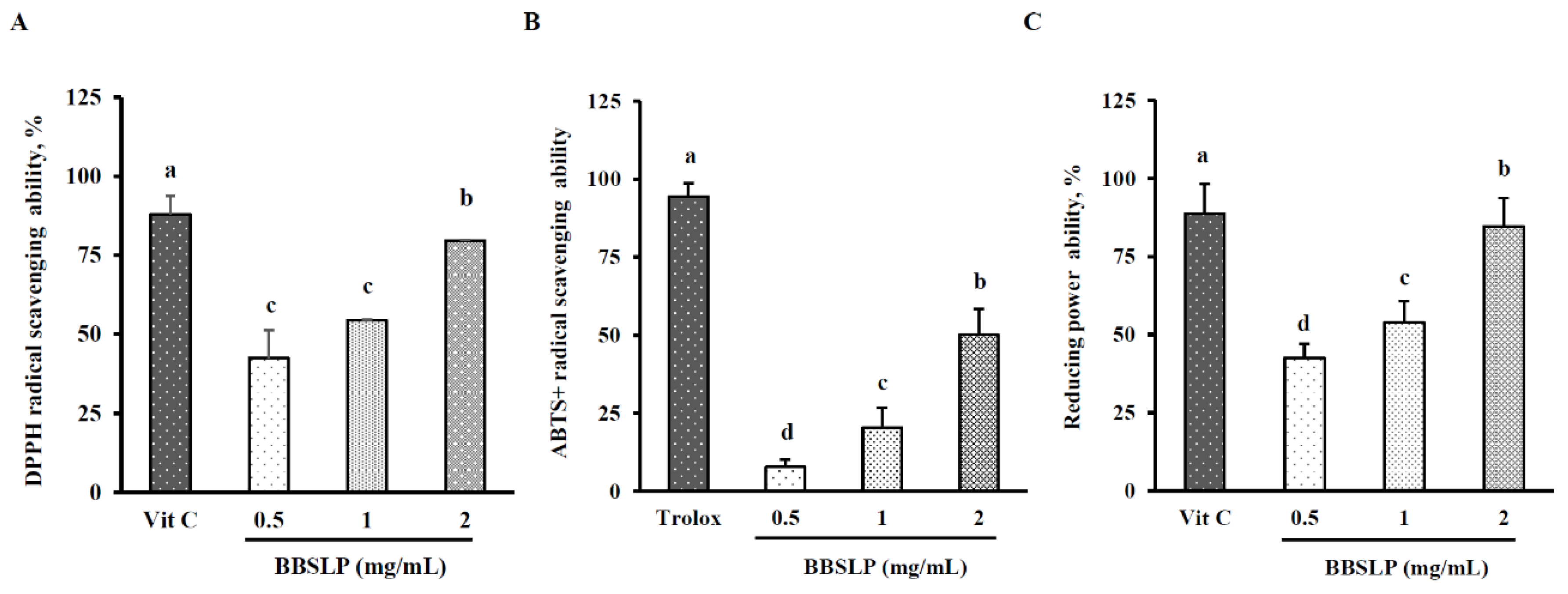
2.2. BBSLP Showed Antioxidant Effects In Vitro Conditions
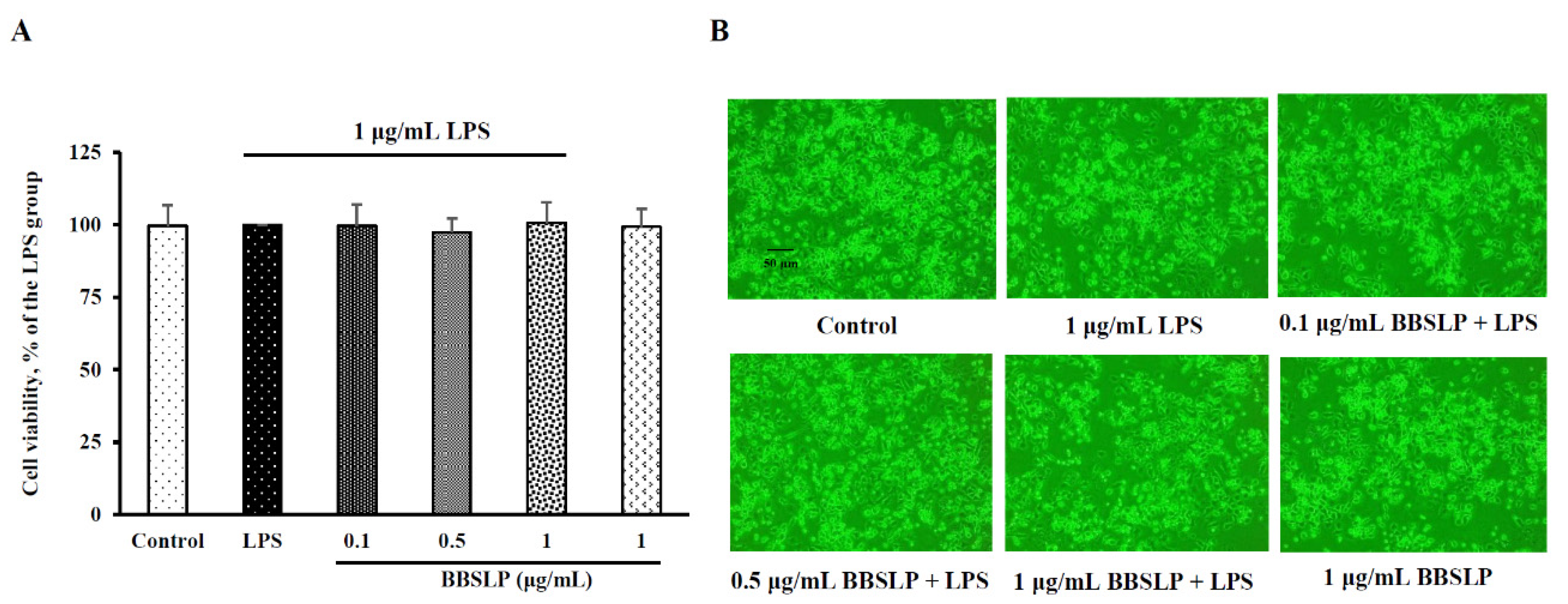
2.3. BBSLP Maintained the Viability of RAW264.7 Macrophages after Lipopolysaccharide (LPS) Induction
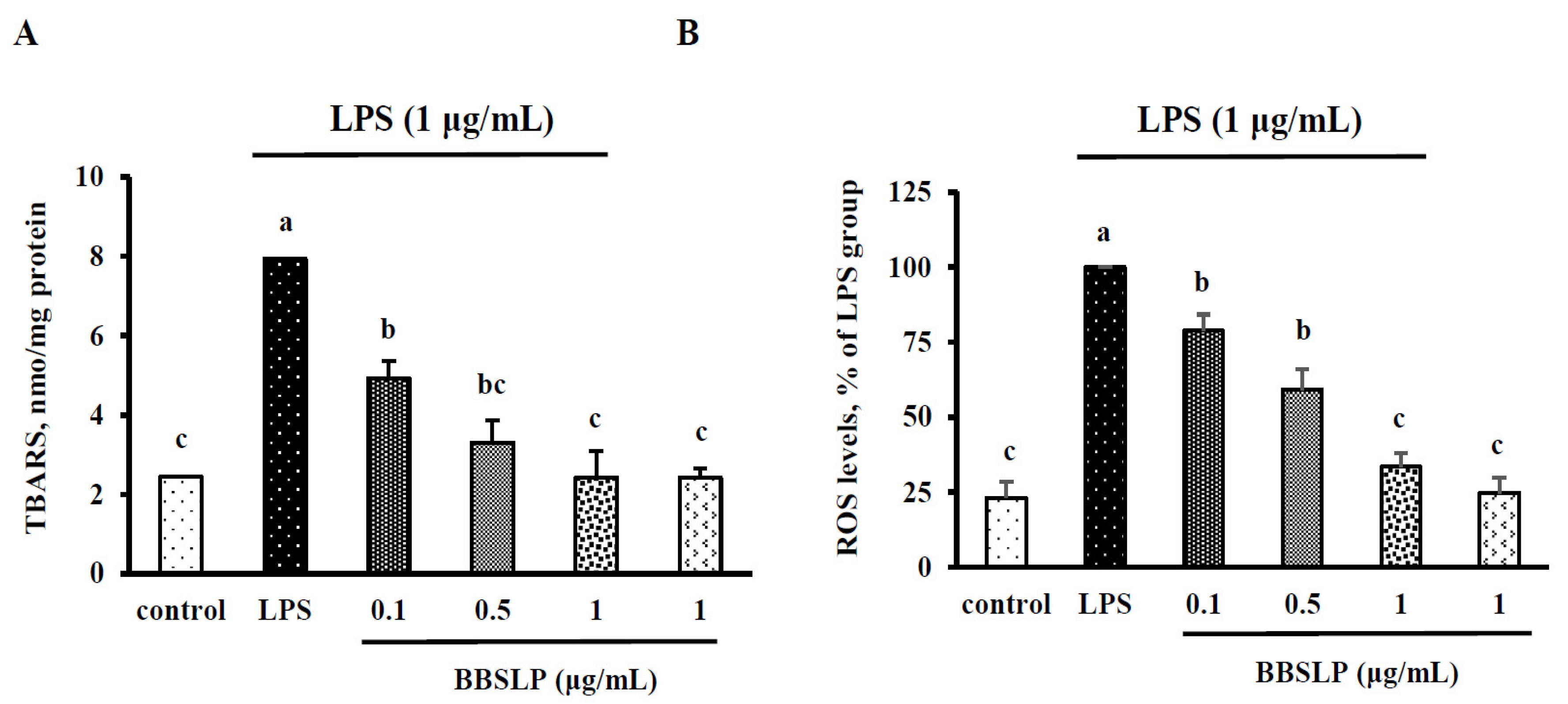
2.4. BBSLP Reduced Oxidative Stress in RAW264.7 Macrophages after LPS Induction
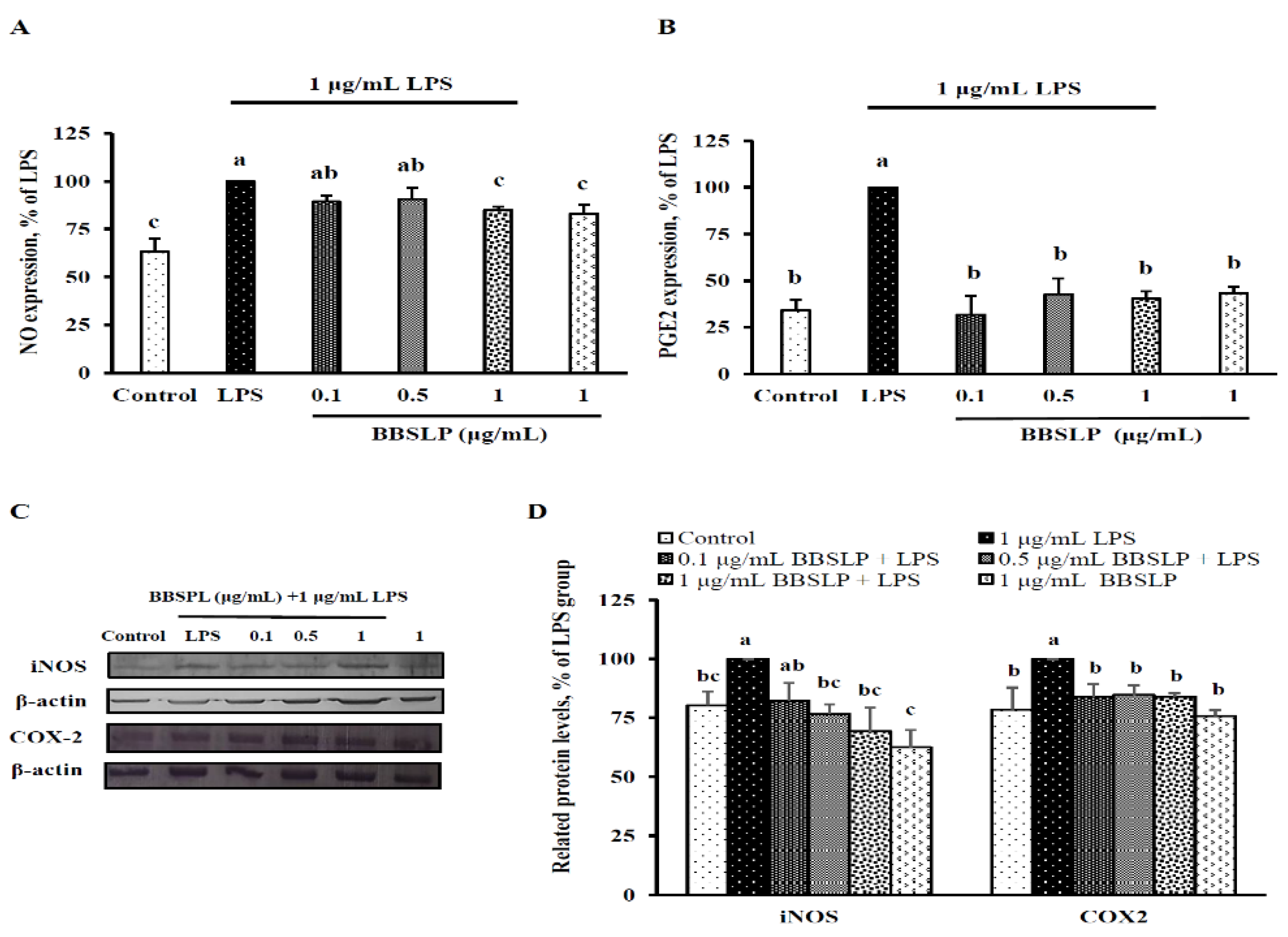
2.5. BBSLP Reduced NO and PGE2 Production in RAW264.7 Macrophages after LPS Induction
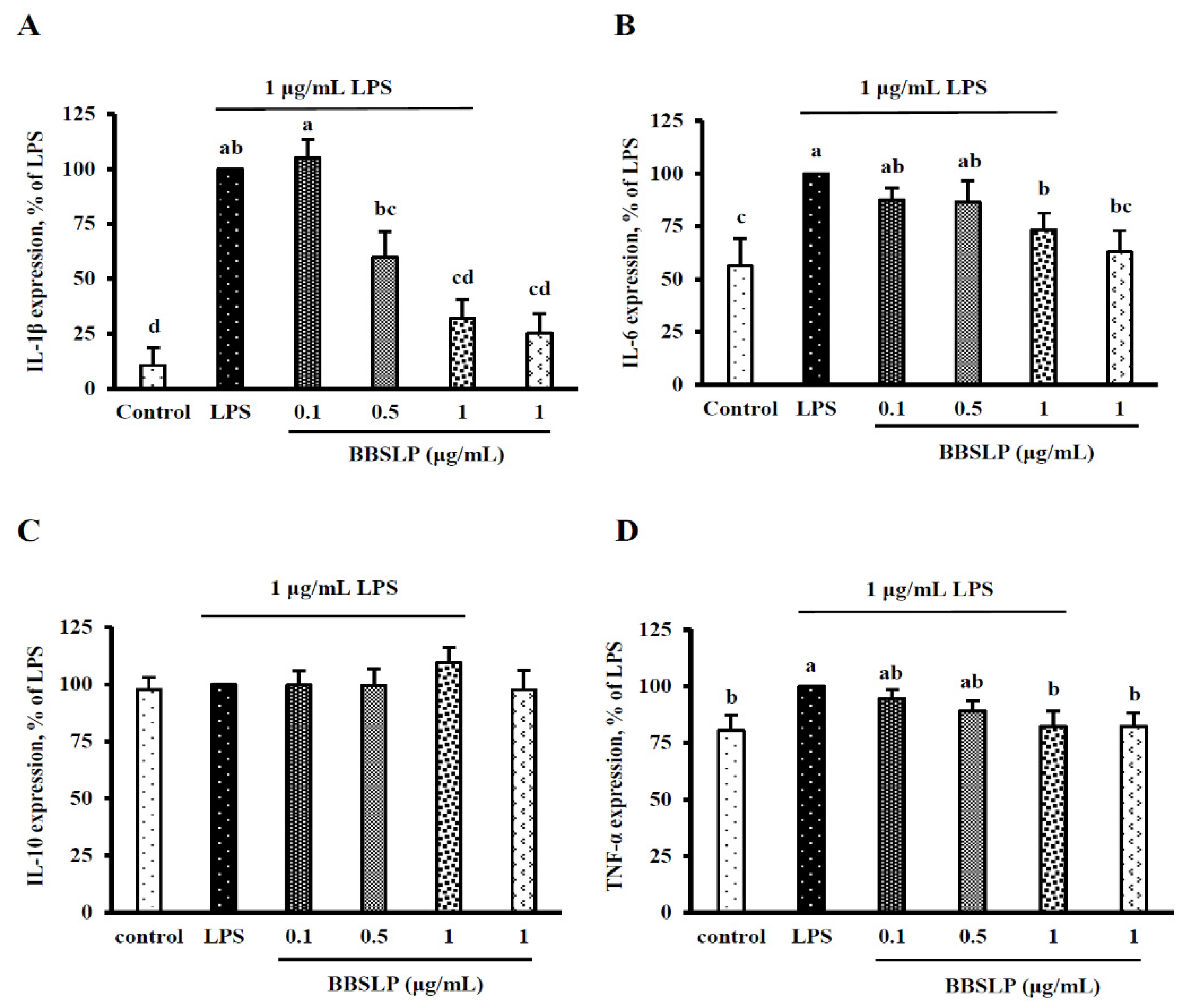
2.6. BBSLP Decreased IL-1β, IL-6 and TNF-α Levels in RAW264.7 Macrophages after LPS Induction
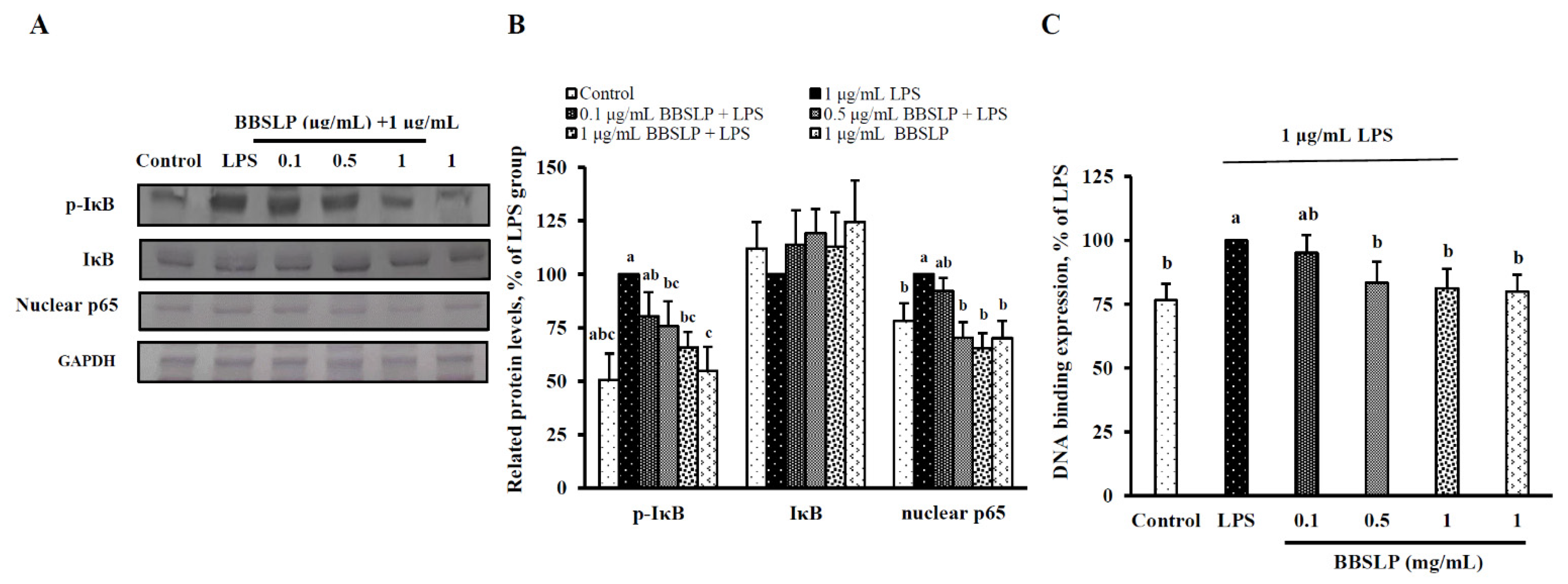
2.7. BBSLP Reduced the Activation of NF-ĸB Signalling in RAW264.7 Cells after LPS Induction
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of BBSLP
4.3. Determination of the pH Value and Total Flavonoids, Phenols and Protein in BBSL and BBSLP
4.4. In Vitro Antioxidant Ability of BBSLP
4.5. Cell Culture and Treatment
4.6. Cell Viability Analysis
4.7. Measurement of Lipid Peroxidation and ROS Levels
4.8. Determination of Nitrite (NO) and Prostaglandin E2 (PGE2)
4.9. Measurement of IL-1β, IL-6 and TNF-α
4.10. Immunoblot Analyses of iNOS, COX-2 and NF-κB Signalling Molecule Expression
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Toole, D.K. The role of microorganisms in soy sauce production. Adv. Appl. Microbiol. 2019, 108, 45–113. [Google Scholar] [CrossRef] [PubMed]
- Teigiserova, D.; Hamelin, L.; Thomsen, M. Towards transparent valorization of food surplus, waste and loss: Clarifying definitions, food waste hierarchy, and role in the circular economy. Sci. Total. Environ. 2019, 706, 136033. [Google Scholar] [CrossRef] [PubMed]
- Domae, C.; Nanba, F.; Maruo, T.; Suzuki, T.; Ashida, H.; Yamashita, Y. Black soybean seed coat polyphenols promote nitric oxide production in the aorta through glucagon-like peptide-1 secretion from the intestinal cells. Food Funct. 2019, 10, 7875–7882. [Google Scholar] [CrossRef] [PubMed]
- Diez-Simon, C.; Eichelsheim, C.; Mumm, R.; Hall, R.D. Chemical and sensory characteristics of soy sauce: A Review. J. Agric. Food Chem. 2020, 68, 11612–11630. [Google Scholar] [CrossRef]
- Choi, Y.-M.; Yoon, H.; Lee, S.; Ko, H.-C.; Shin, M.-J.; Lee, M.C.; Hur, O.S.; Ro, N.Y.; Desta, K.T. Isoflavones, anthocyanins, phenolic content, and antioxidant activities of black soybeans (Glycine max (L.) Merrill) as affected by seed weight. Sci. Rep. 2020, 10, 19960. [Google Scholar] [CrossRef]
- Yamashita, Y.; Nakamura, A.; Nanba, F.; Saito, S.; Toda, T.; Nakagawa, J.; Ashida, H. Black soybean improves vascular function and blood pressure: A randomized, placebo controlled, crossover trial in humans. Nutrients 2020, 12, 2755. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Pan, Y.; Gao, X.; Chen, H. Hypoglycemic and hypolipidemic effects of anthocyanins extract from black soybean seed coat in high fat diet and streptozotocin-induced diabetic mice. Food Funct. 2017, 9, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Takagi, A.; Kano, M.; Kaga, C. Possibility of breast cancer prevention: Use ofsoy isoflavones and fermented soy beverage produced using probiotics. Int. J. Mol. Sci. 2015, 16, 10907–10920. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, K.; Xu, B. A Critical review on polyphenols and health benefits of black soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef] [Green Version]
- Tit, D.M.; Bungau, S.; Iovan, C.; Cseppento, D.C.N.; Endres, L.; Sava, C.; Sabau, A.M.; Furau, G.; Furau, C. Effects of the hormone replacement therapy and of soy isoflavones on bone resorption in postmenopause. J. Clin. Med. 2018, 7, 297. [Google Scholar] [CrossRef] [Green Version]
- Rao, D.E.; Chaitanya, K.V. Changes in the antioxidant intensities of seven different soybean (Glycine max (L.) Merr.) cultivars during drought. J. Food Biochem. 2020, 44, e13118. [Google Scholar]
- Kim, J.N.; Han, S.N.; Ha, T.J.; Kim, H.-K. Black soybean anthocyanins attenuate inflammatory responses by suppressing reactive oxygen species production and mitogen activated protein kinases signaling in lipopolysaccharide-stimulated macrophages. Nutr. Res. Pract. 2017, 11, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.Y.; Jang, G.Y.; Lee, Y.; Li, M.; Ji, Y.M.; Yoon, N.; Lee, S.H.; Kim, K.M.; Lee, J.; Jeong, H.S. Free and bound form bioactive compound profiles in germinated black soybean (Glycine max L.). Food Sci. Biotechnol. 2016, 25, 1551–1559. [Google Scholar] [CrossRef]
- Rajendran, P.; Chen, Y.; Chen, Y.; Chung, L.; Tamilselvi, S.; Shen, C.; Day, C.H.; Chen, R.; Viswanadha, V.P.; Kuo, W.; et al. The multifaceted link between inflammation and human diseases. J. Cell. Physiol. 2018, 233, 6458–6471. [Google Scholar] [CrossRef]
- Infantino, V.; Pierri, C.L.; Iacobazzi, V. Metabolic routes in inflammation: The citrate pathway and its potential as therapeutic target. Curr. Med. Chem. 2020, 26, 7104–7116. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Kuroda, T.; Kobayashi, D. Cytokine networks in the pathogenesis of rheumatoid arthritis. Int. J. Mol. Sci. 2021, 22, 10922. [Google Scholar] [CrossRef]
- Li, L.; Yu, R.; Cai, T.; Chen, Z.; Lan, M.; Zou, T.; Wang, B.; Wang, Q.; Zhao, Y.; Cai, Y. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int. Immunopharmacol. 2020, 88, 106939. [Google Scholar] [CrossRef] [PubMed]
- Donate-Correa, J.; Ferri, C.M.; Sánchez-Quintana, F.; Pérez-Castro, A.; González-Luis, A.; Martín-Núñez, E.; Mora-Fernández, C.; Navarro-González, J.F. Inflammatory cytokines in diabetic kidney disease: Pathophysiologic and therapeutic implications. Front. Med. 2021, 7, 1137. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Carmody, R.J. NF-κB and the transcriptional control of inflammation. Int. Rev. Cell Mol. Biol. 2018, 335, 41–84. [Google Scholar]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal. Transduct. Target. Ther. 2021, 6, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Toker, H.; Görgün, E.P.; Korkmaz, E.M. Analysis of IL-6, IL-10 and NF-kappaB gene polymorphisms in aggressive and chronic periodontitis. Cent. Eur J. Public Health. 2017, 25, 157–162. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Shabnam, B.; Girisa, S.; Harsha, C.; Banik, K.; Devi, T.B.; Choudhury, R.; Sahu, H.; Parama, D.; Sailo, B.L.; et al. Inflammation, NF-kappaB, and chronic diseases: How are they linked? Crit. Rev. Immunol. 2020, 40, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; He, M.; Xiong, X.; Tsang, D.C. Sustainable management and recycling of food waste anaerobic digestate: A review. Bioresour. Technol. 2021, 341, 125915. [Google Scholar] [CrossRef] [PubMed]
- Glevitzky, I.; Dumitrel, G.A.; Glevitzky, M.; Pasca, B.; Otřísal, P.; Bungau, S.; Cioca, G.; Pantis, C.; Popa, M. Statistical analysis of the relationship between antioxidant activity and the structure of flavonoid compounds. Rev. Chim. 2019, 70, 3103–3107. [Google Scholar] [CrossRef]
- de Bhailís, B.; Chrysochou, C.; Kalra, P. Inflammation and oxidative damage in ischaemic renal disease. Antioxidants 2021, 10, 845. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting early atherosclerosis: A focus on oxidative stress and inflammation. Oxid. Med. Cell. Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Ohmori, R.; Kiyose, C.; Momiyama, Y.; Ohsuzu, A.F.; Kondo, K. Antioxidant activities of black and yellow soybeans against low density lipoprotein Oxidation. J. Agric. Food Chem. 2005, 53, 4578–4582. [Google Scholar] [CrossRef]
- Yamashita, Y.; Wang, L.; Nakamura, A.; Nanba, F.; Saito, S.; Toda, T.; Nakagawa, J.; Ashida, H. Black soybean improves the vascular function through an increase in nitric oxide and a decrease in oxidative stress in healthy women. Arch. Biochem. Biophys. 2020, 688, 108408. [Google Scholar] [CrossRef]
- Takekawa, S.; Matsui, T.; Arakawa, Y. The protective effect of the soybean polyphenol genistein against stress-induced gastric mucosal lesions in rats, and its hormonal mechanisms. J. Nutr. Sci. Vitaminol. 2006, 52, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Mo, Y.; Gong, J.; Li, Z.; Peng, H.; Chen, J.; Wang, Q.; Ke, Z.; Xie, J. Puerarin ameliorates cognitive deficits in streptozotocin-induced diabetic rats. Metab. Brain Dis. 2015, 31, 417–423. [Google Scholar] [CrossRef]
- Inoue, H.; Nakata, R. Resveratrol targets in inflammation. Endocr. Metab. Immune Disord. Drug Targets. 2015, 15, 186–195. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [Green Version]
- Thawkar, B.S.; Kaur, G.J. Inhibitors of NF-kappaB and P2X7/NLRP3/Caspase 1 pathway in microglia: Novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer’s disease. J. Neuroimmunol. 2019, 326, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-qB pathways. Int. Immunopharmacol. 2018, 54, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Rathore, A.S.; Singh, S.P. NF-kappaB-mediated neuroinflammation in Parkinson’s disease and potential therapeutic effect of polyphenols. Neurotox. Res. 2020, 37, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Nisbett, N.; Pretty, J.; Robinson, S.; Toulmin, C.; Whiteley, R. The future of global food system. Philos. Trans. R Soc. Lond B Biol. Sci. 2010, 365, 2769–2777. [Google Scholar] [CrossRef] [Green Version]
- Benítez, J.J.; Castillo, P.M.; Del Río, J.C.; León-Camacho, M.; Domínguez, E.; Heredia, A.; Guzmán-Puyol, S.; Athanassiou, A.; Heredia-Guerrero, J.A. Valorization of tomato processing by-products: Fatty acid extraction and production of bio-based materials. Materials 2018, 11, 2211. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Sun, J.; Mohammadtursun, N.; Wu, J.; Dong, J.; Li, L. Curcumin inhibits cigarette smoke-induced inflammation via modulating the PPARγ-NF-κB signaling pathway. Food Funct. 2019, 10, 7983–7994. [Google Scholar] [CrossRef]
- Lee, K.J.; Baek, D.-Y.; Lee, G.-A.; Cho, G.-T.; So, Y.-S.; Lee, J.-R.; Ma, K.-H.; Chung, J.-W.; Hyun, D.Y. Phytochemicals and antioxidant activity of Korean black soybean (Glycine max L.) Landraces. Antioxidants 2020, 9, 213. [Google Scholar] [CrossRef] [Green Version]
- Kerr, B.J.; Urriola, P.E.; Jha, R.; E Thomson, J.; Curry, S.M.; Shurson, G.C. Amino acid composition and digestible amino acid content in animal protein by-product meals fed to growing pigs1. J. Anim. Sci. 2019, 97, 4540–4547. [Google Scholar] [CrossRef]
- Hsieh, S.-L.; Tsai, P.-J.; Liu, Y.-C.; Wu, C.-C. Potential effects of antioxidant and serum cholesterol-lowering effects of Gynura bicolor water extracts in Syrian Hamster. Evid. Based Complement. Altern. Med. 2020, 2020, 2907610. [Google Scholar] [CrossRef]
- Azeem, S.M.A.; Al Mohesen, I.A.; Ibrahim, A.M. Analysis of total phenolic compounds in tea and fruits using diazotized aminobenzenes colorimetric spots. Food Chem. 2020, 332, 127392. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Motta, M.; Marcone, M.; Baptista, J. Variability of antioxidant properties, catechins, caffeine, L-theanine and other amino acids in different plant parts of Azorean Camellia sinensis. Curr. Res. Food Sci. 2020, 3, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.L.; Wang, J.C.; Huang, Y.S.; Wu, C.C. Ethanol extract of Gynura bicolor reduces atherosclerosis risk through enhancing antioxidant capacity and reducing adhesion molecule levels. Pharm. Biol. 2021, 59, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Han, Y.; Yuan, C.; Zhou, X.; Han, Y.; He, Y.; Ouyang, J.; Zhou, W.; Wang, Z.; Wang, H.; Li, G. Anti-inflammatory activity of three triterpene from Hippophae rhamnoides L. in lipopolysaccharide-stimulated RAW264.7 Cells. Int. J. Mol. Sci. 2021, 22, 12009. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Fraga, C.G.; Leibovitz, B.E.; Tappel, A.L. Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: Characterization and comparison with homogenates and microsomes. Free. Radic. Biol. Med. 1988, 4, 155–161. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]-nitrate in biological fluids. Anal. Biochem. 1992, 126, 131–138. [Google Scholar] [CrossRef]
- Hsieh, S.-L.; Wang, J.-J.; Su, K.-H.; Kuo, Y.-L.; Hsieh, S.; Wu, C.-C. Suppressive Effects of the Gynura bicolor Ether Extract on endothelial permeability and leukocyte transmigration in human endothelial cells induced by TNF-α. Evid.-Based Complement. Altern. Med. 2020, 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, S.R. One-dimensional SDS gel electrophoresis of proteins. Curr. Protoc. Toxicol. 2001. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| pH Value | Flavonoids | Total Polyphenols | Crude Protein | |
|---|---|---|---|---|
| BBSL * | 5.84 ± 0.12 | 11.5 ± 1.5 mg RUE mL−1 | 3.83 ± 0.3 mg GAE mL−1 | 3.5 ± 0.8 mg mL−1 |
| BBSLP | - | 0.1 ± 0.01 mg RUE mg−1 | 0.03 ± 0.01 mg GAE mg−1 | 0.31 ± 0.04 mg mg−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, S.-L.; Shih, Y.-W.; Chiu, Y.-M.; Tseng, S.-F.; Li, C.-C.; Wu, C.-C. By-Products of the Black Soybean Sauce Manufacturing Process as Potential Antioxidant and Anti-Inflammatory Materials for Use as Functional Foods. Plants 2021, 10, 2579. https://doi.org/10.3390/plants10122579
Hsieh S-L, Shih Y-W, Chiu Y-M, Tseng S-F, Li C-C, Wu C-C. By-Products of the Black Soybean Sauce Manufacturing Process as Potential Antioxidant and Anti-Inflammatory Materials for Use as Functional Foods. Plants. 2021; 10(12):2579. https://doi.org/10.3390/plants10122579
Chicago/Turabian StyleHsieh, Shu-Ling, Yi-Wen Shih, Ying-Ming Chiu, Shao-Feng Tseng, Chien-Chun Li, and Chih-Chung Wu. 2021. "By-Products of the Black Soybean Sauce Manufacturing Process as Potential Antioxidant and Anti-Inflammatory Materials for Use as Functional Foods" Plants 10, no. 12: 2579. https://doi.org/10.3390/plants10122579
APA StyleHsieh, S.-L., Shih, Y.-W., Chiu, Y.-M., Tseng, S.-F., Li, C.-C., & Wu, C.-C. (2021). By-Products of the Black Soybean Sauce Manufacturing Process as Potential Antioxidant and Anti-Inflammatory Materials for Use as Functional Foods. Plants, 10(12), 2579. https://doi.org/10.3390/plants10122579







