Climate Change Impacts on Sunflower (Helianthus annus L.) Plants
Abstract
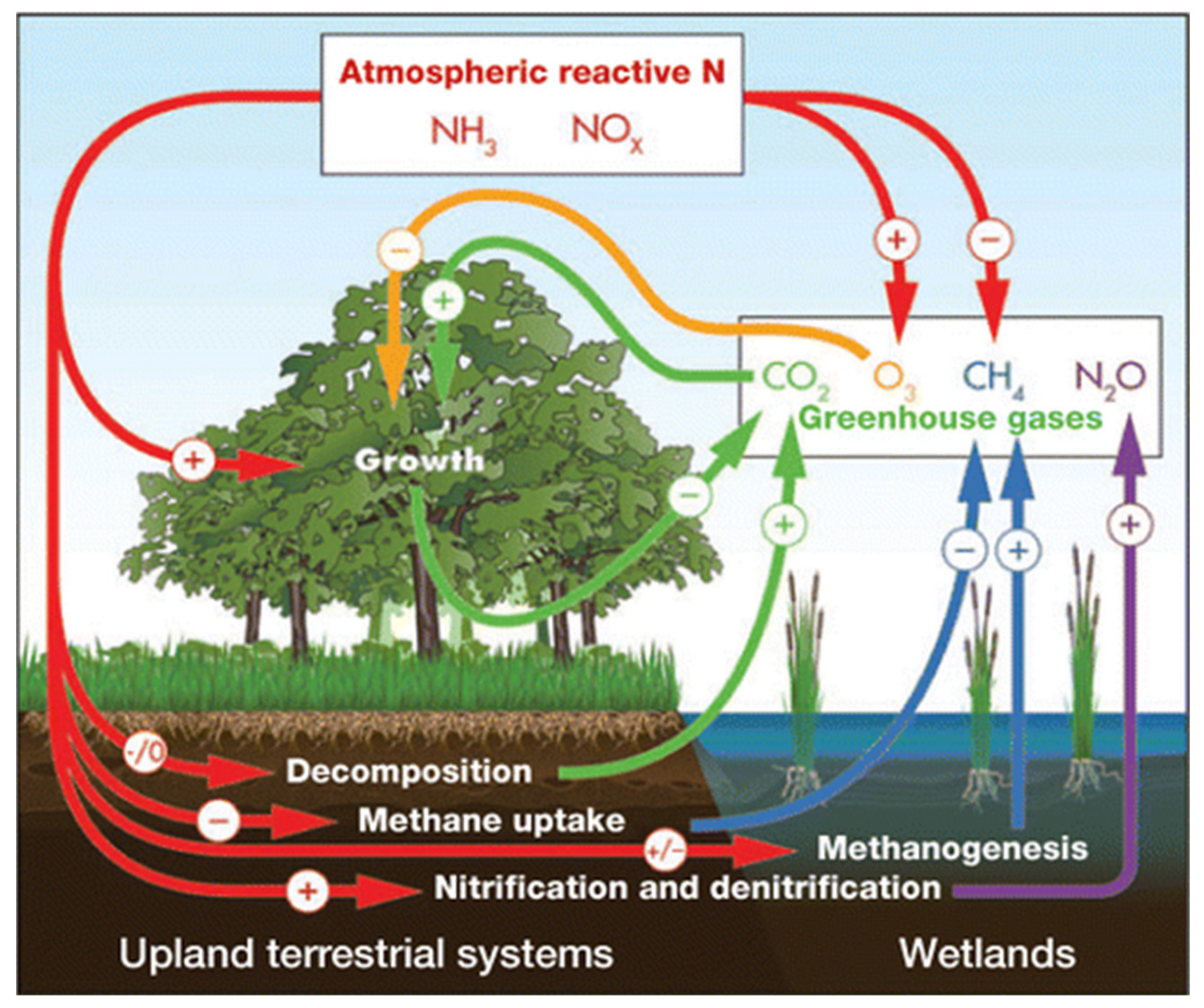
:1. Introduction
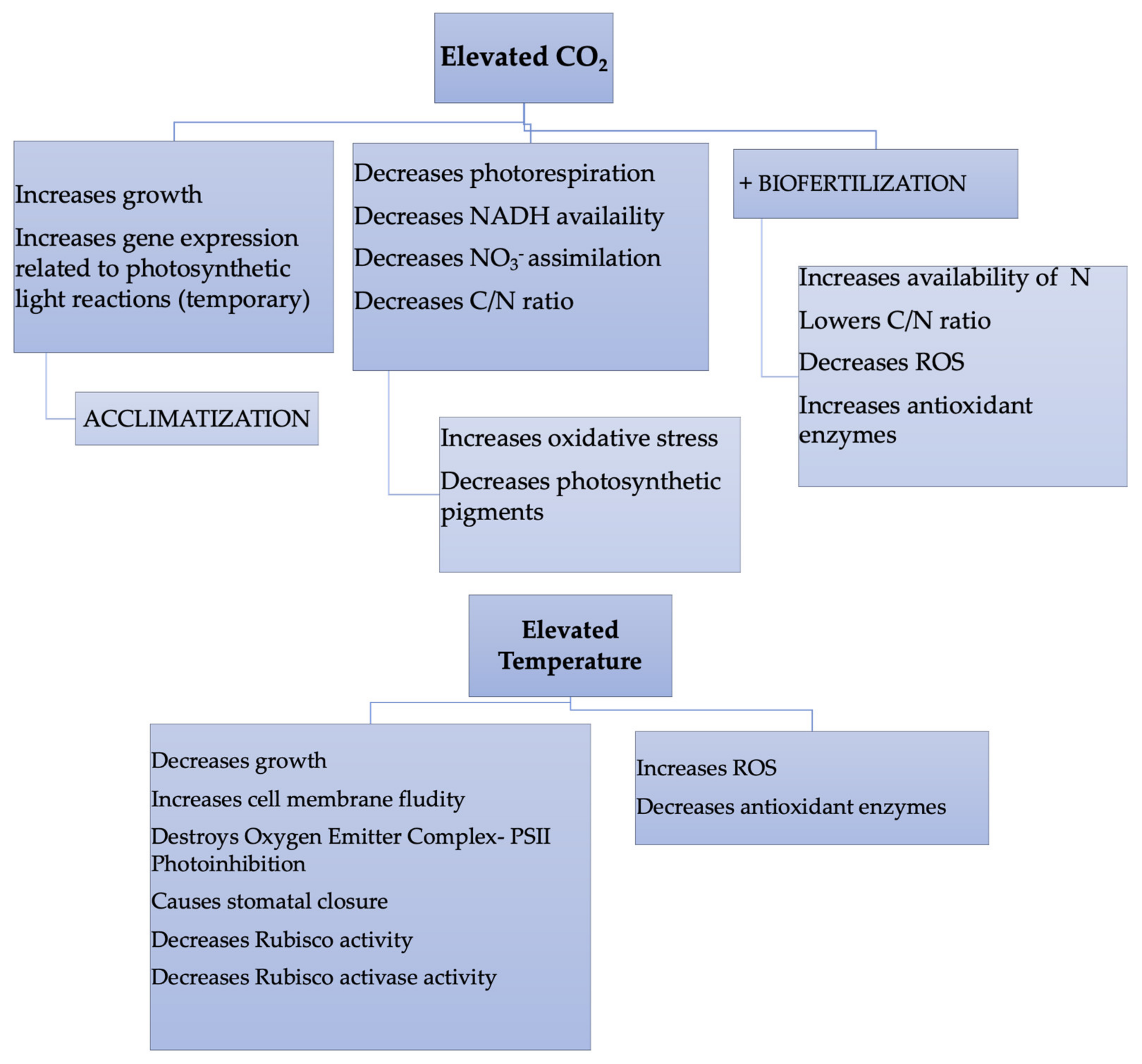
2. Effects of Elevated CO2 and Elevated Temperatures on Sunflower Plants Growth
3. Elevated CO2 Levels and Elevated Temperatures on Carbon Metabolism in Sunflower Plants
4. Elevated CO2 Levels and Elevated Temperatures on Nitrogen Metabolism in Sunflower Plants
5. Oxidative Stress in Sunflower Plants to Elevated CO2 and Temperature
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- NASA Global Climate Change. Vital Sings of Planet. 2019. Available online: http://climate.nasa.gov.vitals-signs/carbon-dioxide (accessed on 8 September 2021).
- IPCC. Intergovernmental panel on climate change 2014: Impacts, adaptations and vulnerability. In Contribution of Working Group II to the Fifth Assessment. Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Eds.; Cambridge University Press: Ginebra, UK, 2014. [Google Scholar]
- Schneider, S.H. The greenhouse effect: Science and policy. Science 1989, 243, 771–781. [Google Scholar] [CrossRef] [Green Version]
- Taylor, K.E.; MacCracken, M.C. Projected effects of increasing concentrations of carbon dioxide and trace gases on climate. In Impact of Carbon Dioxide, Trace Gases, and Climate Change on Global Agriculture; Kimball, B.A., Ed.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 1990; pp. 1–17. [Google Scholar]
- Templer, P.H.; Pinder, R.W.; Goodale, C.L. Effects of nitrogen deposition on greenhouse-gas fluxes for forests and grasslands of North America. Front. Ecol. Environ. 2012, 10, 547–553. [Google Scholar] [CrossRef]
- Fukayama, H.; Sugino, M.; Fukuda, T.; Masumoto, C.; Taniguchi, Y.; Okada, M.; Sameshima, R.; Hatanaka, T.; Misoo, S.; Hasegawa, T.; et al. Gene expression profiling of rice grown in free air CO2 enrichment (FACE) and elevated soil temperature. Field Crops Res. 2011, 121, 195–199. [Google Scholar] [CrossRef]
- Gruissem, W.; Lee, C.; Oliver, M.; Pogson, B. The global plant council: Increasing the impact of plant research to meet global challenges. J. Plant Biol. 2012, 55, 343–345. [Google Scholar] [CrossRef]
- Maas, E.V.; Hoffman, G.J. Crop salt tolerance, current assessment. J. Irrig. Drain Div. ASCE 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Kratsch, H.A.; Wise, R.R. The ultrastructure of chilling stress. Plant Cell Environ. 2000, 23, 337–350. [Google Scholar] [CrossRef]
- Huang, L.; Ye, Z.; Bell, R.W.; Dell, B. Boron nutrition and chilling tolerance of warm climate crop species. Ann. Bot. 2005, 96, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Connor, D.J.; Palta, J.A.; Jones, T.R. Response of sunflower to strategies of irrigation. III. Crop photosynthesis and transpiration. Field Crop Res. 1985, 12, 281–283. [Google Scholar] [CrossRef]
- Connor, D.J.; Jones, T.R. Response of sunflower to strategies of irrigation II. Morphological and physiological responses to water stress. Field Crop Res. 1985, 12, 91–103. [Google Scholar] [CrossRef]
- Sadras, V.O.; Whitfield, D.M.; Connor, D.J. Regulation of evapotranspiration and its partitioning between transpiration and soil evaporation by sunflower crops. A comparison between hybrids of different stature. Field Crop Res. 1991, 28, 17–37. [Google Scholar] [CrossRef]
- Moschen, S.; Di Rienzo, J.A.; Higgins, J.; Tohge, T.; Watanabe, M.; González, S.; Rivarola, M.; García-García, F.; Dopazo, J.; Hopp, H.E.; et al. Integration of transcriptomic and metabolic data reveals hub transcription factors involved in drought stress response in sunflower (Helianthus annuus L.). Plant Mol. Biol. 2017, 94, 549–564. [Google Scholar] [CrossRef]
- Nishimura, C.; Ohashi, Y.; Sato, S.; Kato, T.; Tabata, S.; Ueguchi, C. Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell 2004, 16, 1365–1377. [Google Scholar] [CrossRef] [Green Version]
- Tsukaya, H. Mechanism of leaf-shape determination. Annu. Rev. Plant Biol. 2006, 57, 477–496. [Google Scholar] [CrossRef]
- Riikonen, J.; Percy, K.E.; Kivimäenpää, M.; Kubiske, M.E.; Nelson, N.D.; Vapaavuori, E.; Karnosky, D.F. Leaf size and surface characteristics of Betula papyrifera exposed to elevated CO2 and O3. Environ. Pollut. 2010, 158, 1029–1035. [Google Scholar] [CrossRef]
- Ranasinghe, S.; Taylor, G. Mechanism for increased leaf grwoth in elevated CO2. J. Exp. Bot. 1996, 47, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Kinsman, E.; Lew, D.M.; Young, J.F.D.; Vilhar, B.; Ougham, H. Elevated CO2 stimulates cells to divide in grass meristems: A differential effect in two natural populations of Dactylis glomerata. Plant Cell Environ. 1997, 20, 1309–1316. [Google Scholar] [CrossRef]
- Ferris, R.; Sabatti, M.; Miglietta, F.; Mills, R.; Taylor, G. Leaf area is stimulated in Populus by free air CO2 enrichment (POPFACE), through increased cell expansion and production. Plant Cell Environ. 2001, 24, 305–315. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A.; Vodkin, L.O.; Walter, A.; Schurr, U. The effects of elevated CO2 concentration on soybean gene expression. An analysis of growing and mature leaves. Plant Physiol. 2006, 142, 135–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontunen-Soppela, S.; Parviainen, J.; Ruhanen, H.; Brosche, M.; Keinänen, M.; Thakur, R.C.; Kolehmainen, M.; Kangasjärvi, J.; Oksanen, E.; Karnosky, D.F. Gene expression responses of paper birch (Betula papyrifera) to elevated CO2 and O3 during leaf maturation and senescence. Environ. Pollut. 2010, 158, 959–968. [Google Scholar] [CrossRef]
- Wei, H.; Gou, J.; Yordanov, Y.; Zhang, H.; Thakur, R.; Jones, W.; Burton, A. Global transcriptomic profiling of aspen trees under elevated [CO2] to identify potential molecular mechanisms responsible for enhanced radial growth. J. Plant Res. 2013, 126, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathne, C.L.; Tausz-Posch, S.; Cane, K.; Norton, R.M.; Fitzgerald, G.J.; Tausz, M.; Seneweera, S. Intraspecific variation in leaf growth of wheat (Triticum aestivum) under Australian Grain Free CO2 Enrichment (AGFACE): Is it regulated through carbon and/or nitrogen supply? Func. Plant Biol. 2015, 42, 299–308. [Google Scholar] [CrossRef]
- De la Mata, L.; Cabello, P.; de la Haba, P.; Agüera, E. Growth under elevated atmospheric CO2 concentration accelerates leaf senescence in sunflower (Helianthus annuus L.) plants. J. Plant Physiol. 2012, 169, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Gamage, D.; Thompson, M.; Sutherland, M.; Hirotsu, N.; Makino, A.; Seneweera, S. New insights into the cellular mechanisms of plant growth at elevated atmospheric carbon dioxide concentrations. Plant Cell Environ. 2018, 41, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- De la Haba, P.; De la Mata, L.; Molina, E.; Aguera, E. High temperature promotes early senescence in primary leaves of sunflower (Helianthus annuus L.) plants. Can. J. Plant Sci. 2014, 94, 659–669. [Google Scholar] [CrossRef]
- Ferguson, D.L.; Guikema, J.A.; Paulsen, G.M. Ubiquitin pool modulation and protein degradation in wheat roots during high temperature stress. Plant Physiol. 1990, 92, 740–746. [Google Scholar] [CrossRef] [Green Version]
- Scheurwater, I.; Dunnebacke, M.; Eising, R.; Lambers, H. Respiratory costs and rate of protein turnover in the roots of a fast-growing (Dactylis glomerata L.) and a slow-growing (Festuca ovina L.) grass species. J. Exp. Bot. 2000, 51, 1089–1097. [Google Scholar] [PubMed]
- Benlloch-González, M.; Bochicchio, R.; Berger, J.D.; Bramley, H.; Palta, J. High temperature reduces the positive effect of elevated CO2 on wheat root system growt. Fields Crops Res. 2014, 165, 71–79. [Google Scholar] [CrossRef]
- Lee, I.H.; Sang, W.G.; Baek, J.-K.; Kim, J.-H.; Shin, P.; Seo, M.C.; Cho, J.I. The effect of concurrent elevation in CO2 and temperature on the growth, photosynthesis, and yield of potato crops. PLoS ONE 2020, 15, e0241081. [Google Scholar]
- Awais, M.; Wajid, A.; Saleem, M.F.; Nasim, W.; Ahmad, A.; Raza, M.A.S.; Bashir, M.U.; Mubeen, M.; Hammad, H.M.; Rahman, M.H.; et al. Potential impacts of climate change and adaptation strategies for sunflower in Pakistan. Environ. Sci. Poll Res. 2018, 25, 13719–13730. [Google Scholar] [CrossRef]
- Liu, M.; Xua, X.; Jiang, Y.; Huang, Q.; Huo, Z.; Liu, L.; Huang, G. Responses of crop growth and water productivity to climate change and agricultural water-saving in arid region. Sci. Total Environ. 2020, 703, 134621. [Google Scholar] [CrossRef]
- Nowicka, B.; Ciura, J.; Szymanska, R.; Kruk, J. Improving photosynthesis, plant productivity and abiotic stress tolerance current trends and future perspectives. J. Plant Physiol. 2018, 231, 415–433. [Google Scholar] [CrossRef]
- Drake, B.G.; Gonzàlez-Meler, M.A.; Long, S.P. More efficient plants: A consequence of rising atmospheric CO2? Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 609–639. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, S.G.; Ju, Z.; van Santen, E.; Qiu, J.; Weaver, D.B.; Prior, S.A.; Rogers, H.H. The influence of elevated CO2 on the activities of antioxidative enzymes in two soybean genotypes. Aust. J. Plant Physiol. 2000, 27, 1061–1068. [Google Scholar] [CrossRef]
- Bauwe, H.; Hagemann, M.; Fernie, A.R. Photorespiration: Players, partners and origin. Trends Plant Sci. 2010, 15, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Van Heerden, P.; Swanepoel, J.; Kruger, G. Modulation of photosynthesis by drought in two desert scrub species exhibiting C 3-mode CO2 assimilation. Environ. Ex Bot. 2007, 61, 124–136. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Chen, G.-Y.; Gong, Z.-Y.; Chen, J.; Yong, Z.-H.; Zhu, J.-G.; Xu, D.-Q. Ribulose-1, 5-bisphosphate regeneration limitation in rice leaf photosynthetic acclimation to elevated CO2. Plant Sci. 2008, 175, 348–355. [Google Scholar] [CrossRef]
- Sekhar, K.M.; Sreeharsha, R.V.; Reddy, A.R. Differential responses in photosynthesis, growth and biomass yields in two mulberry genotypes grown under elevated CO2 atmosphere. J. Photochem. Photobiol. B Biol. 2015, 151, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.; Pérez, P.; Martínez-Carrasco, R.; Usadel, B.; Kostadinova, S.; Morcuende, R. Quantitative RT–PCR platform to measure transcript levels of C and N metabolism-related genes in durum wheat: Transcript profiles in elevated [CO2] and high temperature at different levels of N supply. Plant Cell Physiol. 2015, 56, 1556–1573. [Google Scholar] [CrossRef] [Green Version]
- Kant, S.; Seneweera, S.; Rodin, J.; Materne, M.; Burch, D.; Rothstein, S.J.; Spangenberg, G. Improving yield potential in crops under elevated CO2: Integrating the photosynthetic and nitrogen utilization efficiencies. Front. Plant Sci. 2012, 3, 162. [Google Scholar] [CrossRef] [Green Version]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef]
- Kitaoka, S.; Matsuki, S.; Kitao, M.; Tobita, H.; Utsugi, H.; Maruyama, Y.; Koike, T. The photosynthetic response of four seral deciduous broad-leaved tree seedlings grown under elevated CO2 concentrations. J. Agric. Meteorol. 2016, 72, 3–49. [Google Scholar] [CrossRef] [Green Version]
- Bloom, A.J.; Burger, M.; Kimball, B.A.; Pinter, P.J. Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat. Clim. Chang. 2014, 4, 477–480. [Google Scholar] [CrossRef]
- Ruiz-Vera, U.M.; De Souza, A.P.; Long, S.P.; Ort, D.R. The role of sink strength and nitrogen availability in the down-regulation of photosynthetic capacity in field-grown Nicotiana tabacum L. at elevated CO2 concentration. Front. Plant Sci. 2017, 8, 998. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, H.; De Kok, L.J.; Armstrong, R.; Fitzgerald, G.J.; Bourgault, M.; Henty, S.; Tausz, M.; Tausz-Posch, S. The proportion of nitrate in leaf nitrogen, but not changes in root growth, are associated with decreased grain protein in wheat under elevated [CO2]. J. Plant. Physiol. 2017, 216, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Riikonen, J.; Kets, K.; Darbah, J.; Oksanen, E.; Sober, A.; Vapaavuori, E.; Kubiske, M.E.; Nelson, N.; Karnosky, D.F. Carbon gain and bud physiology in Populus tremuloides and Betula papyrifera grown under long-term exposure to elevated concentrations of CO2 and O3. Tree Physiol. 2008, 28, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Larios, B.; Agüera, E.; de la Haba, P.; Pérez-Vicente, R.; Maldonado, J.M. A short-term exposure of cucumber plants to rising atmospheric CO2 increases leaf carbohydrate content and enhances nitrate reductase expression and activity. Planta 2001, 212, 305–312. [Google Scholar] [CrossRef]
- Agüera, E.; Ruano, D.; Cabello, P.; De la Haba, P. Impact of atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in cucumber (Cucumis sativus L.) plants. J. Plant Physiol. 2006, 163, 809–817. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Bae, H.; Sicher, R. Changes of soluble protein expression and leaf metabolite levels in Arabidopsis thaliana grown in elevated atmospheric carbon dioxide. Field Crop Res. 2004, 90, 61–73. [Google Scholar] [CrossRef]
- Araya, T.; Noguchi, K.; Terashima, I. Differences between sink and source leaves in carbohydrate repression of photosynthesis. In Photosynthesis: Fundamental Aspects to Global Perspective; van der Est, A., Bruce, D., Eds.; International Society of Photosynthesis: Monterreal, QC, Canada, 2005; pp. 660–662. [Google Scholar]
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Develop. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcus, V.L.; Prentice, E.J.; Hobbs, J.K.; Mulholland, A.J.; Van der Kamp, M.W.; Pudney, C.R.; Parker, E.J.; Schipper, L.A. On the temperature dependence of enzyme catalyzed rates. Biochemistry 2016, 55, 1681–1688. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, Q.; Shen, W.; Cram, D.; Fowler, D.B.; Wei, Y.; Zou, J. Understanding the biochemical basis of temperature-induced lipid pathway adjustments in plants. Plant Cell. 2015, 27, 86–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenova, G. Structural reorganization of thylakoid systems in response to heat treatment. Photosynthetica 2004, 42, 521–527. [Google Scholar] [CrossRef]
- Aro, E.; Virgin, I.; Andersson, B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1993, 1143, 113–134. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 2007, 1767, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Greer, D.H.; Weedon, M.M. Modelling photosynthetic responses to temperature of grapevine (Vitis vinifera cv. Semillon) leaves on vines grown in a hot climate. Plant Cell Environ. 2012, 35, 1050–1064. [Google Scholar] [CrossRef] [PubMed]
- Greer, D.H.; Weston, C. Heat stress affects flowering, berry growth, sugar accumulation and photosynthesis of Vitis vinifera cv. Semillon grapevines grown in a controlled environment. Func. Plant Biol. 2010, 37, 206–214. [Google Scholar] [CrossRef]
- Feller, U.; Crafts-Brandner, S.J.; Salvucci, M.E. Moderately high temperatures inhibit ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco) activase-mediated activation of rubisco. Plant Physiol. 1998, 116, 539–546. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Lu, Q.; Weng, X.; Zheng, B.; Xi, H. Regulation of rubisco carboxylation activity and photosynthetic rate by rubisco activase during leaf senescence in rice. J. Zhejiang Univ. (Agricult. Life Sci.) 1999, 26, 119–124. [Google Scholar]
- Salvucci, M.E.; Crafts-Brandner, S.J. Inhibition of photosynthesis by heat stress: The activation state of rubisco as a limiting factor in photosynthesis. Physiol. Plant 2004, 120, 179–186. [Google Scholar] [CrossRef]
- Demirevska-Kepova, K.; Holzer, R.; Simova-Stoilova, L.; Feller, U. Heat stress effects on ribulose-1, 5-bisphosphate carboxylase/oxygenase, rubisco binding protein and rubisco activase in wheat leaves. Biol. Plant. 2005, 49, 521–525. [Google Scholar] [CrossRef]
- Carmo-Silva, E.; Scales, J.C.; Madgwick, P.J.; Parry, M.A.J. Optimizing Rubisco and its regulation for greater resource use efficiency. Plant Cell Environ. 2015, 38, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Bracher, A.; Whitney, S.M.; Hartl, F.U.; Hayer-Hartl, M. Biogenesis and metabolic maintenance of Rubisco. Annu. Rev. Plant Biol. 2017, 68, 29–60. [Google Scholar] [CrossRef]
- Crafts-Brandner, S.J.; Salvucci, M.E. Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol. 2002, 129, 1773–1780. [Google Scholar] [CrossRef] [Green Version]
- Itzhak Kurek, O.; Kai Chang, T.; Bertain, S.M.; Madrigal, A.; Liu, L.; Lassner, M.W.; Zhu, G. Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell 2007, 19, 230–3241. [Google Scholar]
- Kumar, A.; Verma, J.P. Does plant-microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef]
- Long, S.P. Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: Has its importance been underestimated? Plant Cell Environ. 1991, 4, 729–739. [Google Scholar] [CrossRef]
- Ku, S.B.; Edwards, G.E. Oxygen inhibition of photosynthesis: I. Temperature dependence and relation to O2/CO2 solubility ratio. Plant Physiol. 1977, 59, 986–990. [Google Scholar] [CrossRef] [Green Version]
- Jordan, D.B.; Ogren, W.L. The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase. Planta 1984, 161, 308–313. [Google Scholar] [CrossRef]
- Ku, S.B.; Edwards, G.E. Oxygen inhibition of photosynthesis: II. Kinetic characteristics as affected by temperature. Plant Physiol. 1977, 59, 991–999. [Google Scholar] [CrossRef] [Green Version]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishorekumar, R.; Bulle, M.; Wany, A.; Gupta, K.J. An overview of important enzymes involved in nitrogen assimilation of plants. In Nitrogen Metabolism in Plants, Methods and Protocols; Gupta, K.J., Ed.; Springer Science-Business Media, LLC, Part of Springer Nature: New York, NY, USA, 2020; pp. 1–13. [Google Scholar]
- Stitt, M.; Krapp, A. The interaction between elevated carbon dioxide and nitrogen nutrition: The physiological and molecular background. Plant Cell Environ. 1999, 22, 583–621. [Google Scholar] [CrossRef]
- De La Mata, L.; De la Haba, P.; Alamillo, M.J.; Pineda, M.; Agüera, E. Elevated CO2 concentrations alter nitrogen metabolism and accelerate senescence in sunflower (Helianthus annuus L.) plants. Plant Soil Environ. 2013, 59, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Bloom, A.J.; Burger, M.; Rubio Asensio, J.S.; Cousins, A.B. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 2010, 328, 899–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igamberdiev, A.U.; Bykova, N.V.; Lea, P.J.; Gardeström, P. The role of photorespiration in redox and energy balance of photosynthetic plant cells: A study with a barley mutant deficient in glycine decarboxylase. Physiol. Plant. 2001, 111, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.; Gómez-García, I.; Fernández, E. Involvement of chloroplast and mitochondria redox valves in nitrate assimilation. Trends Plant Sci. 2000, 5, 463–464. [Google Scholar] [CrossRef]
- Mariscal, V.; Moulin, P.; Orsel, M.; Miller, A.J.; Fernández, E.; Galván, A. Differential regulation of the Chlamydomonas Nar1 gene family by carbon and nitrogen. Protist 2006, 157, 421–433. [Google Scholar] [CrossRef]
- Bloom, A.J.; Smart, D.R.; Nguyen, D.T.; Searles, P.S. Nitrogen assimilation and growth of wheat under elevated carbon dioxide. Proc. Natl. Acad. Sci. USA 2002, 99, 1730–1735. [Google Scholar] [CrossRef] [Green Version]
- Bernard, S.M.; Habash, D.Z. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol. 2009, 182, 608–620. [Google Scholar] [CrossRef]
- Vicente, R.; Martínez-Carrasco, R.; Pérez, P.; Morcuende, R. New insights into the impacts of elevated CO2, nitrogen, and temperature levels on the regulation of C and N metabolism in durum wheat using network analysis. Nat. Biotechnol. 2018, 40, 192–199. [Google Scholar] [CrossRef]
- Canales, F.J.; De la Haba, P.; Barrientos, E.; Aguera, E. Effect of CO2 enrichment and increased nitrogen supply on the induction of sunflower (Helianthus annuus L.) primary leaf senescence. Can. J. Plant. Sci. 2016, 96, 1002–1013. [Google Scholar]
- Bellido, E.; De la Haba, P.; Aguera, E. Physiological Alteration in sunflower plants (Helianthus annuus L.) exposed to high CO2 and arbuscular mycorrhizal fungi. Plants. 2021, 10, 937. [Google Scholar] [CrossRef] [PubMed]
- AbdElgawad, H.; Farfan-Vignolo, E.R.; de Vos, D.; Asard, H. Elevated CO2 mitigates drought and temperature induced oxidative stress differently in grasses and legumes. Plant Sci. 2015, 231, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, D.M.; Gao, Y.; Yu, B.; Xia, C.; Bai, J. Pretreatment with 5-aminolevulinic acid mitigates heat stress of cucumber leaves. Biol. Plant. 2012, 56, 780–784. [Google Scholar] [CrossRef]
- Foyer, C.H.; Harbinson, J.; Mullineaux, P. Oxygen metabolism and the regulation of photosynthetic electron transport. In Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants; Foyer, C.H., Mullineaux, P.M., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 1–42. [Google Scholar]
- Dat, J.F.; Lopez-Delgado, H.; Foyer, C.; Scott, I.M. Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol. 1998, 116, 1351–1357. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Li, J.; Zhang, X.; Wei, H.; Cui, L. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ. Exp. Bot. 2006, 56, 274–285. [Google Scholar] [CrossRef]
- Srivastava, S.; Pathak, A.D.; Gupta, P.S.; Shrivastava, A.K.; Srivastava, A.K. Hydrogen peroxide-scavenging enzymes impart tolerance to high temperature induced oxidative stress in surgarcane. Biol. J. Environ. 2012, 33, 656–661. [Google Scholar]
- Haba, P.; Amil-Ruiz, F.; Aguera, E. Physiological and proteomic characterization of the elevated temperature effect on sunflower (Helianthus annuus L.) primary leaves. Russian J. Plant Physiol. 2020, 67, 1094–1104. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agüera, E.; de la Haba, P. Climate Change Impacts on Sunflower (Helianthus annus L.) Plants. Plants 2021, 10, 2646. https://doi.org/10.3390/plants10122646
Agüera E, de la Haba P. Climate Change Impacts on Sunflower (Helianthus annus L.) Plants. Plants. 2021; 10(12):2646. https://doi.org/10.3390/plants10122646
Chicago/Turabian StyleAgüera, Eloísa, and Purificación de la Haba. 2021. "Climate Change Impacts on Sunflower (Helianthus annus L.) Plants" Plants 10, no. 12: 2646. https://doi.org/10.3390/plants10122646
APA StyleAgüera, E., & de la Haba, P. (2021). Climate Change Impacts on Sunflower (Helianthus annus L.) Plants. Plants, 10(12), 2646. https://doi.org/10.3390/plants10122646





