Chemical Genetics Applied to Elucidate the Physiological Role of Stress-Signaling Molecules on the Wound-Induced Accumulation of Glucosinolates in Broccoli
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Signaling Molecule Inhibitor Concentrations on Glucosinolate (GS) Accumulation
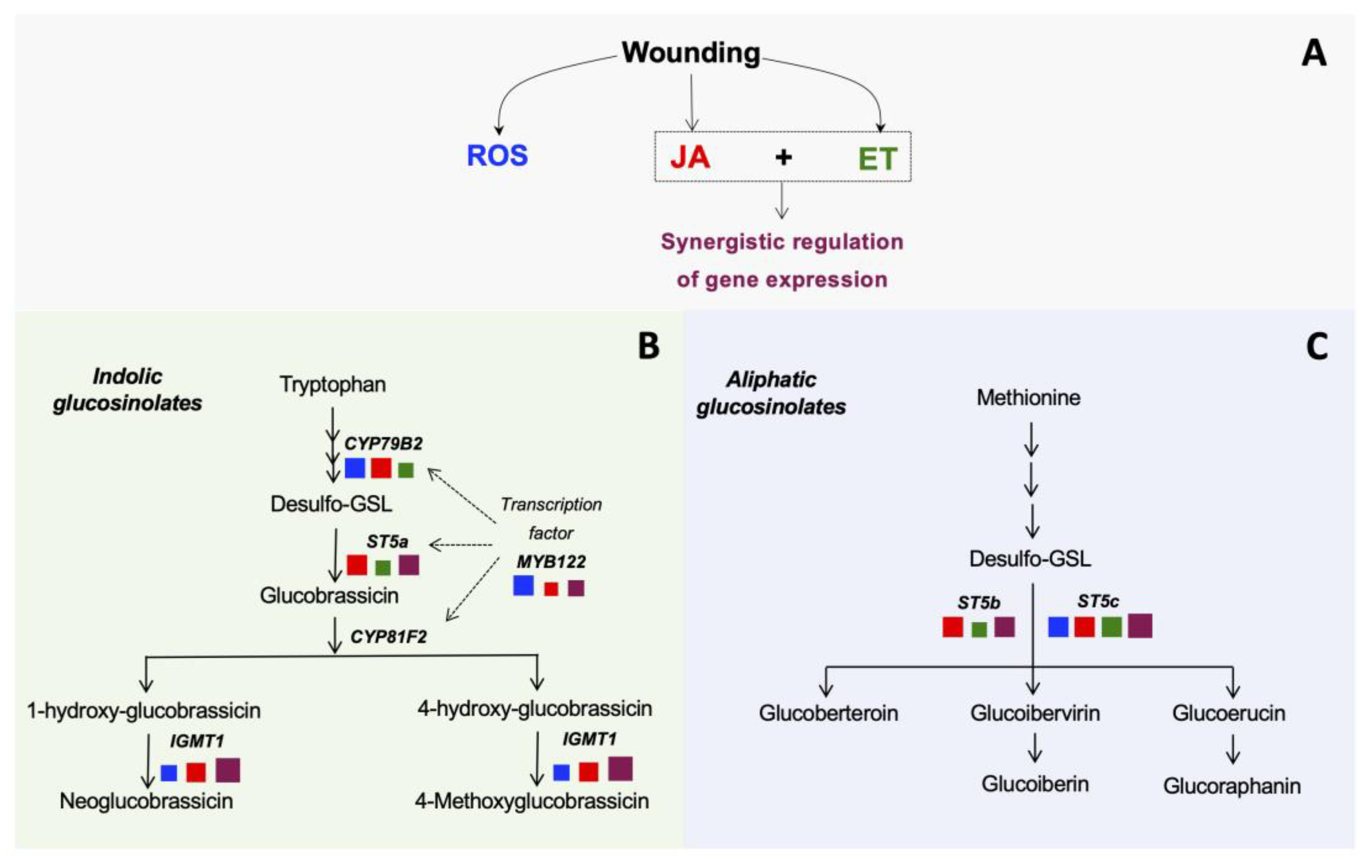
2.2. Role of JA, ET, and ROS on the Wound-Induced Activation of Glucosinolate Biosynthetic Genes
2.3. Role of JA, ET, and ROS on the Wound-Induced Accumulation of Glucosinolates
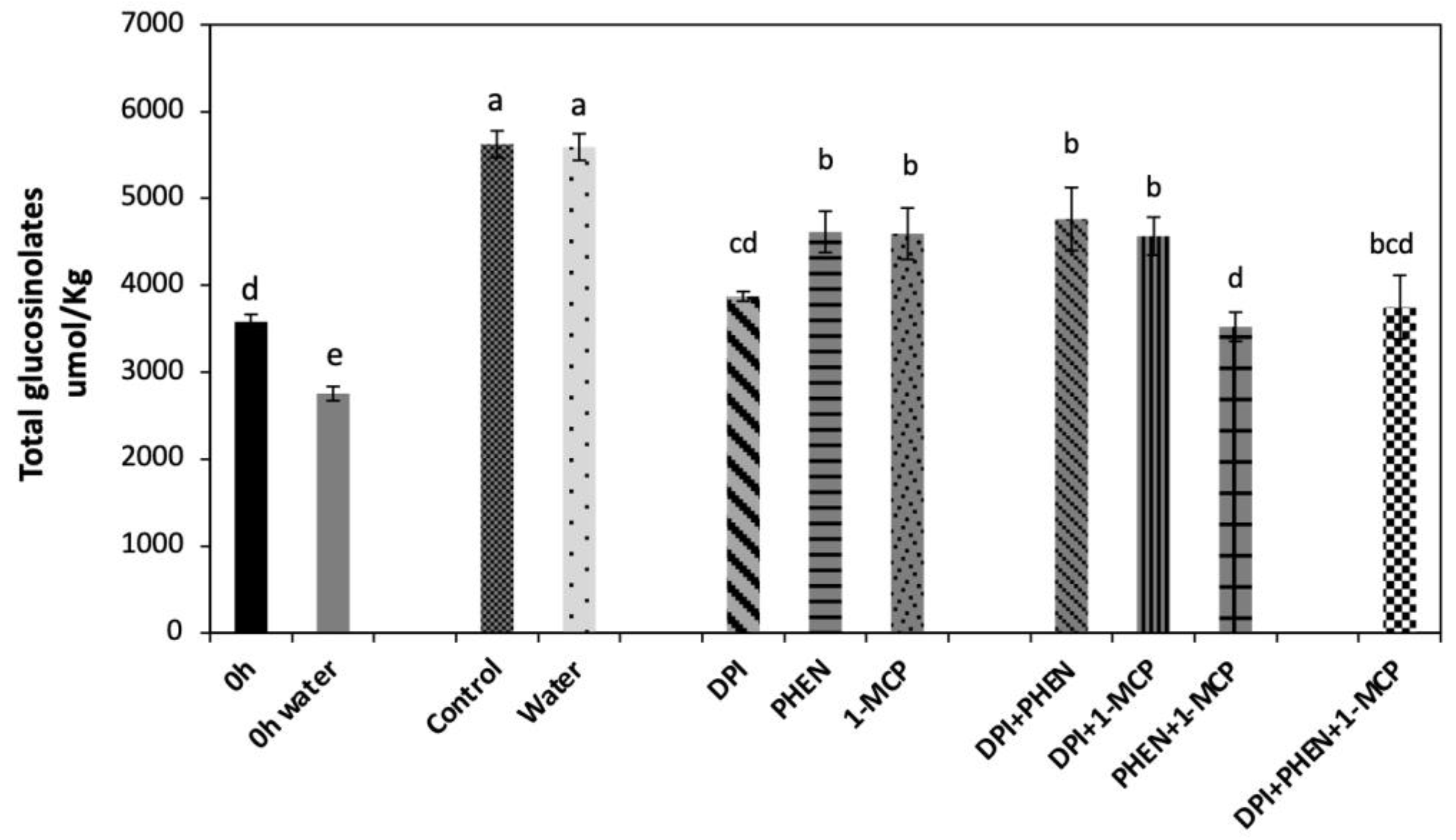
2.3.1. Total Glucosinolate Accumulation
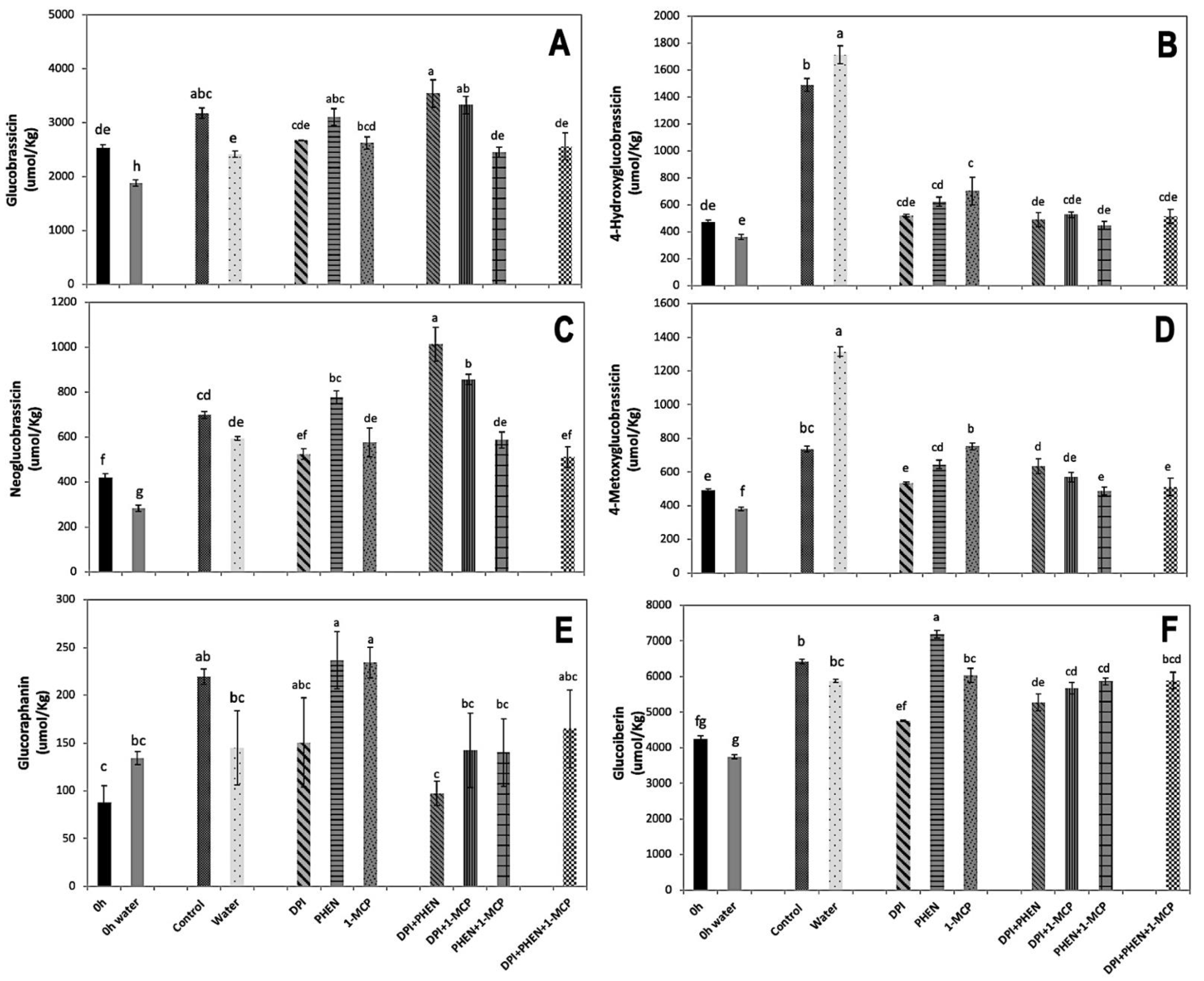
2.3.2. Individual Glucosinolate Accumulation
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material, Processing, and Storage of Broccoli Samples
3.3. Application of Stress-Signaling Molecule Inhibitors
3.4. RNA Extraction and Quantitative Real-Time Reverse Transcription-PCR (qRT-PCR)
3.5. Glucosinolate Analysis
3.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Villarreal-García, D.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as biofactories: Postharvest stress-induced accumulation of phenolic compounds and glucosinolates in broccoli subjected to wounding stress and exogenous phytohormones. Front. Plant Sci. 2016, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Stability of bioactive compounds in broccoli as affected by cutting styles and storage time. Molecules 2017, 22, 636. [Google Scholar] [CrossRef] [Green Version]
- Torres-Contreras, A.M.; Senés-Guerrero, C.; Pacheco, A.; González-Agüero, M.; Perla, A.; Ramos-Parra, P.A.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Genes differentially expressed in broccoli as an early and late response to wounding stress. Postharvest Biol. Technol. 2018, 145, 171–182. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, W.; Xu, Y.; Yang, X.; Ji, Y.; Feng, K.; Sarengaowa. Metabolomics and physiological analyses validates previous findings on the mechanism of response to wounding stress of different intensities in broccoli. Food Res. Int. 2021, 140, 110058. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Hu, W.; Xu, Y.; Sarengowa; Ji, Y.; Yang, X.; Feng, K. Proteomic analysis validates previous findings on wounding-responsive plant hormone signaling and primary metabolism contributing to the biosynthesis of secondary metabolites based on metabolomic analysis in harvested broccoli (Brassica oleracea L. var. italica). Food Res. Int. 2021, 145, 110388. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Controlled abiotic stresses revisited: From homeostasis through hormesis to extreme stresses and the impact on nutraceuticals and quality during pre-and postharvest applications in horticultural crops. J. Agric. Food Chem. 2020, 68, 11877–11879. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; González-Agüero, M.; Cisneros-Zevallos, L. Cross-talk between signaling pathways: The link between plant secondary metabolite production and wounding stress response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surjadinata, B.B.; Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Physiological role of reactive oxygen species, ethylene, and jasmonic acid on UV light induced phenolic biosynthesis in wounded carrot tissue. Postharvest Biol. Technol. 2021, 172, 111388. [Google Scholar] [CrossRef]
- Blackwell, H.E.; Zhao, Y. Chemical genetic approaches to plant biology. Plant Physiol. 2003, 133, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.F.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.G.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef]
- Heil, M.; Greiner, S.; Meimberg, H.; Krüger, R.; Noyer, J.-L.; Heubl, G.; Linsenmair, E.; Boland, W. Evolutionary change from induced to constitutive expression of an indirect plant resistance. Nature 2004, 430, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J. Selective inhibition of jasmonic acid accumulation by a small α, β-unsaturated carbonyl and phenidone reveals different modes of octadecanoid signalling activation in response to insect elicitors and green leaf volatiles in Zea mays. BMC Res. Notes 2011, 4, 377. [Google Scholar] [CrossRef] [Green Version]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.; Dong, C.; Terry, L.A.; Watkins, C.B.; Yu, Z.; Cheng, Z.-M.M. Meta-analysis of the effects of 1-methylcyclopropene (1-MCP) treatment on climacteric fruit ripening. Hortic. Res. 2020, 7, 208. [Google Scholar] [CrossRef]
- Mikkelsen, M.D.; Hansen, C.H.; Wittstock, U.; Halkier, B.A. Cytochrome P450 CYP79B2 from Arabidopsis catalyzes the conversion of tryptophan to indole-3-acetaldoxime, a precursor of indole glucosinolates and indole-3-acetic acid. J. Biol. Chem. 2000, 275, 33712–33717. [Google Scholar] [CrossRef] [Green Version]
- Sønderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of glucosinolates–gene discovery and beyond. Trends Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Frerigmann, H.; Gigolashvili, T. MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Mol. Plant 2014, 7, 814–828. [Google Scholar] [CrossRef] [Green Version]
- Pfalz, M.; Mikkelsen, M.D.; Bednarek, P.; Olsen, C.E.; Halkier, B.A.; Kroymann, J. Metabolic engineering in Nicotiana benthamiana reveals key enzyme functions in Arabidopsis indole glucosinolate modification. Plant Cell 2011, 23, 716–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Pujante, P.J.; Sabater-Jara, A.B.; Belchí-Navarro, S.; Pedreño, M.A.; Almagro, L. Increased glucosinolate production in Brassica oleracea var. italica cell cultures due to coronatine activated genes involved in glucosinolate biosynthesis. J. Agric. Food Chem. 2018, 67, 102–111. [Google Scholar] [CrossRef]
- Ku, K.M.; Choi, J.H.; Kim, H.S.; Kushad, M.M.; Jeffery, E.H.; Juvik, J.A. Methyl jasmonate and 1-methylcyclopropene treatment effects on quinone reductase inducing activity and post-harvest quality of broccoli. PLoS ONE 2013, 8, e77127. [Google Scholar] [CrossRef]
- Klein, M.; Papenbrock, J. Kinetics and substrate specificities of desulfo-glucosinolate sulfotransferases in Arabidopsis thaliana. Physiol. Plant. 2009, 135, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Reichelt, M.; Gershenzon, J.; Papenbrock, J. The three desulfoglucosinolate sulfotransferase proteins in Arabidopsis have different substrate specificities and are differentially expressed. FEBS J. 2006, 273, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, M.; Ding, X.; Chu, Z.; Liu, H. Activating the MYB51 and MYB122 to upregulate the transcription of glucosinolates biosynthesis genes by copper ions in Arabidopsis. Plant Physiol. Biochem. 2021, 162, 496–505. [Google Scholar] [CrossRef]
- Clarke, D.B. Glucosinolates, structures and analysis in food. Anal. Methods 2010, 2, 310–325. [Google Scholar] [CrossRef]
- Aguilar-Camacho, M.; Welti-Chanes, J.; Jacobo-Velázquez, D.A. Combined effect of ultrasound treatment and exogenous phytohormones on the accumulation of bioactive compounds in broccoli florets. Ultrason. Sonochem. 2019, 50, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.D.; Petersen, B.L.; Glawischnig, E.; Jensen, A.B.; Andreasson, E.; Halkier, B.A. Modulation of CYP79 genes and glucosinolate profiles in Arabidopsis by defense signaling pathways. Plant Phys. 2003, 131, 298–308. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.G.; Juvik, J.A.; Jeffery, E.H.; Berman-Booty, L.D.; Clinton, S.K.; Erdman, J.W.J. Enhancement of broccoli indole glucosinolates by methyl jasmonate treatment and effects on prostate carcinogenesis. J. Med. Food 2014, 17, 1177–1182. [Google Scholar] [CrossRef]
- Yi, G.-E.; Robin, A.H.K.; Yang, K.; Park, J.-I.; Hwang, B.H.; Nou, I.-S. Exogenous methyl jasmonate and salicylic acid induce subspecies-specific patterns of glucosinolate accumulation and gene expression in Brassica oleracea L. Molecules 2016, 21, 1417. [Google Scholar] [CrossRef] [Green Version]
- Natella, F.; Maldini, M.; Nardini, M.; Azzini, E.; Foddai, M.S.; Giusti, A.M.; Baima, S.; Morelli, G.; Scaccini, C. Improvement of the nutraceutical quality of broccoli sprouts by elicitation. Food Chem. 2016, 201, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB light, and methyl jasmonate, alone or combined, redirect the biosynthesis of glucosinolates, phenolics, carotenoids, and chlorophylls in broccoli sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef] [Green Version]
- Wan, C.Y.; Wilkins, T.A. A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.). Anal. Biochem. 1994, 223, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Salzman, R.A.; Brady, J.A.; Finlayson, S.A.; Buchanan, C.D.; Summer, E.J.; Sun, F.; Klein, P.E.; Klein, R.R.; Pratt, L.; Cordonnier-Pratt, M.M.; et al. Transcriptional profiling of sorghum induced by methyl jasmonate, salicylic acid, and aminocyclopropane carboxylic acid reveals cooperative regulation and novel gene responses. Plant Physiol. 2005, 138, 352–368. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kiddle, G.; Bennett, R.N.; Botting, N.P.; Davidson, N.E.; Robertson, A.A.B.; Wallsgrove, R.M. High-performance liquid chromatographic separation of natural and synthetic desulphoglucosinolates and their chemical validation by UV, NMR and chemical ionisation-MS methods. Phytochem. Anal. 2001, 12, 226–242. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Glucosinolate Concentration (μmol/Kg DW) abc | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucoraphanin | 4-Hydroxyglucobrassicin | Glucobrassicin | 4-Metoxyglucobrassicin | Neoglucobrassicin | Total | |||||||
| 0 h | 10502.5 ± 748.1 | def | 1670.2 ± 93.7 | hi | 8157.7 ± 615.7 | bc | 1914.9 ± 122.9 | f | 4207.1 ± 579.5 | a | 22245.4 ± 1472.8 | efg |
| 0 h water | 10129.6 ± 1140.2 | ef | 1180.7 ± 165.4 | i | 7273.5 ± 556.6 | cde | 1518.9 ± 112.8 | gh | 3337.7 ± 485.1 | ab | 20102.8 ± 1669.0 | fg |
| Control | 13255.3 ± 104.9 | b | 4423.6 ± 373.5 | a | 9425.1 ± 604.4 | a | 2503.2 ± 153.7 | cde | 4010.7 ± 272.1 | a | 29607.2 ± 1214.5 | a |
| Water | 11443.9 ± 454.0 | cde | 4194.9 ± 92.8 | ab | 7367.8 ± 114.5 | cd | 2881.7 ± 3.2 | ab | 3703.8 ± 284.9 | ab | 25887.4 ± 358.7 | bc |
| 1-MCP 500ppb | 12813.7 ± 322.6 | bc | 4158.9 ± 106.4 | abc | 8470.9 ± 188.0 | ab | 2628.0 ± 63.7 | abcd | 1679.8 ± 386.4 | c | 28071.5 ± 635.1 | ab |
| 1-MCP 1000 ppb | 15115.9 ± 835.7 | a | 3681.9 ± 141.2 | bcde | 8802.3 ± 291.8 | ab | 1734.8 ± 44.5 | fg | 2306.5 ± 589.6 | b | 29334.9 ± 814.8 | a |
| 1-MCP 2000 ppb | 8964.4 ± 336.8 | fg | 1250.4 ± 63.5 | i | 7204.1 ± 35.4 | cde | 2451.3 ± 14.0 | cde | 3159.7 ± 666.3 | abc | 22583.1 ± 356.6 | def |
| DPI 3.17 μM | 12041.6 ± 95.9 | bcd | 3348.9 ± 103.6 | de | 6729.9 ± 38.1 | de | 2587.2 ± 34.4 | bcd | 3458.4 ± 232.1 | ab | 24707.6 ± 233.1 | cde |
| DPI 31.7 μM | 9941.5 ± 126.5 | ef | 2223.1 ± 163.4 | gh | 6734.9 ± 146.5 | de | 2266.9 ± 46.2 | e | 2650.9 ± 929.9 | abc | 21166.4 ± 220.9 | fg |
| DPI 317 μM | 15843.6 ± 538.4 | a | 1100.4 ± 33.3 | i | 7227.0 ± 131.4 | cde | 1417.9 ± 32.4 | h | 3160.8 ± 468.3 | abc | 25588.9 ± 658.6 | def |
| PHEN 0.1 mM | 13131.7 ± 625.2 | b | 3505.8 ± 422.0 | cde | 6181.7 ± 329.6 | ef | 2751.2 ± 183.8 | abc | 2272.1 ± 235.9 | bc | 25570.5 ± 1431.5 | bcd |
| PHEN 1.0 mM | 8220.8 ± 436.8 | g | 3038.1 ± 246.6 | ef | 5624.4 ± 186.7 | f | 2383.8 ± 110.5 | de | 3255.6 ± 281.6 | abc | 19267.1 ± 888.9 | g |
| PHEN 10 mM | 15843.5 ± 538.3 | a | 2569.4 ± 77.9 | fg | 4479.5 ± 77.4 | g | 2903.8 ± 26.3 | a | 3232.7 ± 257.8 | abc | 25144.9 ± 356.2 | bcde |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Contreras, A.M.; Nair, V.; Senés-Guerrero, C.; Pacheco, A.; González-Agüero, M.; Ramos-Parra, P.A.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chemical Genetics Applied to Elucidate the Physiological Role of Stress-Signaling Molecules on the Wound-Induced Accumulation of Glucosinolates in Broccoli. Plants 2021, 10, 2660. https://doi.org/10.3390/plants10122660
Torres-Contreras AM, Nair V, Senés-Guerrero C, Pacheco A, González-Agüero M, Ramos-Parra PA, Cisneros-Zevallos L, Jacobo-Velázquez DA. Chemical Genetics Applied to Elucidate the Physiological Role of Stress-Signaling Molecules on the Wound-Induced Accumulation of Glucosinolates in Broccoli. Plants. 2021; 10(12):2660. https://doi.org/10.3390/plants10122660
Chicago/Turabian StyleTorres-Contreras, Ana M., Vimal Nair, Carolina Senés-Guerrero, Adriana Pacheco, Mauricio González-Agüero, Perla A. Ramos-Parra, Luis Cisneros-Zevallos, and Daniel A. Jacobo-Velázquez. 2021. "Chemical Genetics Applied to Elucidate the Physiological Role of Stress-Signaling Molecules on the Wound-Induced Accumulation of Glucosinolates in Broccoli" Plants 10, no. 12: 2660. https://doi.org/10.3390/plants10122660
APA StyleTorres-Contreras, A. M., Nair, V., Senés-Guerrero, C., Pacheco, A., González-Agüero, M., Ramos-Parra, P. A., Cisneros-Zevallos, L., & Jacobo-Velázquez, D. A. (2021). Chemical Genetics Applied to Elucidate the Physiological Role of Stress-Signaling Molecules on the Wound-Induced Accumulation of Glucosinolates in Broccoli. Plants, 10(12), 2660. https://doi.org/10.3390/plants10122660








