Problems and Prospects of Improving Abiotic Stress Tolerance and Pathogen Resistance of Oil Palm
Abstract
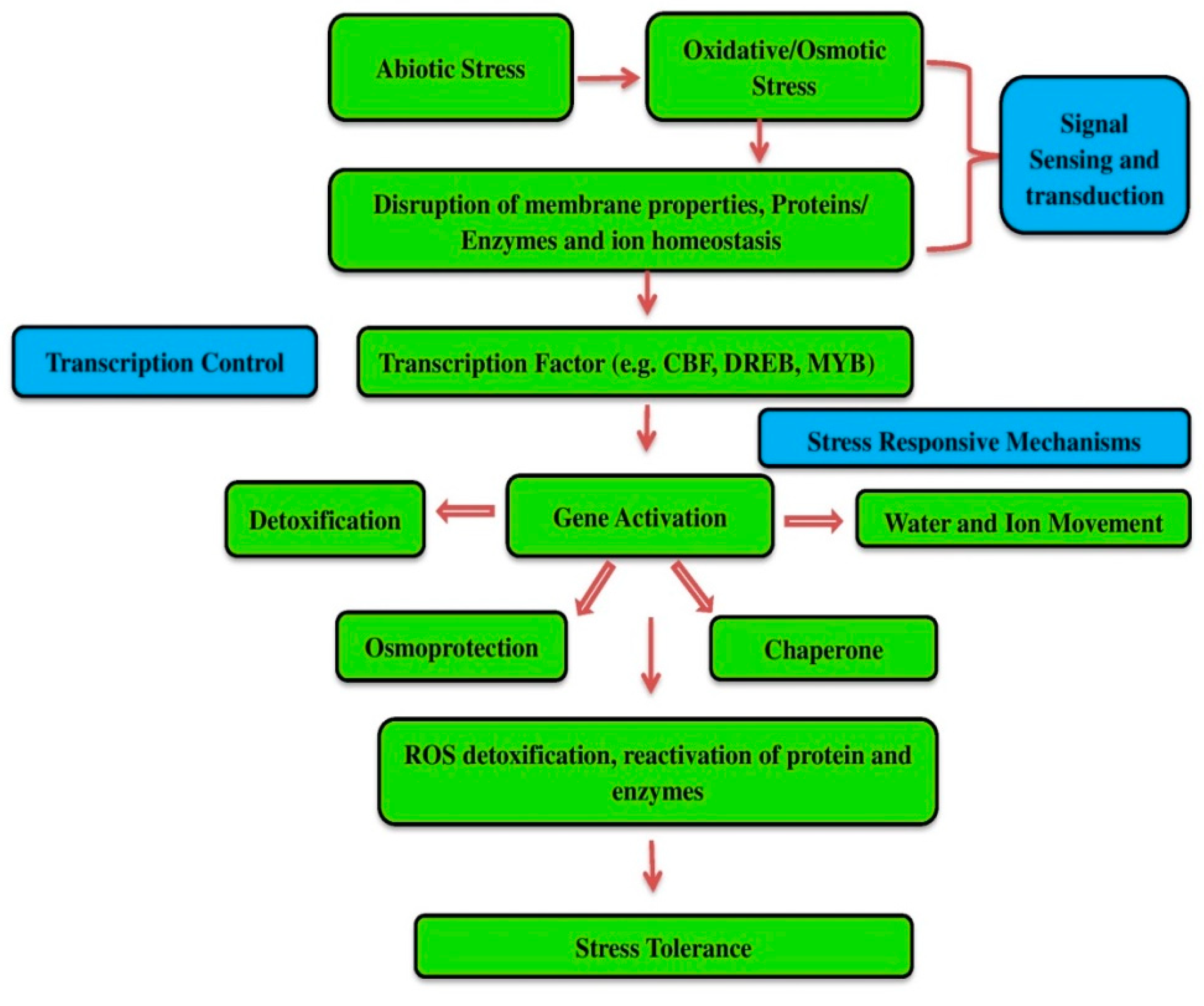
:1. Introduction
2. Morphological Changes of Oil Palm under Abiotic Stress
3. Anatomical Structure of Oil Palm under Abiotic Stress
4. Role of Antioxidant Defense of Oil Palm under Abiotic Stress
5. Role of Photosynthesis Change in Oil Palm under Abiotic Stress
6. Molecular Biology of Oil Palm with Cold Resistance
7. Molecular Biology of Oil Palm with Drought Resistance
8. Cultivation Techniques of Oil Palm with Drought Resistance
9. Research on Disease Resistance of Oil Palm
9.1. Pathogens of Oil Palm Disease
9.2. Detection Technology for Oil Palm Disease Resistance
9.3. Biocontrol Bacteria, Fungicides and Cultivation Techniques of Oil Palm Disease Resistance
9.4. Molecular Biology of Oil Palm Disease Resistance
10. Problems and Prospects of Oil Palm Resistance
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, L.; Liu, Y.J. The evolution of world oil palm production and trade in time and space and its development forecast. World Geogr. Res. 2012, 21, 70–76. [Google Scholar]
- Zeng, X.H.; Li, W.F.; Liu, Z.; Li, Z.; Zou, J.X.; Pan, D.L.; Lin, W.F. Research status and trends of oil palm resistant cul-tivation in China. China Trop. Agric. 2014, 5, 24–29. [Google Scholar]
- Rhebergen, T.; Fairhurst, T.; Giller, K.E.; Zingore, S. The influence of water and nutrient management on oil palm yield trends on a large-scale plantation in Ghana. Agric. Water Manag. 2019, 221, 377–387. [Google Scholar] [CrossRef]
- Danso, I.; Nuertey, B.N.; Andoh-Mensah, E.; Osei-Bonsu, A.; E O Asamoah, T. Response of Oil Palm to Planting Density and water deficit in three Climatic Zones of Southern Ghana. J. Ghana Sci. Assoc. 2009, 10, 93–102. [Google Scholar] [CrossRef]
- Antonini, J.C.d.A.; de Oliveira, A.D. Potencial de cultivo da palma de óleo irrigada nas condições do Cerrado—Planaltina. Embrapa Cerrados Doc. 2021, 2, 40. [Google Scholar]
- You, L.J.; Wang, R.M. The impact of climate and its changes on agricultural production in Southeast Asia. Geogr. Educ. 2010, Z1, 29. [Google Scholar]
- Fang, F.C. Hainan island climate and oil palm planting. Chin. J. Trop. Crop. 1982, 1, 81–93. [Google Scholar]
- Cheng, Q.R.; Liu, Z.F.; Zeng, X.H.; Zeng, J. Research progress in oil palm cold resistance. Trop. Agric. Sci. 2020, 40, 50–56. [Google Scholar]
- Mathivanan, S. Abiotic Stress-Induced Molecular and Physiological Changes and Adaptive Mechanisms in Plants, Abiotic Stress in Plants. Abiotic Stress Plants 2021, 315, 93367. [Google Scholar]
- Mattos, L.M.; Moretti, C.L. Oxidative Stress in Plants under Drought Conditions and the Role of Different Enzymes. Enzym. Eng. 2016, 5. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Fahad, S.; Bajwa, A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Yu, X.; Cheng, Z.; Yu, X.; Ruan, M.; Li, W.; Peng, M. Global Gene Expression Analysis Reveals Crosstalk between Response Mechanisms to Cold and Drought Stresses in Cassava Seedlings. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Hanumantharao, B.; Nair, R.M.; Nayyar, H. Salinity and High Temperature Tolerance in Mungbean [Vigna radiata (L.) Wilczek] from a Physiological Perspective. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Liliane, T.; Mutengwa, C. Factors Affecting Yield of Crops. In Agronomy-Climate Change & Food Security; IntechOpen: London, UK, 2020; Volume 24, pp. 1–16. [Google Scholar] [CrossRef]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and Abiotic Stresses in Plants. In Abiotic and Biotic Stress in Plants; De Oliveira, A.B., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Malinowska, M.; Donnison, I.; Robson, P. Morphological and Physiological Traits that Explain Yield Response to Drought Stress in Miscanthus. Agronomy 2020, 10, 1194. [Google Scholar] [CrossRef]
- Su, Z.; Ma, X.; Guo, H.; Sukiran, N.L.; Guo, B.; Assmann, S.M.; Ma, H. Flower development under drought stress: Morphological and transcriptomic analyses reveal acute responses and long-term acclimation in Arabidopsis. Plant Cell 2013, 25, 3785–3807. [Google Scholar] [CrossRef] [Green Version]
- Subronto, A.M.; Taniputra, B. Correlation between vegetative characters of the oil palm in the nursery and yield. Bul. Perkeb. 1989, 20, 107–116. [Google Scholar]
- Lucas, E.O. Relations Between Growth Parameters in Oil Palm Seedlings Grown in Polybags. Exp. Agric. 1980, 16, 275–278. [Google Scholar] [CrossRef]
- Balakrishna, P.; Pinnamaneni, R.; Pavani, K.; Mathur, R. Correlation and Path Coefficient Analysis in Indian Oil Palm genotypes. J. Pure Appl. Microbiol. 2018, 12, 195–206. [Google Scholar] [CrossRef]
- Marjuni, M.; Rafii, M.; Afizi, M.M.A.; Arolu, I.W.; Noh, A.; Din, A.; Kushairi, A.; Abdullah, N.; Rajanaidu, N.; Latif, M.A.; et al. Genetic variability in yield and vegetative traits in elite germplasm of MPOB-Nigerian dura × AVROS pisifera progenies. J. Food Agric. Environ. 2013, 11, 515–519. [Google Scholar]
- Corley, R.H.V.; Tinker, P.B. The Oil Palm; John Wiley & Sons: Oxford, UK, 2003. [Google Scholar]
- Thirasak, S.; Sompong, T.-c.; Sureerat, Y. Determination of Salt Tolerance of Oil Palm SUP-PSU 1 Using Cell Suspension Culture. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 4, 1–14. [Google Scholar] [CrossRef]
- Luis, Z.G.; Bezerra, K.M.G.; Scherwinski-Pereira, J.E. Adaptability and leaf anatomical features in oil palm seedlings produced byembryo rescue and pre-germinated seeds. Braz. J. Plant Physiol. 2010, 22, 209–215. [Google Scholar] [CrossRef]
- Zeng, X.H.; Jiao, Y.F.; Liao, Z.R.; Pan, D.L.; Lin, W.F. Effects of the anatomical structure of oil palm leaves in different regions of Guangdong on the cold resistance of oil palm. Guangdong Agric. Sci. 2018, 45, 50–58. [Google Scholar]
- Sari, N.Y.; Putra, E.T.S. The Contribution of Calcium to Changes in Leaf Anatomical Character of Oil Palm Seedlings (Elaeis guineensis Jacq.) under Drought Stress. Ilmu Pertan. Agric. Sci. 2019, 4, 23–32. [Google Scholar] [CrossRef]
- Arteaga, S.; Yabor, L.; Díez, M.J.; Prohens, J.; Boscaiu, M.; Vicente, O. The Use of Proline in Screening for Tolerance to Drought and Salinity in Common Bean (Phaseolus vulgaris L.) Genotypes. Agronomy 2020, 10, 817. [Google Scholar] [CrossRef]
- Najihah, T.S.; Ibrahim, M.H.; Razak, A.A.; Nulit, R.; Wahab, P.E.M. Effects of water stress on the growth, physiology and biochemical properties of oil palm seedlings. AIMS Agric. Food 2019, 4, 854–868. [Google Scholar] [CrossRef]
- Zali, A.G.; Ehsanzadeh, P. Exogenously applied proline as a tool to enhance water use efficiency: Case of fennel. Agric. Water Manag. 2018, 197, 138–146. [Google Scholar] [CrossRef]
- Rahman, S.; Miyake, H.; Takeoka, Y. Effects of Exogenous Glycinebetaine on Growth and Ultrastructure of Salt-Stressed Rice Seedlings (Oryza sativa L.). Plant Prod. Sci. 2002, 5, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Ali, Q.; Ashraf, M.; Athar, H. Exogenously applied proline at different growth stages enhances growth of two maize cultivars grown under water deficit conditions. Pak. J. Bot. 2007, 39, 1133–1144. [Google Scholar]
- Talat, A.; Nawaz, K.; Hussian, K.; Bhatti, K.H.; Siddiqi, E.; Khalid, A.; Anwer, S.; Sharif, M. Foliar application of proline for salt tolerance of two wheat (Triticum aestivum L.) cultivars. World Appl. Sci. J. 2013, 22, 547–554. [Google Scholar]
- Noreen, S.; Faiz, S.; Akhter, M.S.; Shah, K.H. Influence of Foliar Application of Osmoprotectants to Ameliorate Salt Stress in Sunflower (Helianthus annuus L.). Sarhad J. Agric. 2019, 35. [Google Scholar] [CrossRef]
- Jaleel, C.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.; Somasundaram, R.; Panneerselvam, R. Drought Stress in Plants: A Review on Morphological Characteristics and Pigments Composition. Int. J. Agric. Biol. 2009, 6, 112. [Google Scholar]
- Shukla, S.D.; Bhatnagar, M.; Khurana, S. Critical evaluation of ayurvedic plants for stimulating intrinsic anti-oxidant response. Front. Neurosci. 2012, 6, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turhadi, T.; Minarsih, H.; Riyadi, I.; Priyono; Budiani, A. Physiological responses and P5CS gene expression of transgenic oil palm plantlet induced by drought stress. E-J. Menara Perkeb. 2020, 88, 69–78. [Google Scholar] [CrossRef]
- Bandurska, H.; Niedziela, J.; Pietrowska-Borek, M.; Nuc, K.; Chadzinikolau, T.; Radzikowska, D. Regulation of proline biosynthesis and resistance to drought stress in two barley (Hordeum vulgare L.) genotypes of different origin. Plant Physiol. Biochem. 2017, 118, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.S.; Ahmad, R.; Ihsan, M.Z.; Ashraf, M.Y.; Hussain, Y.; Fahad, S. Foliar calcium spray confers drought stress tolerance in maize via modulation of plant growth, water relations, proline content and hydrogen peroxide activity. Arch. Agron. Soil Sci. 2017, 64, 116–131. [Google Scholar] [CrossRef]
- Cao, H.X.; Zhang, J.C.; Lei, X.T.; Liu, Y.J.; Zhang, R.L. Physiological and biochemical responses of different oil palm resources to low temperature stress. J. Yunnan Agric. Univ. Nat. Sci. 2017, 32, 316–321. [Google Scholar]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Ditmarová, L.; Kurjak, D.; Palmroth, S.; Kmeť, J.; Střelcová, K. Physiological responses of Norway spruce (Picea abies) seedlings to drought stress. Tree Physiol. 2009, 30, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Gong, L.; Wei, L.; Ni, S.; Liu, S. Effects of continuous drought on chlorophyll fluorescence kinetic parameters of oil palm seedlings. Chin. Agric. Sci. Bull. 2016, 32, 1–6. [Google Scholar]
- Cha-Um, S.; Takabe, T.; Kirdmanee, C. Physio-Biochemical Responses of Oil Palm (Elaeis guineensis Jacq.) Seedlings to Mannitol- and Polyethylene Glycol-Induced Iso-Osmotic Stresses. Plant Prod. Sci. 2012, 15, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Cha-Um, S.; Yamada, N.; Takabe, T.; Kirdmanee, C. Physiological features and growth characters of oil palm (Elaeis guineensis jacq.) in response to reduced water-deficit and rewatering. Aust. J. Crop. Sci. 2013, 7, 432–439. [Google Scholar]
- Silva, P.A.; Oliveira, I.V.; Rodrigues, K.C.B.; Cosme, V.S.; Bastos, A.J.R.; Detmann, K.S.C.; Cunha, R.L.; Festucci-Buselli, R.A.; DaMatta, F.M.; Pinheiro, H.A. Leaf gas exchange and multiple enzymatic and non-enzymatic antioxidant strategies related to drought tolerance in two oil palm hybrids. Trees 2015, 30, 203–214. [Google Scholar] [CrossRef]
- Yeap, W.; Namasivayam, P.; Ooi, T.E.K.; Appleton, D.R.; Kulaveerasingam, H.; Ho, C. EgRBP 42 from oil palm enhances adaptation to stress in Arabidopsis through regulation of nucleocytoplasmic transport of stress-responsive mRNAs. Plant Cell Environ. 2018, 42, 1657–1673. [Google Scholar] [CrossRef] [PubMed]
- Azzeme, A.M.; Abdullah, S.N.A.; Aziz, M.A.; Wahab, P.E.M. Oil palm drought inducible DREB1 induced ex-pression of DRE/CRT and non-DRE/CRT containing genes in lowland transgenic tomato under cold and PEG treatments. Plant Physiol. Biochem. 2017, 112, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Z.; Wang, L.; Wang, H.R. Identification and characterization of a plastidial ω-3 fatty acid desaturase EgFAD8 from oil palm (Elaeis guineensis Jacq.) and its promoter response to light and low temperature. PLoS ONE 2018, 13, e0196693. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, R.; Zheng, Y.; Yuan, Y.; Li, D. Isolation and characterization of the EgWRI1 promoter from oil palm (Elaeis guineensis Jacq.) and its response to environmental stress and ethylene. PLoS ONE 2019, 14, e0225115. [Google Scholar] [CrossRef]
- Zhou, L.; Yarra, R.; Jin, L.; Cao, H. Genome-wide identification and expression analysis of MYB gene family in oil palm (Elaeis guineensis Jacq.) under abiotic stress conditions. Environ. Exp. Bot. 2020, 180, 104245. [Google Scholar] [CrossRef]
- Zhou, L.X.; Cao, H.X. Analysis of the expression characteristics of oil palm WRKY transcription factor genes under low temperature stress. South. Agric. J. 2018, 49, 1490–1497. [Google Scholar]
- Xia, W.; Mason, A.S.; Xiao, Y.; Liu, Z.; Yang, Y.; Lei, X.; Wu, X.; Ma, Z.; Peng, M. Analysis of multiple transcriptomes of the African oil palm (Elaeis guineensis) to identify reference genes for RT-qPCR. J. Biotechnol. 2014, 184, 63–73. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Iqbal, A.; Qadri, R.; Shi, P.; Wang, Y.; Wu, Y.; Fan, H.; Wu, G. Correlation analysis of cold-related gene expression with physiological and biochemical indicators under cold stress in oil palm. PLoS ONE 2019, 14, e0225768. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhou, L.; Lei, X.; Cao, H.; Wang, Y.; Dou, Y.; Tang, W.; Xia, W. Genome-wide identification of WRKY genes and their expression profiles under different abiotic stresses in Elaeis guineensis. PLoS ONE 2017, 12, e0189224. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Rajesh, Y. Genome-Wide Identification and Characterization of AP2/ERF Transcription Factor Family Genes in Oil Palm under Abiotic Stress Conditions. Int. J. Mol. Sci. 2021, 22, 2821. [Google Scholar] [CrossRef]
- Zhou, L.; Yarra, R. Genome-wide identification and expression analysis of bZIP transcription factors in oil palm (Elaeis guineensis Jacq.) under abiotic stress. Protoplasma 2021, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Yarra, R.; Zhou, L.; Cao, H. The auxin response factor (ARF) gene family in Oil palm (Elaeis guineensis Jacq.): Genome-wide identification and their expression profiling under abiotic stresses. Protoplasma 2021, 1–14. [Google Scholar] [CrossRef]
- Hualkasin, W.; Thongin, W.; Petsean, K.; Phongdara, A.; Nakkaew, A. Molecular cloning and characterization of the late embryogenesis abundant group 4 (EgLEA4) gene from oil palm (Elaeis guineensis Jacq). Songklanakarin J. Sci. Technol. 2013, 35, 275–285. [Google Scholar]
- Wang, L.; Lee, M.; Ye, B.; Yue, G.H. Genes, pathways and networks responding to drought stress in oil palm roots. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Brum, M.; Oliveira, R.S.; López, J.G.; Licata, J.; Pypker, T.; Chia, G.S.; Tinôco, R.S.; Asbjornsen, H. Effects of irrigation on oil palm transpiration during ENSO-induced drought in the Brazilian Eastern Amazon. Agric. Water Manag. 2020, 245, 106569. [Google Scholar] [CrossRef]
- Safitri, L. Manajemen Irigasi Pembibitan Sawit (Elaeis guineensis) Presisi dengan Cropwat 8.0. J. Tek. Pertan. Lampung J. Agric. Eng. 2019, 8, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Nurwahyuni, E.; Putra, E.T.S. The Effect of Calcium on Photosynthetic Rate due to ABA and Proline Behaviour of Oil Palm (Elaeis guineensis Jacq.) Seedlings under Drought Conditions. Caraka Tani J. Sustain. Agric. 2018, 34, 31–42. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, J.; Fan, J.; Li, Y.; Huang, Y.; Zhang, J. Current status and prospects of research on plant disease resistance. Natl. Sci. Found. China 2020, 34, 433–440. [Google Scholar]
- Hanum, H.; Tantawi, A.R. Survey of Basal Stem Rot Disease on Oil Palms (Elaeis guineensis Jacq.) in Kebun Bukit Kijang, North Sumatera, Indonesia. IOP Conf. Series: Earth Environ. Sci. 2016, 41, 12007. [Google Scholar] [CrossRef] [Green Version]
- Rakib, M.; Borhan, A.; Jawahir, A. The relationship between SPAD chlorophyll and disease severity index in Ganoderma-infected oil palm seedlings. J. Bangladesh Agric. Univ. 2019, 17, 355–358. [Google Scholar] [CrossRef] [Green Version]
- Shafri, H.; Anuar, I. Hyperspectral Signal Analysis for Detecting Disease Infection in Oil Palms. Am. J. Appl. Sci. 2008, 6, 1031–1035. [Google Scholar]
- Ahmadi, P.; Muharam, F.M.; Ahmad, K.; Mansor, S.; Abu Seman, I. Early Detection of Ganoderma Basal Stem Rot of Oil Palms Using Artificial Neural Network Spectral Analysis. Plant Dis. 2017, 101, 1009–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aucique-Perez, C.E.; Daza, E.S.; Ávila-Diazgranados, R.A.; Romero, H.M. Chlorophyll a fluorescence and leaf temperature are early indicators of oil palm diseases. Sci. Agricola 2020, 77, 106. [Google Scholar] [CrossRef] [Green Version]
- Ntsomboh Ntsefong, G.; Ngando, G.; Paul, K.; Bell, J.M.; Youmbi, E.; Bille, N.; Gervais, B.; Galdima, M.; Bienvenu, A. Control Approaches against Vascular Wilt Disease of Elaeis guineensis Jacq. Caused by Fusarium oxysporum f. sp. elaeidis. J. Biol. Life Sci. 2012, 3, 1–14. [Google Scholar] [CrossRef]
- Corley, R.V.H.; Tinker, P.B. The Oil Palm, 5th ed.; Blackwell Science: Oxford, UK, 2015. [Google Scholar]
- Navia, E.A.; Restrepo, E.F.; Romero, H.M. Response of six sources of oil palm planting materials from Malaysia planted in the eastern plains of Colombia to bud rot. J. Oil Palm Res. 2014, 26, 73–83. [Google Scholar]
- Yu, F.; Qin, W.; Zhu, H.; Ma, Z. Research review of oil palm fusarium wilt. China Trop. Agric. 2009, 2, 46–48. [Google Scholar]
- Koussinou, C.K.S.L.; Adandonon, A.; Nodichao, L. Distribution and incidence of Fusarium wilt in oil palm in Benin. J. Appl. Biosci. 2019, 135, 13831. [Google Scholar] [CrossRef]
- Zheng, L.; Shen, H.; Li, J.; He, S.; Zeng, X.; Feng, C.; Qin, X.; Xie, C. Investigation of oil palm diseases and preliminary identification of pathogenic fungi of leaf diseases. Guangdong Agric. Sci. 2014, 41, 66–69. [Google Scholar]
- Chen, X.; Zeng, H.; He, H.; Peng, Y. Identification of Pythium splendens, a new record in Hainan and its pathogenicity test to oil palm. J. Yunnan Agric. Univ. 2008, 23, 321–324. [Google Scholar]
- Zheng, L.; Zhang, H.; Lin, J.; Zeng, X.; Li, J.; Shen, H.; Xie, C.; Qin, X. Biological characteristics and viru-lence determination of the pathogen of oil palm grass stem spot mold leaf spot. Chin. J. Trop. Crop. 2017, 38, 1694–1699. [Google Scholar]
- Ayoib, A.; Hashim, U.; Gopinath, S.C. Automated, high-throughput DNA extraction protocol for disposable label free, microfluidics integrating DNA biosensor for oil palm pathogen, Ganoderma boninense. Process. Biochem. 2020, 92, 447–456. [Google Scholar] [CrossRef]
- Saad, M.M.; Ali, N.S.; Meon, S. Relationship between ganoderma ergosterol concentration and basal stem rot disease progress on Elaeis guineensis. Trop. Life Sci. Res. 2020, 31, 19–43. [Google Scholar] [CrossRef]
- Khaleda, A.Y.; Aziza, S.A.; Bejoa, S.K.; Nawi, N.M.; Seman, I.A. Spectral features selection and classification of oil palm leaves infected by Basal stem rot (BSR) disease using dielectric spectroscopy. Agric. Week 2018, 144, 297–309. [Google Scholar] [CrossRef]
- Santoso, H.; Tani, H.; Wang, X. Random Forest classification model of basal stem rot disease caused by Ganoderma boninense in oil palm plantations. Int. J. Remote Sens. 2017, 38, 4683–4699. [Google Scholar] [CrossRef]
- Izzuddin, A.M.; Idris, A.S.; Nisfariza, M.N.; Nordiana, A.A.; Shafri, H.Z.M.; Ezzati, B. The development of spectral indices for early detection of Ganoderma disease in oil palm seedlings. Int. J. Remote Sens. 2017, 38, 6505–6527. [Google Scholar] [CrossRef]
- Golhani, K.; Balasundram, S.K.; Vadamalai, G.; Pradhan, B. Selection of a Spectral Index for Detection of Orange Spotting Disease in Oil Palm (Elaeis guineensis Jacq.) Using Red Edge and Neural Network Techniques. J. Indian Soc. Remote Sens. 2019, 47, 639–646. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, W.; Zhang, J.; Wang, J.; Xu, Y.; Chen, X. Study on detection of oil palm damping-off bacteria by loop-mediated isothermal amplification technique. J. Zhejiang Agric. Sci. 2013, 25, 303–308. [Google Scholar]
- Nababan, M.; Laia, Y.; Sitanggang, D.; Sihombing, O.; Indra, E.; Siregar, S.; Purba, W.; Mancur, R. The diagnose of oil palm disease using Naive Bayes Method based on Expert System Technology. J. Phys. Conf. Ser. 2018, 1007, 012015. [Google Scholar] [CrossRef]
- Cao, X.; Shang, L.; Li, J.; Hu, J. Research on the prevention and treatment of oil palm stem basal infection with Ganoderma boninense by microorganisms. Heilongjiang Sci. 2020, 11, 1–3. [Google Scholar]
- Sujarit, K.; Pathom-Aree, W.; Mori, M.; Dobashi, K.; Shiomi, K.; Lumyong, S. Streptomyces palmae CMU-AB204T, an antifungal producing-actinomycete, as a potential biocontrol agent to protect palm oil producing trees from basal stem rot disease fungus, Ganoderma boninense. Biol. Control 2020, 148, 104307. [Google Scholar] [CrossRef]
- Sunpapao, A.; Chairin, T.; Ito, S.-I. The biocontrol by Streptomyces and Trichoderma of leaf spot disease caused by Curvularia oryzae in oil palm seedlings. Biol. Control 2018, 123, 36–42. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z.; Idris, A.S. An Overview of the Oil Palm Industry: Challenges and Some Emerging Opportunities for Nanotechnology Development. Agronomy 2020, 10, 356. [Google Scholar] [CrossRef] [Green Version]
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Idris, A.S.; Hilmi, N.H.Z.; Daim, L.D.J. Chitosan-Based Agronanofungicides as a Sustainable Alternative in the Basal Stem Rot Disease Management. J. Agric. Food Chem. 2020, 68, 4305–4314. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Rodríguez-Dimaté, F.A.; Campos, J.M.; Serro, J.E. Exposure to insecticides reduces populations of rhynchophorus palmarum in oil palm plantations with bud rot disease. Insects 2019, 10, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puspita, F.; Hadiwiyono; Poromorto, S.H.; Roslim, D.I. The application of different Bacillus subtilis contained formula as bio fungicide tablet to control Ganoderma boninense in oil palm nurseries. IOP Conf. Series Earth Environ. Sci. 2019, 250, 012052. [Google Scholar] [CrossRef] [Green Version]
- Rebitanim, N.A.; Hanafi, M.M.; Idris, A.S.; Abdullah, S.N.A. GanoCare® improves oil palm growth and resistance against ganoderma basal stem rot disease in nursery and field trials. BioMed Res. Int. 2020, 2020, 3063710. [Google Scholar] [CrossRef]
- Bivi, M.S.H.R.; Paiko, A.S.; Khairulmazmi, A.; Akhtar, M.; Idris, A.S. Control of Basal Stem Rot Disease in Oil Palm by Supplementation of Calcium, Copper, and Salicylic Acid. Plant Pathol. J. 2016, 32, 396–406. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, R.K.; Radziah, O. Influence of pH levels on disease development in oil palm seedling roots infected with Ganoderma boninensis. Rhizosphere 2020, 13, 100181. [Google Scholar]
- Shukri, I.M.; Izzuddin, M.A.; Hefni, R.M.; Idris, A.S. Geostatistics of oil palm trees affected by Ganoderma disease in low and high planting density. IOP Conf. Ser. Earth Environ. Sci. 2020, 540, 012065. [Google Scholar] [CrossRef]
- Benítez, E.; García, C. The history of research on oil palm bud rot (Elaeis guineensis Jacq.) in Colombia. Agron. Colomb. 2014, 32, 390–398. [Google Scholar] [CrossRef]
- Navia, E.A.; Ávila, R.A.; Daza, E.E.; Restrepo, E.F.; Romero, H.M. Assessment of tolerance to bud rot in oil palm under field conditions. Eur. J. Plant Pathol. 2014, 140, 711–720. [Google Scholar] [CrossRef]
- Hanin, A.N.; Parveez, G.K.A.; Rasid, O.A.; Masani, M.Y.A. Biolistic-mediated oil palm transformation with alfalfa glucanase (AGLU1) and rice chitinase (RCH10) genes for increasing oil palm resistance towards Ganoderma boninense. Ind. Crop. Prod. 2019, 144, 112008. [Google Scholar] [CrossRef]
- Ramzi, A.B.; Me, M.L.C.; Ruslan, U.S.; Baharum, S.N.; Muhammad, N.A.N. Insight into plant cell wall degradation and pathogenesis of Ganoderma boninense via comparative genome analysis. PeerJ 2019, 7, e8065. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, S.V.D.; Magalhães, M.M.; Cunha, R.L.; Costa, P.H.D.O.; Alves, R.C.D.O.; De Oliveira, G.C.; Valadares, R.B.D.S. Differential accumulation of proteins in oil palms affected by fatal yellowing disease. PLoS ONE 2018, 13, e0195538. [Google Scholar] [CrossRef] [Green Version]

| Family | Gene Name | Types of Stress Condition |
Degree/ Dose | Function | Reference |
|---|---|---|---|---|---|
| MYB | EgMYB38, EgMYB43, EgMYB57, EgMYB76, EgMYB82, EgMYB91, EgMYB104, EgMYB106, EgMYB111, EgMYB127, EgMYB133, EgMYB146, EgMYB 151, EgMYB155 | Cold, Drought, and Salt | NA | Up-regulated under all abiotic stress conditions (cold, drought, and salt). | [52] |
| WRKY | EgWRKY18, EgWRKY64 | Cold | 8 °C | Involved in cold stress and negative regulator of cold response. | [56] |
| EgWRKY07, EgWRKY52 | Salt | 400 mmol/L of NACL | WRKY gene was strongly induced and up-regulated gene in leaves after Salt stress. | [56] | |
| AP2/ERF/RAV | EgAP2.15, EgAP2.34, EgERF23, EgERF104, EgERF130 | Cold | 8 °C | Increase expression of AP2/ERF genes in re-sponse to cold exposure. | [57] |
| EgAP2.09, EgERF26, EgERF90, EgER104 | Drought | NA | Drought stress-induced AP2 and ERF genes. | [57] | |
| EgERF14, EgERF73, EgRAV02 | Salinity | 300 mmol/L of NACL | Salt stress were induced and upregulated by ERF/RAV gene members. | [57] | |
| bZIP | EgbZIP1, EgbZIP4, EgbZIP27, EgbZIP44, EgbZIP52, EgbZIP68, EgbZIP77, EgbZIP85, EgbZIP86, EgbZIP89, EgbZIP95 | Cold, Salt, and Drought | NA | The bZIP genes were up-regulated in response to cold, salt, or drought stress, suggesting that EgbZIP plays a significant role in stress response. | [58] |
| ARF | EgARF4, EgARF5, EgARF6, EgARF9, EgARF10, EgARF12, EgARF13, EgARF15, EgARF21, EgARF22 | Cold (Up-regulated) | 8 °C | Different types of abiotic stresses can induce the expression of EgARFs (cold, drought, and salt). The ARF gene functional investigations in oil palm and serve as a genetic resource platform for oil palm abiotic stress resistance breeding. | [59] |
| EgARF1, EgARF3, EgARF8, EgARF14, EgARF17, EgARF18, EgARF19, and EgARF20 | Cold (Down-regulated) | ||||
| EgARF4, EgARF6, EgARF9, EgARF10, EgARF12, EgARF13, EgARF15, EgARF16, and EgARF22 | Drought (Up-regulated) | NA | |||
| EgARF1, EgARF14, EgARF17, EgARF18, EgARF19, EgARF20, and EgARF21 | Drought (Down-regulated) | ||||
| EgARF9, EgARF10, EgARF17, and EgARF22 | Salt (Up-regulated) | 300 mmol/L | |||
| EgARF3, EgARF4, EgARF5, EgARF8, EgARF14, EgARF15, EgARF16, EgARF18, EgARF19, EgARF20, and EgARF21 | Salt (Down-regulated) | ||||
| LEA | EgLEA4 | Drought | NA | Enhance drought tolerance. | [60] |
| Disease | Symptoms | Control |
|---|---|---|
| Spindle Bug | Necrotic sores and dry ground spots on leaves spindle fail to open | Keep perforated poly sachets loaded with porate (2 g) in the leaf axil. |
| Tussock Caterpillar | Defoliation of leaves | Damaged leaves should be cut and burned. If the infestation is severe, spray monocrotophos (0.36%) or carbaryl (0.1%). |
| Root Grubs | Sudden death of young plants | Fill the seedling bags with soil that is free of root grubs. While planting the sprouts, apply 50 gms of phorate per seedling. |
| Termites | Hindered growth of the plant | Destroy termite mounds and drench with chlropyriphos (0.5%). |
| Lesser Bandicoot, Bandicota bengalensis | Destruction of apical region | In a suitable bait station, ideally composed of earthen pots, anticoagulant baiting with bromadiolone (0.05%) may be injected. |
| Wild Boar | Destruction of boll region | Wild boar scaring device may be utilized. |
| Rhinoceros beetle (Oryctes rhinoceros L.) | The leaf silhouette has “V” shaped gaps. | Damaged and dead palms, as well as decaying bunches, must be removed from the orchard. To prevent egg-laying, apply “tar” to wounds and cuts on the stem section. Use logs or pheromone baits to catch the adult beetles. Maintain a clean environment in the orchard. Trunk injection of carbaryl % WP at 1% or endosulfan 35 EC at 0.1%. |
| Red Palm Weevil (Rhynchophorus ferrugineus Oliver) | Palm wilts and dries gradually. Grubs feeding inside the trunk generate a distinctive sound. | |
| Case Worm | Sporadic defoliation | Spray carbaryl (0.1%) on infected leaves. If the infestation is severe, root feeding with monocrotophos may be practiced. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.; John Martin, J.J.; Zhang, H.; Zhang, R.; Cao, H. Problems and Prospects of Improving Abiotic Stress Tolerance and Pathogen Resistance of Oil Palm. Plants 2021, 10, 2622. https://doi.org/10.3390/plants10122622
Wei L, John Martin JJ, Zhang H, Zhang R, Cao H. Problems and Prospects of Improving Abiotic Stress Tolerance and Pathogen Resistance of Oil Palm. Plants. 2021; 10(12):2622. https://doi.org/10.3390/plants10122622
Chicago/Turabian StyleWei, Lu, Jerome Jeyakumar John Martin, Haiqing Zhang, Ruining Zhang, and Hongxing Cao. 2021. "Problems and Prospects of Improving Abiotic Stress Tolerance and Pathogen Resistance of Oil Palm" Plants 10, no. 12: 2622. https://doi.org/10.3390/plants10122622
APA StyleWei, L., John Martin, J. J., Zhang, H., Zhang, R., & Cao, H. (2021). Problems and Prospects of Improving Abiotic Stress Tolerance and Pathogen Resistance of Oil Palm. Plants, 10(12), 2622. https://doi.org/10.3390/plants10122622





