Anti-Inflammatory and Anticancer Properties of Birch Bark-Derived Betulin: Recent Developments
Abstract
:1. Introduction
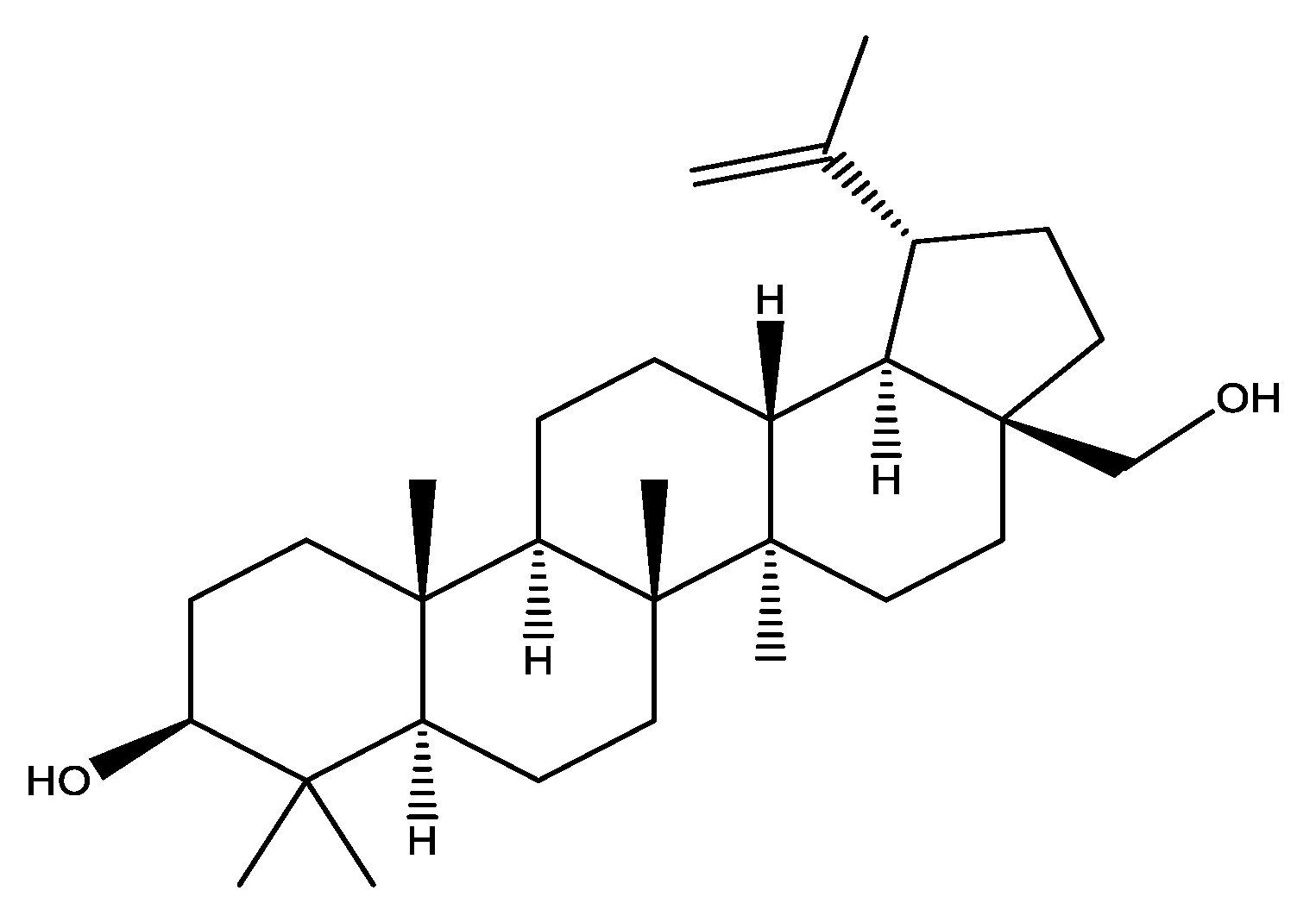
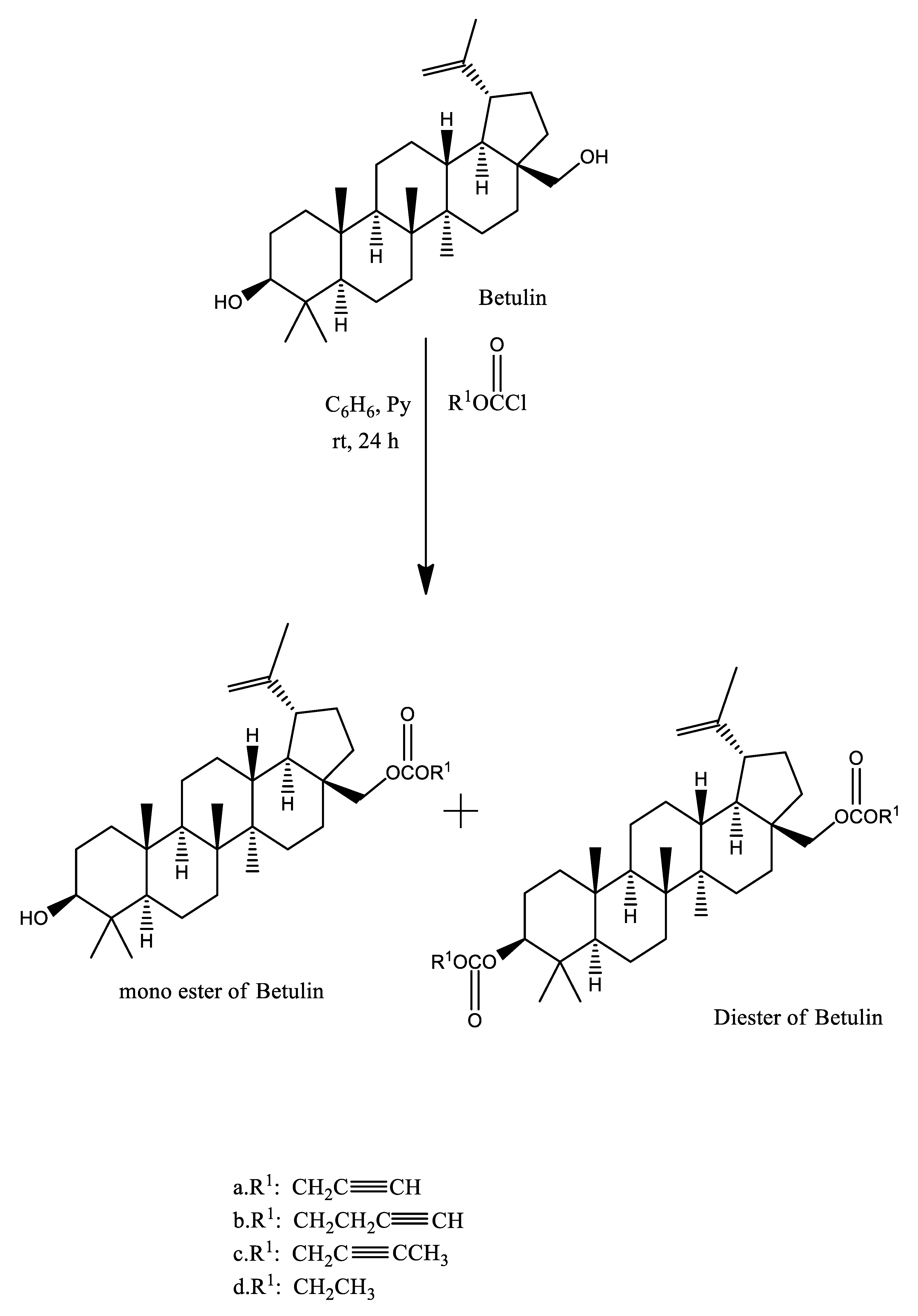
2. Chemistry of Betulin
3. Absorption, Metabolic Conversion, and Bioavailability of Betulin
4. Anti-Inflammatory Mechanisms Involved in Anticancer Action of Betulin
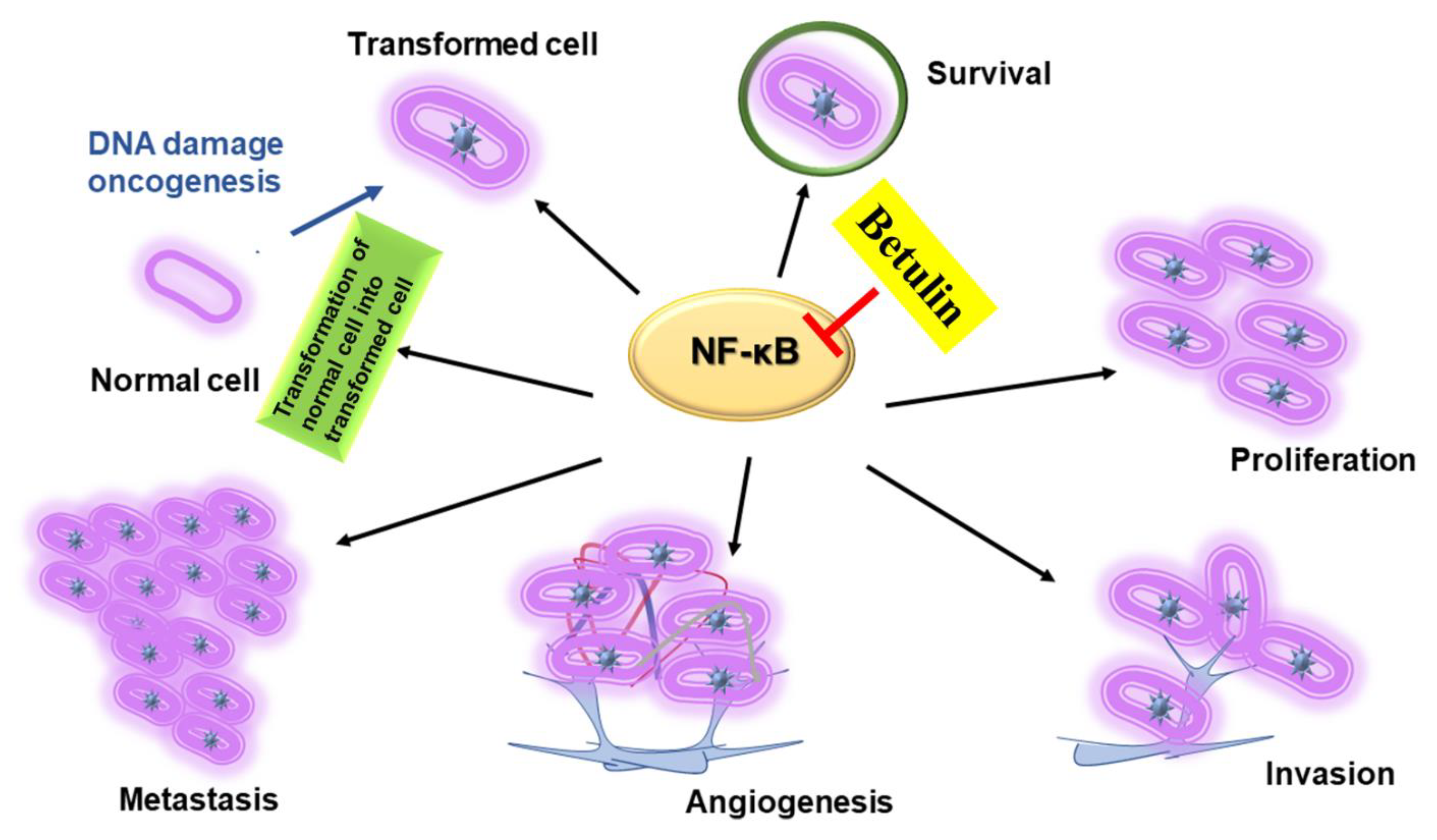
4.1. NF-kB-Mediated Signaling
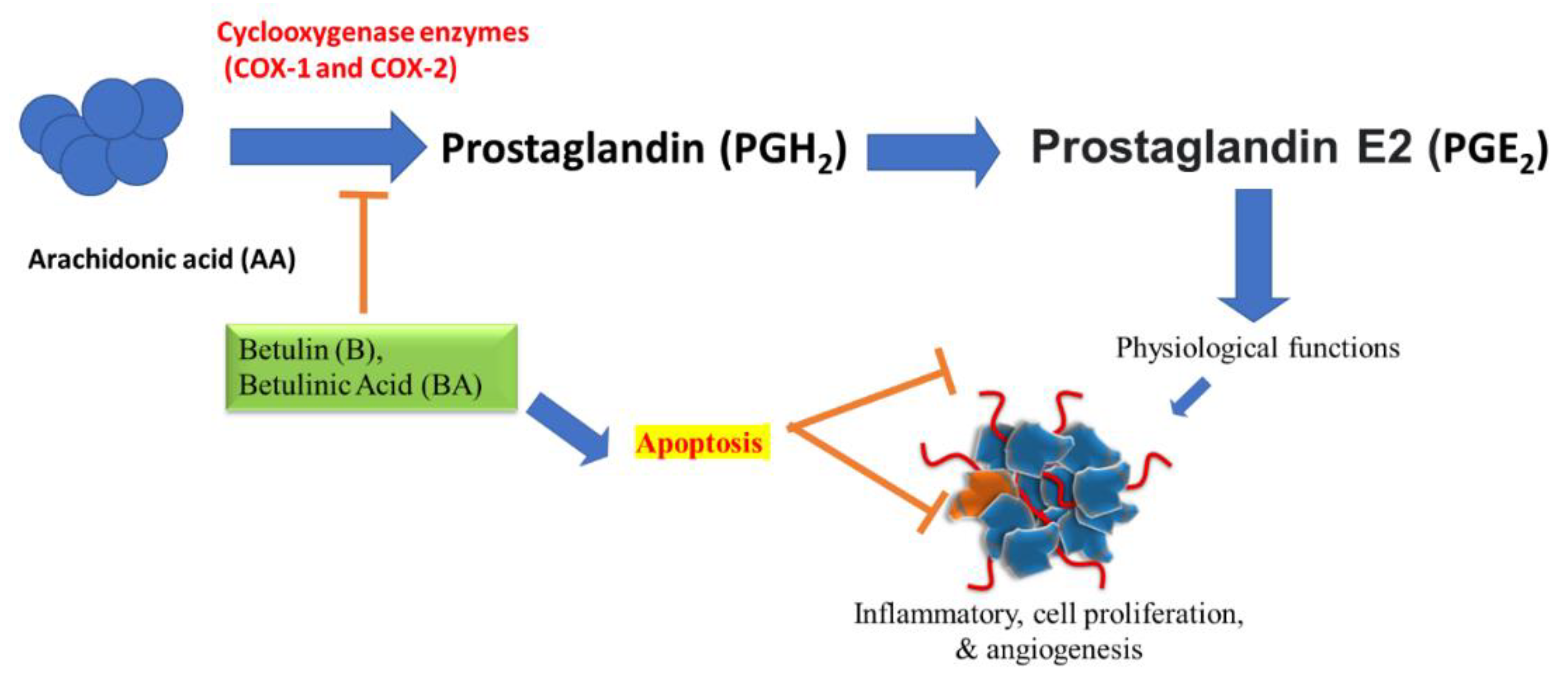
4.2. Prostaglandin/COX-2-Regulated Inflammatory Events
4.3. Nrf2-Associated Signaling
5. Betulin as a Treatment Strategy for Cancer
5.1. Co-Effects of Betulin with Standard Anticancer Therapies
5.2. Role of Nanotechnology in Delivery of Betulin to Target Tissues
5.3. Safety Issue of Betulin
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gullo, V.P.; McAlpine, J.; Lam, K.S.; Baker, D.; Petersen, F. Drug discovery from natural products. J. Ind. Microbiol. Biotechnol. 2006, 33, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Verma, S.S.; Rai, V.; Awasthee, N.; Chava, S.; Hui, K.M.; Kumar, A.P.; Challagundla, K.B.; Sethi, G.; Gupta, S.C. Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases. Cell. Mol. Life Sci. 2019, 76, 1947–1966. [Google Scholar] [CrossRef]
- Tewari, D.; Nabavi, S.F.; Nabavi, S.M.; Sureda, A.; Farooqi, A.A.; Atanasov, A.G.; Vacca, R.A.; Sethi, G.; Bishayee, A. Targeting activator protein 1 signaling pathway by bioactive natural agents: Possible therapeutic strategy for cancer prevention and intervention. Pharmacol. Res. 2018, 128, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.M.; Nagulapalli Venkata, K.C.; Bhattacharyya, P.; Sethi, G.; Bishayee, A. Potential of neem (Azadirachta indica L.) for prevention and treatment of oncologic diseases. Semin. Cancer Biol. 2016, 40–41, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Arellano, E.; Díaz, V.; Rodríguez, J. Current status and future directions in unresectable stage III non-small cell lung cancer. J. Clin. Transl. Res. 2020, 6, 109–120. [Google Scholar] [CrossRef]
- De Dios, N.; Murcia-Mejía, M. Current and future strategies in radiotherapy for small-cell lung cancer. J. Clin. Transl. Res. 2020, 6, 97–108. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Sandberg, E.N.; Goel, N.; Bishayee, A. Oncogenic and tumor suppressive components of the cell cycle in breast cancer progression and prognosis. Pharmaceutics 2021, 13, 569. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Garg, V.K.; Goel, N.; Bishayee, A. Oncogenic and Tumor-Suppressive Roles of MicroRNAs with Special Reference to Apoptosis: Molecular Mechanisms and Therapeutic Potential. Mol. Diagn. Ther. 2018, 22, 179–201. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Goel, N. Intrinsic and Extrinsic Pathways of Apoptosis: Role in Cancer Development and Prognosis. Adv. Protein. Chem. Struct. Biol. 2021, 125, 73–120. [Google Scholar] [PubMed]
- Aggarwal, V.; Kashyap, D.; Sak, K.; Tuli, H.S.; Jain, A.; Chaudhary, A.; Garg, V.K.; Sethi, G.; Yerer, M.B. Molecular mechanisms of action of tocotrienols in cancer: Recent trends and advancements. Int. J. Mol. Sci. 2019, 20, 656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashyap, D.; Garg, V.K.; Tuli, H.S.; Yerer, M.B.; Sak, K.; Sharma, A.K.; Kumar, M.; Aggarwal, V.; Sandhu, S.S. Fisetin and quercetin: Promising flavonoids with chemopreventive potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, D.; Tuli, H.S.; Garg, V.K.; Bhatnagar, S.; Sharma, A.K. Ursolic acid and quercetin: Promising anticancer phytochemicals with antimetastatic and antiangiogenic potential. Tumor Microenviron. 2018, 1, 9–15. [Google Scholar] [CrossRef]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular mechanisms of action of genistein in cancer: Recent advances. Front. Pharmacol. 2019, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, D.; Tuli, H.S.; Sharma, A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016, 146, 201–213. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Mukherjee, T.; Bishayee, A. Molecular targets of celastrol in cancer: Recent trends and advancements. Crit. Rev. Oncol. Hematol. 2018, 128, 70–81. [Google Scholar] [CrossRef]
- Tuli, H.S.; Yerer, M.B.; Sak, K. Editorial: Current aspects in chemopreventive strategies. Front. Pharmacol. 2021, 11, 2020–2021. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef]
- Fulda, S. Betulinic Acid for cancer treatment and prevention. Int. J. Mol. Sci. 2008, 9, 1096–1107. [Google Scholar] [CrossRef] [Green Version]
- Blondeau, D.; St-Pierre, A.; Bourdeau, N.; Bley, J.; Lajeunesse, A.; Desgagné-Penix, I. Antimicrobial activity and chemical composition of white birch (Betula papyrifera Marshall) bark extracts. Microbiologyopen 2020, 9, e00944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwiebs, A.; Radeke, H.H. Immunopharmacological Activity of Betulin in Inflammation-associated Carcinogenesis. Anticancer. Agents Med. Chem. 2017, 18, 645–651. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef] [Green Version]
- Green, B.; Bentley, M.D.; Chung, B.Y.; Lynch, N.G.; Jensen, B.L. Isolation of betulin and rearrangement to allobetulin. A biomimetic natural product synthesis. J. Chem. Educ. 2007, 84, 1985–1987. [Google Scholar] [CrossRef]
- Bȩbenek, E.; Jastrzȩbska, M.; Kadela-Tomanek, M.; Chrobak, E.; Orzechowska, B.; Zwolińska, K.; Latocha, M.; Mertas, A.; Czuba, Z.; Boryczka, S. Novel triazole hybrids of betulin: Synthesis and biological activity profile. Molecules 2017, 22, 1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boryczka, S.; Bebenek, E.; Wietrzyk, J.; Kempińska, K.; Jastrzebska, M.; Kusz, J.; Nowak, M. Synthesis, structure and cytotoxic activity of new acetylenic derivatives of betulin. Molecules 2013, 18, 4526–4543. [Google Scholar] [CrossRef] [Green Version]
- Mullauer, F.B.; Kessler, J.H.; Medema, J.P. Betulinic acid, a natural compound with potent anticancer effects. Anticancer. Drugs 2010, 21, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.H.L.; Chen, Z.; Van Nguyen, T.; Pezzuto, J.M.; Qiu, S.; Lu, Z.Z. A concise semi-synthetic approach to betulinic acid from betulin. Synth. Commun. 1997, 27, 1607–1612. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Z.; Luo, S.; James, M.O.; Wang, Y. Phase II metabolism of betulin by rat and human UDP-glucuronosyltransferases and sulfotransferases. Chem. Biol. Interact. 2019, 302, 190–195. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Jin, M.; Wang, Q.; Sun, Q.; Du, Y.; Cao, L.; Xu, H. UHPLC-Q-TOF-MS/MS based screening and identification of the metabolites in vivo after oral administration of betulin. Fitoterapia 2018, 127, 29–41. [Google Scholar] [CrossRef]
- Zichri, S.B.; Kolusheva, S.; Shames, A.I.; Schneiderman, E.A.; Poggio, J.L.; Stein, D.E.; Doubijensky, E.; Levy, D.; Orynbayeva, Z.; Jelinek, R. Mitochondria membrane transformations in colon and prostate cancer and their biological implications. Biochim. Biophys. Acta Biomembr. 2021, 1863, 18347. [Google Scholar] [CrossRef]
- Drag-Zalesińska, M.; Drag, M.; Poreba, M.; Borska, S.; Kulbacka, J.; Saczko, J. Anticancer properties of ester derivatives of betulin in human metastatic melanoma cells (Me-45). Cancer Cell Int. 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bębenek, E.; Chrobak, E.; Marciniec, K.; Kadela-Tomanek, M.; Trynda, J.; Wietrzyk, J.; Boryczka, S. Biological activity and in silico study of 3-modified derivatives of betulin and betulinic aldehyde. Int. J. Mol. Sci. 2019, 20, 1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puar, Y.R.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; Sethi, G.; Tergaonkar, V. Evidence for the involvement of the master transcription factor NF-κB in cancer initiation and progression. Biomedicines 2018, 6, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwang, S.A.; Sethi, G.; Jain, A.K.; Jaiswal, A.K.; Aggarwal, B.B. Genetic deletion of NAD(P)H:quinone oxidoreductase 1 abrogates activation of nuclear factor-κB, IκBα kinase, c-Jun N-terminal kinase, Akt, p38, and p44/42 mitogen-activated protein kinases and potentiates apoptosis. J. Biol. Chem. 2006, 281, 19798–19808. [Google Scholar] [CrossRef] [Green Version]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Siveen, K.S.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Arfuso, F.; Kumar, A.P.; et al. Isorhamnetin augments the anti-tumor effect of capeciatbine through the negative regulation of NF-κB signaling cascade in gastric cancer. Cancer Lett. 2015, 363, 28–36. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Ong, T.H.; Kumar, A.P.; Lun, C.K.; Ho, P.C.; Wong, P.T.H.; Hui, K.M.; Sethi, G. Ursolic acid inhibits the initiation, progression of prostate cancer and prolongs the survival of TRAMP mice by modulating pro-inflammatory pathways. PLoS ONE 2012, 7, e32476. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Li, F.; Chen, L.; Siveen, K.S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J. Mol. Med. 2014, 92, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Shanmugam, M.K.; Chen, L.; Chatterjee, S.; Basha, J.; Kumar, A.P.; Kundu, T.K.; Sethi, G. Garcinol, a polyisoprenylated benzophenone modulates multiple proinfl ammatory signaling cascades leading to the suppression of growth and survival of head and neck carcinoma. Cancer Prev. Res. 2013, 6, 843–854. [Google Scholar] [CrossRef] [Green Version]
- Matilla, J.; Zabaleta, M.; Martínez-Téllez, E.; Abal, J.; Rodríguez-Fuster, A.; Hernández-Hernández, J. New TNM staging in lung cancer (8th edition) and future perspectives. J. Clin. Transl. Res. 2020, 6, 145–154. [Google Scholar] [CrossRef]
- Maqueda, L.; Falcón, R.; Tsai, C.; García-Pérez, A.; Minasyan, A.; Gonzalez-Rivas, D. Current role of Uniportal Video Assisted Thoracic Surgery for lung cancer treatment. J. Clin. Transl. Res. 2020, 6, 135–144. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A. Betulin and betulinic acid in cancer research. J. Pre-Clin. Clin. Res. 2018, 12, 72–75. [Google Scholar] [CrossRef]
- Mastron, J.K.; Siveen, K.S.; Sethi, G.; Bishayee, A. Silymarin and hepatocellular carcinoma: A systematic, comprehensive, and critical review. Anticancer Drugs 2014, 26, 475–486. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Ho, P.C.L.; Wong, F.C.; Sethi, G.; Wang, L.Z.; Goh, B.C. Garcinol: Current status of its anti-oxidative, anti-inflammatory and anti-cancer effects. Cancer Lett. 2015, 362, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.; Kim, S.H.; Sethi, G.; Ahn, K.S. Farnesol inhibits tumor growth and enhances the anticancer effects of bortezomib in multiple myeloma xenograft mouse model through the modulation of STAT3 signaling pathway. Cancer Lett. 2015, 360, 280–293. [Google Scholar] [CrossRef]
- Rzeski, W.; Stepulak, A.; Szymański, M.; Sifringer, M.; Kaczor, J.; Wejksza, K.; Zdzisińska, B.; Kandefer-Szerszeń, M. Betulinic acid decreases expression of bcl-2 and cyclin D1, inhibits proliferation, migration and induces apoptosis in cancer cells. Naunyn. Schmiedebergs. Arch. Pharmacol. 2006, 374, 11–20. [Google Scholar] [CrossRef]
- Zhao, J.; Li, R.; Pawlak, A.; Henklewska, M.; Sysak, A.; Wen, L.; Yi, J.E.; Obmińska-Mrukowicz, B. Antitumor activity of betulinic acid and betulin in canine cancer cell lines. In Vivo 2018, 32, 1081–1088. [Google Scholar] [CrossRef] [Green Version]
- Rzeski, W.; Stepulak, A.; Szymański, M.; Juszczak, M.; Grabarska, A.; Sifringer, M.; Kaczor, J.; Kandefer-Szerszeń, M. Betulin Elicits Anti-Cancer Effects in Tumour Primary Cultures and Cell Lines in Vitro. Basic Clin. Pharmacol. Toxicol. 2009, 105, 425–432. [Google Scholar] [CrossRef] [PubMed]
- PA, K. Birch bark research and development. Nat. Prod. Rep. 2006, 23, 919–942. [Google Scholar] [CrossRef]
- Tang, J.J.; Li, J.G.; Qi, W.; Qiu, W.W.; Li, P.S.; Li, B.L.; Song, B.L. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell Metab. 2011, 13, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Van Loo, G.; Saelens, X.; Van Gurp, M.; MacFarlane, M.; Martin, S.J.; Vandenabeele, P. The role of mitochondrial factors in apoptosis: A Russian roulette with more than one bullet. Cell Death Differ. 2002, 9, 1031–1042. [Google Scholar] [CrossRef]
- Ahn, K.S.; Sethi, G.; Aggarwal, B.B. Reversal of chemoresistance and enhancement of apoptosis by statins through down-regulation of the NF-κB pathway. Biochem. Pharmacol. 2008, 75, 907–913. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Wang, L.; Deivasigamni, A.; Looi, C.Y.; Karthikeyan, C.; Trivedi, P.; Chinnathambi, A.; Alharbi, S.A.; Arfuso, F.; Dharmarajan, A.; et al. A novel benzimidazole derivative, MBIC inhibits tumor growth and promotes apoptosis via activation of ROS-dependent JNK signaling pathway in hepatocellular carcinoma. Oncotarget 2017, 8, 12831–12842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Kan, H.; Yinghui, H.; Daxin, Z.; Chang, G.; Lin, C.; Ying Hua, J. Betulin induces mitochondrial cytochrome c release associated apoptosis in human cancer cells. Mol. Carcinog. 2010, 49, 630–640. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Jiang, D.; Lin, Y.; Wang, Y.; Li, Q.; Liu, L.; Jin, Y.H. Betulin induces reactive oxygen species-dependent apoptosis in human gastric cancer SGC7901 cells. Arch. Pharm. Res. 2016, 39, 1257–1265. [Google Scholar] [CrossRef]
- Takada, Y.; Aggarwal, B.B. Betulinic Acid Suppresses Carcinogen-Induced NF-κB Activation Through Inhibition of IκBα Kinase and p65 Phosphorylation: Abrogation of Cyclooxygenase-2 and Matrix Metalloprotease-9. J. Immunol. 2003, 171, 3278–3286. [Google Scholar] [CrossRef] [Green Version]
- Anaya-Eugenio, G.D.; Eggers, N.A.; Ren, Y.; Rivera-Chávez, J.; Douglas Kinghorn, A.; Carcache De Blanco, E.J. Apoptosis induced by (+)-Betulin through NF-ĸB inhibition in MDA-MB-231 breast cancer cells. Anticancer Res. 2020, 40, 6637–6647. [Google Scholar] [CrossRef] [PubMed]
- Zeldin, D.C. Epoxygenase pathways of arachidonic acid metabolism. J. Biol. Chem. 2001, 276, 36059–36062. [Google Scholar] [CrossRef] [Green Version]
- Yarla, N.S.; Bishayee, A.; Sethi, G.; Reddanna, P.; Kalle, A.M.; Dhananjaya, B.L.; Dowluru, K.S.V.G.K.; Chintala, R.; Duddukuri, G.R. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin. Cancer Biol. 2016, 40–41, 48–81. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.M.; Ribeiro, D.; Silva, A.M.S.; Fernandes, E. 2,3-Diarylxanthones as potential inhibitors of arachidonic acid metabolic pathways. Inflammation 2017, 40, 956–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, Y.; Inazumi, T.; Tsuchiya, S. Roles of prostaglandin receptors in female reproduction. J. Biochem. 2015, 157, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Shih, R.H.; Wang, C.Y.; Yang, C.M. NF-kappaB signaling pathways in neurological inflammation: A mini review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aid, S.; Langenbach, R.; Bosetti, F. Neuroinflammatory response to lipopolysaccharide is exacerbated in mice genetically deficient in cyclooxygenase-2. J. Neuroinflamm. 2008, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Morita, I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002, 68–69, 165–175. [Google Scholar] [CrossRef]
- Kurumbail, R.G.; Kiefer, J.R.; Marnett, L.J. Cyclooxygenase enzymes: Catalysis and inhibition. Curr. Opin. Struct. Biol. 2001, 11, 752–760. [Google Scholar] [CrossRef]
- Langenbach, R.; Loftin, C.; Lee, C.; Tiano, H. Cyclooxygenase knockout mice Models for elucidating isoform-specific functions. Biochem. Pharmacol. 1999, 58, 1237–1246. [Google Scholar] [CrossRef]
- Vadlamudi, R.; Mandal, M.; Adam, L.; Steinbach, G.; Mendelsohn, J.; Kumar, R. Regulation of cyclooxygenase-2 pathway by HER2 receptor. Oncogene 1999, 18, 305–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howe, L.R.; Subbaramaiah, K.; Chung, W.J.; Dannenberg, A.J.; Brown, A.M.C. Transcriptional activation of cyclooxygenase-2 in Wnt-1-transformed mouse mammary epithelial cells. Cancer Res. 1999, 59, 1572–1577. [Google Scholar] [PubMed]
- McAdam, B.F.; Mardini, I.A.; Habib, A.; Burke, A.; Lawson, J.A.; Kapoor, S.; FitzGerald, G.A. Effect of regulated expression of human cyclooxygenase isoforms on eicosanoid and isoeicosanoid production in inflammation. J. Clin. Invest. 2000, 105, 1473–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attiq, A.; Jalil, J.; Husain, K.; Ahmad, W. Raging the war against inflammation with natural products. Front. Pharmacol. 2018, 9, 976. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Charles, K.A.; Mantovani, A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005, 7, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, L.; Yang, H.; G, B.; Li, W.; Wang, Y.; Wang, H.; Du, G.; Tang, B.; Wang, J. Research progress on anti-angiogenesis drugs in hepatocellular carcinoma. Cancer Plus 2021, 3, S1. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, H.; Su, H.; Lai, S.; Dai, L.; Liu, X.; Wang, Y.; Shu, G.; Tang, B.; Li, Y. Research progress on the role of enzymes involved in histone methylation in hepatocellular carcinoma. Cancer Plus 2021, 2, S1. [Google Scholar]
- Raja, S.A.; Ashraf, M.; Anjum, A.A.; Javeed, A.; Ijaz, T.; Attiq, A. Antibacterial activity of essential oils extracted from medicinal plants against multi-drug resistant Staphylococcus aureus. J. Anim. Plant. Sci. 2016, 26, 415–423. [Google Scholar]
- Yadav, V.R.; Prasad, S.; Sung, B.; Kannappan, R.; Aggarwal, B.B. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins 2010, 2, 2428–2466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, S.M.; Silva, G.N.; Pitta, I.D.; Rêgo, M.J.; Gnoato, S.C.; Pitta, M.G. Novel betulin derivatives inhibit IFN-γ and modulates COX-2 expression. Nat. Prod. Res. 2020, 34, 1702–1711. [Google Scholar] [CrossRef]
- Viji, V.; Helen, A.; Luxmi, V.R. Betulinic acid inhibits endotoxin-stimulated phosphorylation cascade and pro-inflammatory prostaglandin E2 production in human peripheral blood mononuclear cells. Br. J. Pharmacol. 2011, 162, 1291–1303. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.K.; Tseng, C.K.; Chen, K.H.; Wu, S.H.; Liaw, C.C.; Lee, J.C. Betulinic acid exerts anti-hepatitis C virus activity via the suppression of NF-κB- and MAPK-ERK1/2-mediated COX-2 expression. Br. J. Pharmacol. 2015, 172, 4481–4492. [Google Scholar] [CrossRef] [Green Version]
- Jalil, J.; Sabandar, C.W.; Ahmat, N.; Jamal, J.A.; Jantan, I.; Aladdin, N.A.; Muhammad, K.; Buang, F.; Mohamad, H.F.; Sahidin, I. Inhibitory effect of triterpenoids from Dillenia serrata (Dilleniaceae) on prostaglandin e2 production and quantitative HPLC analysis of its koetjapic acid and betulinic acid contents. Molecules 2015, 20, 3206–3220. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef] [Green Version]
- De la Vega, M.R.; Dodson, M.; Gross, C.; Mansour, H.M.; Lantz, R.C.; Chapman, E.; Wang, T.; Black, S.M.; Garcia, J.G.N.; Zhang, D.D. Role of Nrf2 and autophagy in acute lung injury. Curr. Pharmacol. Rep. 2016, 2, 91–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghunath, A.; Sundarraj, K.; Arfuso, F.; Sethi, G.; Perumal, E. Dysregulation of Nrf2 in hepatocellular carcinoma: Role in cancer progression and chemoresistance. Cancers 2018, 10, 481. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, N.; Slocum, S.L.; Skoko, J.J.; Shin, S.; Kensler, T.W. When NRF2 talks, who’s listening? Antioxid. Redox Signal. 2010, 13, 1649–1663. [Google Scholar] [CrossRef] [Green Version]
- Cebula, M.; Schmidt, E.E.; Arnér, E.S.J. TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Antioxid. Redox Signal. 2015, 23, 823–853. [Google Scholar] [CrossRef] [PubMed]
- Carling, D.; Thornton, C.; Woods, A.; Sanders, M.J. AMP-activated protein kinase: New regulation, new roles? Biochem. J. 2012, 445, 11–27. [Google Scholar] [CrossRef] [Green Version]
- Xing, H.Y.; Cai, Y.Q.; Wang, X.F.; Wang, L.L.; Li, P.; Wang, G.Y.; Chen, J.H. The cytoprotective effect of hyperoside against oxidative stress is mediated by the Nrf2-ARE signaling pathway through GSK-3β inactivation. PLoS ONE 2015, 10, e0145183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhou, B.; Sun, J.; Chen, H.; Yang, Z. Betulin ameliorates 7,12-dimethylbenz(a)anthracene-induced rat mammary cancer by modulating MAPK and AhR/Nrf-2 signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22779. [Google Scholar] [CrossRef] [PubMed]
- Ci, X.; Zhou, J.; Lv, H.; Yu, Q.; Peng, L.; Hua, S. Betulin exhibits anti-inflammatory activity in lps-stimulated macrophages and endotoxin-shocked mice through an ampk/akt/nrf2-dependent mechanism. Cell Death Dis. 2017, 8, e2798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loboda, A.; Rojczyk-Golebiewska, E.; Bednarczyk-Cwynar, B.; Zaprutko, L.; Jozkowicz, A.; Dulak, J. Targeting Nrf2-mediated gene transcription by triterpenoids and their derivatives. Biomol. Ther. 2012, 20, 499–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.Y.; Yan, D.; Zhou, H.Y.; Li, W.X.; Lou, Y.Y.; Zhou, X.R.; Qian, L.B.; Xiao, C. Betulinic acid attenuates lipopolysaccharide-induced vascular hyporeactivity in the rat aorta by modulating Nrf2 antioxidative function. Inflammopharmacology 2020, 28, 165–174. [Google Scholar] [CrossRef]
- Kong, X.; Thimmulappa, R.; Kombairaju, P.; Biswal, S. NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. J. Immunol. 2010, 185, 569–577. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirtonia, A.; Sethi, G.; Garg, M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell. Mol. Life Sci. 2020, 77, 4459–4483. [Google Scholar] [CrossRef]
- Cuadrado, A.; Pajares, M.; Benito, C.; Jiménez-Villegas, J.; Escoll, M.; Fernández-Ginés, R.; Garcia Yagüe, A.J.; Lastra, D.; Manda, G.; Rojo, A.I.; et al. Can activation of NRF2 be a strategy against COVID-19? Trends Pharmacol. Sci. 2020, 41, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.M.; Jawad, M.J.; Ahjel, S.W.; Singh, R.B.; Singh, J.; Awad, S.M.; Hadi, N.R. The Nrf2 activator (DMF) and Covid-19: Is there a possible role? Med. Arch. 2020, 74, 134–138. [Google Scholar] [CrossRef]
- Gomez, J.C.; Dang, H.; Martin, J.R.; Doerschuk, C.M. Nrf2 Modulates host defense during streptococcus pneumoniae pneumonia in mice. J. Immunol. 2016, 197, 2864–2879. [Google Scholar] [CrossRef] [Green Version]
- Staitieh, B.S.; Ding, L.; Neveu, W.A.; Spearman, P.; Guidot, D.M.; Fan, X. HIV-1 decreases Nrf2/ARE activity and phagocytic function in alveolar macrophages. J. Leukoc. Biol. 2017, 102, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Bonay, M.; Vanhee, V.; Vinit, S.; Deramaudt, T.B. Comparative effectiveness of 4 natural and chemical activators of Nrf2 on inflammation, oxidative stress, macrophage polarization, and bactericidal activity in an in vitro macrophage infection model. PLoS ONE 2020, 15, e0234484. [Google Scholar] [CrossRef]
- Iddir, M.; Brito, A.; Dingeo, G.; Del Campo, S.S.F.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: Considerations during the covid-19 crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef]
- Bousquet, J.; Cristol, J.P.; Czarlewski, W.; Anto, J.M.; Martineau, A.; Haahtela, T.; Fonseca, S.C.; Iaccarino, G.; Blain, H.; Fiocchi, A.; et al. Nrf2-interacting nutrients and COVID-19: Time for research to develop adaptation strategies. Clin. Transl. Allergy 2020, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Asemi, Z. Quercetin: A natural compound for ovarian cancer treatment. J. Ovarian Res. 2019, 12, 55. [Google Scholar] [CrossRef]
- Esposito, S.; Bianco, A.; Russo, R.; Di Maro, A.; Isernia, C.; Pedone, P.V. Therapeutic perspectives of molecules from urtica dioica extracts for cancer treatment. Molecules 2019, 24, 2753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.U.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [Green Version]
- Tay, K.-C.; Tan, L.T.-H.; Chan, C.K.; Hong, S.L.; Chan, K.-G.; Yap, W.H.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Formononetin: A review of its anticancer potentials and mechanisms. Front. Pharmacol. 2019, 10, 820. [Google Scholar] [CrossRef] [Green Version]
- Man, S.; Gao, W.; Zhang, Y.; Huang, L.; Liu, C. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia 2010, 81, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Novío, S.; Cartea, M.E.; Soengas, P.; Freire-Garabal, M.; Núñez-Iglesias, M.J. Effects of brassicaceae isothiocyanates on prostate cancer. Molecules 2016, 21, 626. [Google Scholar] [CrossRef] [Green Version]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M.C. Curcumin and health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid. Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-inflammatory activity of natural products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Kunnumakkara, A.B.; Kumar, A.; Samec, M.; Kubatka, P.; Aggarwal, B.B.; Shakibaei, M. Multitargeting effects of calebin a on malignancy of CRC cells in multicellular tumor microenvironment. Front. Oncol. 2021, 11, 650603. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Brockmueller, A.; Shakibaei, M. Resveratrol suppresses cross-talk between colorectal cancer cells and stromal cells in multicellular tumor microenvironment: A bridge between in vitro and in vivo tumor microenvironment study. Molecules 2020, 25, 4292. [Google Scholar] [CrossRef]
- Buhrmann, C.; Kraehe, P.; Lueders, C.; Shayan, P.; Goel, A.; Shakibaei, M. Curcumin suppresses crosstalk between colon cancer stem cells and stromal fibroblasts in the tumor microenvironment: Potential role of EMT. PLoS ONE 2014, 9, e107514. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; Lee, J.H.; Sethi, G.; Kim, C.; Baek, S.H.; Nam, D.; Chung, W.S.; Kim, S.H.; Shim, B.S.; Ahn, K.S. Bergamottin, a natural furanocoumarin obtained from grapefruit juice induces chemosensitization and apoptosis through the inhibition of STAT3 signaling pathway in tumor cells. Cancer Lett. 2014, 354, 153–163. [Google Scholar] [CrossRef]
- Sethi, G.; Shanmugam, M.K.; Warrier, S.; Merarchi, M.; Arfuso, F.; Kumar, A.P.; Bishayee, A. Pro-apoptotic and anti-cancer properties of diosgenin: A comprehensive and critical review. Nutrients 2018, 10, 645. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, V.; Tuli, H.S.; Kaur, J.; Aggarwal, D.; Parashar, G.; Parashar, N.C.; Kulkarni, S.; Kaur, G.; Sak, K.; Kumar, M.; et al. Garcinol exhibits anti-neoplastic effects by targeting diverse oncogenic factors in tumor cells. Biomedicines 2020, 8, 103. [Google Scholar] [CrossRef]
- Tuli, H.S.; Kashyap, D.; Sharma, A.K.; Sandhu, S.S. Molecular aspects of melatonin (MLT)-mediated therapeutic effects. Life Sci. 2015, 135, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ahn, K.S.; Kim, C.; Shanmugam, M.K.; Siveen, K.S.; Arfuso, F.; Samym, R.P.; Deivasigamanim, A.; Lim, L.H.K.; Wang, L.; et al. Nimbolide-induced oxidative stress abrogates STAT3 signaling cascade and inhibits tumor growth in transgenic adenocarcinoma of mouse prostate model. Antioxid. Redox Signal. 2016, 24, 575–589. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.G.; Yang, W.M.; Arfuso, F.; Um, J.Y.; Kumar, A.P.; Bian, J.; Sethi, G.; Ahn, K.S. Formononetin-induced oxidative stress abrogates the activation of STAT3/5 signaling axis and suppresses the tumor growth in multiple myeloma preclinical model. Cancer Lett. 2018, 431, 123–141. [Google Scholar] [CrossRef]
- Li, F.; Shanmugam, M.K.; Siveen, K.S.; Wang, F.; Ong, T.H.; Loo, S.Y.; Swamy, M.M.M.; Mandal, S.; Kumar, A.P.; Goh, B.C.; et al. Garcinol sensitizes human head and neck carcinoma to cisplatin in a xenograft mouse model despite downregulation of proliferative biomarkers. Oncotarget 2015, 6, 5147–5163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soica, C.; Danciu, C.; Savoiu-Balint, G.; Borcan, F.; Ambrus, R.; Zupko, I.; Bojin, F.; Coricovac, D.; Ciurlea, S.; Avram, S.; et al. Betulinic acid in complex with a gamma-cyclodextrin derivative decreases proliferation and in vivo tumor development of non-metastatic and metastatic B164A5 cells. Int. J. Mol. Sci. 2014, 15, 8235–8255. [Google Scholar] [CrossRef] [Green Version]
- Bache, M.; Bernhardt, S.; Passin, S.; Wichmann, H.; Hein, A.; Zschornak, M.; Kappler, M.; Taubert, H.; Paschke, R.; Vordermark, D. Betulinic acid derivatives NVX-207 and B10 for treatment of glioblastoma—An in vitro study of cytotoxicity and radiosensitization. Int. J. Mol. Sci. 2014, 15, 19777–19790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selzer, E.; Pimentel, E.; Wacheck, V.; Schlegel, W.; Pehamberger, H.; Jansen, B.; Kodym, R. Effects of betulinic acid alone and in combination with irradiation in human melanoma cells. J. Invest. Dermatol. 2000, 114, 935–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eder-Czembirek, C.; Erovic, B.M.; Czembirek, C.; Brunner, M.; Selzer, E.; Pötter, R.; Thurnher, D. Betulinic acid a radiosensitizer in head and neck squamous cell carcinoma cell lines. Strahlenther. Onkol. 2010, 186, 143–148. [Google Scholar] [CrossRef]
- Wang, Y.J.; Liu, J.B.; Dou, Y.C. Sequential treatment with betulinic acid followed by 5-fluorouracil shows synergistic cytotoxic activity in ovarian cancer cells. Int. J. Clin. Exp. Pathol. 2015, 8, 252–259. [Google Scholar] [PubMed]
- Xu, Y.; Li, J.; Li, Q.J.; Feng, Y.L.; Pan, F. Betulinic acid promotes TRAIL function on liver cancer progression inhibition through p53/Caspase-3 signaling activation. Biomed. Pharmacother. 2017, 88, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zheng, Y.; Gu, J.; Wang, S.; Wang, N.; Yang, B.; Zhang, F.; Wang, D.; Fu, W.; Wang, Z. Betulinic acid chemosensitizes breast cancer by triggering ER stress-mediated apoptosis by directly targeting GRP78 article. Cell Death Dis. 2018, 9, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Li, Y.; Wang, X.; Fang, X.; He, K.; Guo, X.; Zhan, Z.; Sun, C.; Jin, Y.H. Co-treatment with ginsenoside Rh2 and betulinic acid synergistically induces apoptosis in human cancer cells in association with enhanced capsase-8 activation, bax translocation, and cytochrome c release. Mol. Carcinog. 2011, 50, 760–769. [Google Scholar] [CrossRef]
- Yamai, H.; Sawada, N.; Yoshida, T.; Seike, J.; Takizawa, H.; Kenzaki, K.; Miyoshi, T.; Kondo, K.; Bando, Y.; Ohnishi, Y.; et al. Triterpenes augment the inhibitory effects of anticancer drugs on growth of human esophageal carcinoma cells in vitro and suppress experimental metastasis in vivo. Int. J. Cancer 2009, 125, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 2021, 69, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Nasery, M.M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin delivery mediated by bio-based nanoparticles: A review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullauer, F.B.; Van Bloois, L.; Daalhuisen, J.B.; Ten Brink, M.S.; Storm, G.; Medema, J.P.; Schiffelers, R.M.; Kessler, J.H. Betulinic acid delivered in liposomes reduces growth of human lung and colon cancers in mice without causing systemic toxicity. Anticancer. Drugs 2011, 22, 223–233. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, D.; Zhang, X.; Liu, Z.; Dai, K.; Ji, B.; Wang, Q.; Luo, L. Antitumor drug effect of betulinic acid mediated by polyethylene glycol modified liposomes. Mater. Sci. Eng. C 2016, 64, 124–132. [Google Scholar] [CrossRef]
- Shu, Q.; Wu, J.; Chen, Q. Synthesis, characterization of liposomes modified with biosurfactant MEL-A loading betulinic acid and its anticancer effect in HepG2 cell. Molecules 2019, 24, 3939. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Gao, C.; Yin, C.; Fan, J.; Wu, X.; Guo, C. Improved anticancer activity of betulinic acid on breast cancer through a grafted copolymer-based micelles system. Drug Deliv. 2021, 28, 1962–1971. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Feflea, S.; Gheorgheosu, D.; Ganta, S.; Cimpean, A.M.; Muntean, D.; Amiji, M.M. Anti-angiogenic and anti-cancer evaluation of betulin nanoemulsion in chicken chorioallantoic membrane and skin carcinoma in Balb/c mice. J. Biomed. Nanotechnol. 2013, 9, 577–589. [Google Scholar] [CrossRef]
- Tan, J.M.; Karthivashan, G.; Abd Gani, S.; Fakurazi, S.; Hussein, M.Z. Biocompatible polymers coated on carboxylated nanotubes functionalized with betulinic acid for effective drug delivery. J. Mater. Sci. Mater. Med. 2016, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Şoica, C.; Dehelean, C.; Danciu, C.; Wang, H.M.; Wenz, G.; Ambrus, R.; Bojin, F.; Anghel, M. Betulin complex in γ-cyclodextrin derivatives: Properties and antineoplasic activities in in vitro and in vivo tumor models. Int. J. Mol. Sci. 2012, 13, 14992–15011. [Google Scholar] [CrossRef] [PubMed]
- Urandur, S.; Banala, V.T.; Shukla, R.P.; Gautam, S.; Marwaha, D.; Rai, N.; Sharma, M.; Sharma, S.; Ramarao, P.; Mishra, P.R. Theranostic lyotropic liquid crystalline nanostructures for selective breast cancer imaging and therapy. Acta Biomater. 2020, 113, 522–540. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, A.K.; Raj, V.; Rai, A.; Keshari, A.K.; Kumar, D.; Maity, B.; Prakash, A.; Maiti, S.; Saha, S. Poly(Lactic-co-glycolic acid)-loaded nanoparticles of betulinic acid for improved treatment of hepatic cancer: Characterization, in vitro and in vivo evaluations. Int. J. Nanomed. 2018, 13, 975–990. [Google Scholar] [CrossRef] [Green Version]
- Pinzaru, I.; Sarau, C.; Coricovac, D.; Marcovici, I.; Utescu, C.; Tofan, S.; Popovici, R.A.; Manea, H.C.; Pavel, I.E.; Soica, C.; et al. Article silver nanocolloids loaded with betulinic acid with enhanced antitumor potential: Physicochemical characterization and in vitro evaluation. Nanomaterials 2021, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Kambhampati, N.S.V.; Kar, S.; Pinnepalli, S.S.K.; Chelli, J.; Doble, M. Microbial cyclic β-(1→3),(1→6)-glucans as potential drug carriers: Interaction studies between cyclic β-glucans isolated from Bradyrhizobium japonicum and betulinic acid. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2018, 203, 494–500. [Google Scholar] [CrossRef]
- Kirtonia, A.; Gala, K.; Fernandes, S.G.; Pandya, G.; Pandey, A.K.; Sethi, G.; Khattar, E.; Garg, M. Repurposing of drugs: An attractive pharmacological strategy for cancer therapeutics. Semin. Cancer Biol. 2021, 68, 258–278. [Google Scholar] [CrossRef] [PubMed]
- Merarchi, M.; Sethi, G.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; Ahn, K.S. Role of natural products in modulating histone deacetylases in cancer. Molecules 2019, 24, 1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäger, S.; Laszczyk, M.N.; Scheffler, A. A preliminary pharmacokinetic study of betulin, the main pentacyclic triterpene from extract of outer bark of birch (Betulae alba cortex). Molecules 2008, 13, 3224–3235. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, E.H.; Tribuiani, N.; Sabadim, G.; Moreno, D.A.N.; Varanda, E.A.; Oshima-Franco, Y. Evaluation of betulin mutagenicity by Salmonella/microsome test. Adv. Pharm. Bull. 2016, 6, 443–447. [Google Scholar] [CrossRef] [Green Version]
- Pflugfelder, A.; Andonov, E.; Weide, B.; Dirschka, T.; Schempp, C.; Stockfleth, E.; Stratigos, A.; Krüger-Krasagakis, S.; Bauer, J.; Garbe, C.; et al. Lack of activity of betulin-based Oleogel-S10 in the treatment of actinic keratoses: A randomized, multicentre, placebo-controlled double-blind phase II trial. Br. J. Dermatol. 2015, 172, 926–932. [Google Scholar] [CrossRef]
- Schwieger-Briel, A.; Kiritsi, D.; Schempp, C.; Has, C.; Schumann, H. Betulin-based oleogel to improve wound healing in dystrophic epidermolysis bullosa: A prospective controlled proof-of-concept study. Dermatol. Res. Pract. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Frew, Q.; Rennekampff, H.O.; Dziewulski, P.; Moiemen, N.; Zahn, T.; Hartmann, B. Betulin wound gel accelerated healing of superficial partial thickness burns: Results of a randomized, intra-individually controlled, phase III trial with 12-months follow-up. Burns 2019, 45, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Małaczewska, J.; Kaczorek-Łukowska, E.; Kazuń, B. High cytotoxicity of betulin towards fish and murine fibroblasts: Is betulin safe for nonneoplastic cells? BMC Vet. Res. 2021, 17, 198. [Google Scholar] [CrossRef]
- Laiolo, J.; Barbieri, C.L.; Joray, M.B.; Lanza, P.A.; Palacios, S.M.; Vera, D.M.A.; Carpinella, M.C. Plant extracts and betulin from Ligaria cuneifolia inhibit P-glycoprotein function in leukemia cells. Food Chem. Toxicol. 2021, 147, 111922. [Google Scholar] [CrossRef]
- Król, S.K.; Bębenek, E.; Sławińska-Brych, A.; Dmoszyńska-Graniczka, M.; Boryczka, S.; Stepulak, A. Synthetic betulin derivatives inhibit growth of glioma cells in Vitro. Anticancer Res. 2020, 40, 6151–6158. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chen, H.Y.; Hsieh, C.P.; Huang, Y.F.; Chang, I.L. Betulin inhibits mTOR and induces autophagy to promote apoptosis in human osteosarcoma cell lines. Environ. Toxicol. 2020, 35, 879–887. [Google Scholar] [CrossRef]
- Shen, H.; Liu, L.; Yang, Y.; Xun, W.; Wei, K.; Zeng, G. Betulinic acid inhibits cell proliferation in human Oral squamous cell carcinoma via modulating ROS-regulated p53 signaling. Oncol. Res. 2017, 25, 1141–1152. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Bębenek, E.; Chrobak, E.; Marciniec, K.; Latocha, M.; Kuśmierz, D.; Jastrzębska, M.; Boryczka, S. Betulin-1,4-quinone hybrids: Synthesis, anticancer activity and molecular docking study with NQO1 enzyme. Eur. J. Med. Chem. 2019, 177, 302–315. [Google Scholar] [CrossRef]
- Pfarr, K.; Danciu, C.; Arlt, O.; Neske, C.; Dehelean, C.; Pfeilschifter, J.M.; Radeke, H.H. Simultaneous and dose dependent melanoma cytotoxic and immune stimulatory activity of betulin. PLoS ONE 2015, 10, e0118802. [Google Scholar] [CrossRef] [PubMed]
- Drag-Zalesińska, M.; Wysocka, T.; Borska, S.; Drag, M.; Poreba, M.; Choromańska, A.; Kulbacka, J.; Saczko, J. The new esters derivatives of betulin and betulinic acid in epidermoid squamous carcinoma treatment—In vitro studies. Biomed. Pharmacother. 2015, 72, 91–97. [Google Scholar] [CrossRef]
- John, R.; Dalal, B.; Shankarkumar, A.; Devarajan, P.V. Innovative Betulin Nanosuspension exhibits enhanced anticancer activity in a Triple Negative Breast Cancer Cell line and Zebrafish angiogenesis model. Int. J. Pharm. 2021, 600, 120511. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, P.; Wang, N.; Wang, S.; Yang, B.; Li, M.; Chen, J.; Situ, H.; Xie, M.; Lin, Y.; et al. Betulinic acid suppresses breast cancer metastasis by targeting GRP78-mediated glycolysis and ER stress apoptotic pathway. Oxid. Med. Cell. Longev. 2019, 2019, 8781690. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Feflea, S.; Molnár, J.; Zupko, I.; Soica, C. Betulin as an antitumor agent tested in vitro on A431, HeLa and MCF7, and as an angiogenic inhibitor in vivo in the CAM assay. Nat. Prod. Commun. 2012, 7, 981–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kıran, İ.; Çiftçi, G.A.; Eklioğlu, Ö.A.; Akkaya, Ş.G.K. Bacterial biotransformation and anticancer activities of betulin against A549, HepG2 and 5RP7 cancer cell lines. Anticancer. Agents Med. Chem. 2020, 21, 1581–1593. [Google Scholar] [CrossRef]
- Zhao, H.; Mu, X.; Zhang, X.; You, Q. Lung cancer inhibition by betulinic acid nanoparticles via adenosine 5’-Triphosphate (ATP)-binding cassette transporter G1 gene downregulation. Med. Sci. Monit. 2020, 26, e922092. [Google Scholar] [CrossRef]
- Zehra, B.; Ahmed, A.; Sarwar, R.; Khan, A.; Farooq, U.; Ali, S.A.; Al-Harrasi, A. Apoptotic and antimetastatic activities of betulin isolated from Quercus incana against non-small cell lung cancer cells. Cancer Manag. Res. 2019, 11, 1667–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jae, S.P.; Si, H.R.; Dae, K.K.; Jin, G.L.; Yong, Y.L.; Soon, S.H.; Sung, W.K.; Jeong, H.P. Anti-cancer effect of betulin on a human lung cancer cell line: A pharmacoproteomic approach using 2 D SDS PAGE coupled with nano-HPLC tandem mass spectrometry. Planta Med. 2009, 75, 127–131. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, X.; Zhang, Z.; Gong, P.; Yin, W.; Jiang, Q.; Xu, J.; Xu, X.; Gao, Y.; Chen, W.; et al. Betulinic acid inhibits cell proliferation and migration in gastric cancer by targeting the NF-κB/VASP pathway. Eur. J. Pharmacol. 2020, 889, 173493. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Hwangbo, H.; Kim, M.Y.; Ji, S.Y.; Kim, D.H.; Lee, H.; Kim, G.Y.; Moon, S.K.; Leem, S.H.; Yun, S.J.; et al. Betulinic acid restricts human bladder cancer cell proliferation in vitro by inducing caspase-dependent cell death and cell cycle arrest, and decreasing metastatic potential. Molecules 2021, 26, 1381. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhu, C.; Cai, Z.; Zhao, F.; He, L.; Lou, X.; Qi, X. Betulin induces cytochrome c release and apoptosis in colon cancer cells via NOXA. Oncol. Lett. 2018, 15, 7319–7327. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cao, J.; Chen, K.; Cheng, L.; Zhou, C.; Yan, B.; Qian, W.; Li, J.; Duan, W.; Ma, J.; et al. Betulinic acid inhibits stemness and EMT of pancreatic cancer cells via activation of AMPK signaling. Int. J. Oncol. 2019, 54, 98–110. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Liu, M.; Zhang, Y.; Yang, T.; Li, D.; Huang, Y.; Li, Q.; Bai, G.; Shi, L. Betulinic acid induces apoptosis and suppresses metastasis in hepatocellular carcinoma cell lines in vitro and in vivo. J. Cell. Mol. Med. 2019, 23, 586–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Li, Y.; Fu, L.; Jiang, T.; Meng, F. Betulinic acid induces apoptosis and inhibits metastasis of human renal carcinoma cells in vitro and in vivo. J. Cell. Biochem. 2018, 119, 8611–8622. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Ji, S.; Zhang, H.; Han, Z.; Liu, Q.; Wang, J.; Ping, H. mTOR activation is critical for betulin treatment in renal cell carcinoma cells. Biochem. Biophys. Res. Commun. 2017, 482, 1030–1036. [Google Scholar] [CrossRef]
- Yim, N.H.; Jung, Y.P.; Kim, A.; Kim, T.; Ma, J.Y. Induction of apoptotic cell death by betulin in multidrug-resistant human renal carcinoma cells. Oncol. Rep. 2015, 34, 1058–1064. [Google Scholar] [CrossRef] [Green Version]
- Härmä, V.; Haavikko, R.; Virtanen, J.; Ahonen, I.; Schukov, H.P.; Alakurtti, S.; Purev, E.; Rischer, H.; Yli-Kauhaluoma, J.; Moreira, V.M.; et al. Optimization of invasion-specific effects of betulin derivatives on prostate cancer cells through lead development. PLoS ONE 2015, 10, e0126111. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Liu, C.; Xie, X.; Zhou, J. Betulinic acid induces apoptosis and impairs migration and invasion in a mouse model of ovarian cancer. J. Food Biochem. 2020, 44, e13278. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, S.R.; Kang, K.S.; Ko, Y.; Pang, C.; Yamabe, N.; Kim, K.H. Betulinic acid suppresses ovarian cancer cell proliferation through induction of apoptosis. Biomolecules 2019, 9, 257. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Cho, H.S.; Ban, H.S.; Nakamura, H. Suppression of HIF-1α accumulation by betulinic acid through proteasome activation in hypoxic cervical cancer. Biochem. Biophys. Res. Commun. 2020, 523, 726–732. [Google Scholar] [CrossRef]
- Weber, L.A.; Meißner, J.; Delarocque, J.; Kalbitz, J.; Feige, K.; Kietzmann, M.; Michaelis, A.; Paschke, R.; Michael, J.; Pratscher, B.; et al. Betulinic acid shows anticancer activity against equine melanoma cells and permeates isolated equine skin in vitro. BMC Vet. Res. 2020, 16, 44. [Google Scholar] [CrossRef]
- Zeng, A.; Hua, H.; Liu, L.; Zhao, J. Betulinic acid induces apoptosis and inhibits metastasis of human colorectal cancer cells in vitro and in vivo. Bioorg. Med. Chem. 2019, 27, 2546–2552. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Liu, C.; Zhou, Y. Betulinic acid triggers apoptosis and inhibits migration and invasion of gastric cancer cells by impairing EMT progress. Cell Biochem. Funct. 2020, 38, 702–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Type of Cancer | Cell Lines | Effects | Mechanisms | Concentration | References |
|---|---|---|---|---|---|
| Leukemia | Lucena 1 and K562 | Blocking of the efflux mediated by P-gp | ↑ restore sensitivity to doxorubicin in Lucena 1 cells, did not exhibit erythrocyte hemolysis | 0.39–50 µM | [153] |
| Myeloma | RPMI 8226 | Induces apoptosis | ↓ proliferation, migration and invasion by tumor cells, ↓ bcl-2, ↑ bax, ↓ cyclin D1, No change in CREB phosphorylation, | 0, 2.5, 5, 10 and 25 μM | [46] |
| Human T-cell leukemia | Jurkat E6.1 | Induces apoptosis | ↓ proliferation, migration and invasion by tumor cells, ↓ bcl-2, ↑ bax, ↓ cyclin D1, No change in CREB phosphorylation, Amounts of the CREB protein, and ERK1/2, Akt, CaMKII kinases remained unchanged | 0, 2.5, 5, 10 and 25 μM | [46] |
| Glioma | T98G and C6 | Induces apoptosis | ↓ cell viability/survival and proliferation, ↓ % age of T98G cells in G1 phase, ↑ in cell number in S phase, significant activation of caspase 3 | 0.0–25 μM for EB5 or 0.0–50 μM for EB25/1 | [154] |
| Osteosarcoma | HOS and MG-63 | Induces autophagy↓↑ | ↑ LC 3-II, ↑ phospho-Akt (Ser473), ↓ activation of mTOR | 0, 0.5, 1, 2, 4, 5, 10 and 20 μM | [155] |
| Medulloblastoma | TE671 | Induces apoptosis | ↓ proliferation, migration and invasion by tumor cells, ↓ bcl-2, ↑ bax, ↓ cyclin D1, No change in CREB phosphorylation, Amounts of the CREB protein, and ERK1/2, Akt, CaMKII kinases remained unchanged | 0, 2.5, 5, 10 and 25 μM | [46] |
| Oral squamous | KB | Induced apoptosis | ↓ cell proliferation, ↑ TUNEL+ cells in KB cells, ↑ caspase 3, ↑ caspase 9, ↑ Bax, ↓ Bcl-2, ↓ oxygen consumption rate, Induced a significant mitochondrial dysfunction, ↑ cell number in the G 0/G1 phase, | 0, 12.5, 25, 50 and 100 μM | [156] |
| Thyroid | FTC 238 | Induces apoptosis | ↓ proliferation, migration and invasion by tumor cells, ↓ bcl-2, ↑ bax, ↓ cyclin D1, No change in CREB phosphorylation, | 0, 2.5, 5, 10 and 25 μM | [46] |
| Melanoma | Colo-829 | Induces apoptosis | ↓ NQO1 protein, ↑ formation of superoxide, ↑ oxidative stress, ↑ TP53 ↑ CDKN1A genes, ↓ p53 protein | 0.1 to 100 μg/mL | [157] |
| C-32 | Induces apoptosis | ↓ transcription of the gene encoding the histone H3, ↓ NQO1 protein, ↑ formation of superoxide, ↑ oxidative stress, ↑ TP53 ↑ CDKN1A genes, ↑ BAX gene, ↓ BCL-2 gene, ↑ BAX/BCL-2 ratio, ↓ p53 protein | 0.1 to 100 μg/mL | [157] | |
| Me-45 | Induces apoptosis | ↑ apoptotic nuclei, ↑ cytotoxicity towards malignant cells, ↑ apoptosis arte, ↑ pro-apoptotic effects, ↑ PARP-1, ↓ expression of caspase-3 | 0.75–100 µM | [32] | |
| B164A5 and B16F10 | Induced apoptosis | ↓ mitochondrial oxidoreductase, ↓ cell division rate, ↑ Bax, ↓ Bcl-2, ↑ IL-12p70 secretion, ↑ cleaved caspase 3, ↑ cleaved PARP | 0, 40, 80, 120 and 160 μM | [158] | |
| Epidermoid squamous | A431 | Induces apoptosis | ↑ apoptotic cells, ↑ Increased cytotoxicity for cancerous cells, ↑ PARP-1, ↓ amounts of caspase-3 | 0.75–100 μM | [159] |
| Breast | MDA-MB-231 | Anti-angiogenic | ↑ betulin uptake, ↓ cell viability of the cancer cells, ↑ in vitro cytotoxicity, ↑ mononucleated cells, ↓in binucleated cells | Nanosuspension of betulin equivalent to 5, 10, 25, 50, 100, 150 and 200 µM | [160] |
| MDA-MB-231 | Induces apoptosis | ↓ cell size, ↑ shrinkage of the cytoplasm, ↓ NF-ĸB p65 and p50, ↓ IKK α and β, ↓ ICAM-1, ↓ bcl-2 expressions, significantly induced loss of mitochondrial transmembrane potential | 0–50 μM | [58] | |
| MCF-7 and MDA-MB-231 | Induces apoptosis | ↓ histone H3, ↓ NQO1 protein, ↑ formation of superoxide, ↑ oxidative stress, ↑ TP53 ↑ CDKN1A genes, ↑ BAX gene, ↓ BCL-2 gene, ↑ BAX/BCL-2 ratio, ↓ p53 protein | 0.1 to 100 μg/mL | [157] | |
| MDA-MB-231 and BT-549 | Inhibited metastasis | ↓ aerobicglycolysis, ↓reduction of lactate production, ↓ down regulation of aerobic glycolysis-related proteins, ↑ GRP78 overexpression, ↓ c-Myc-mediated glycolysis, ↓ MMP-2 and MMP-9, ↑ LDHB, ↑ PERK signaling, ↑ phosphorylation of eIF2α | 0, 2.5, 5, 10, 15, 20, 25, 30, 40 and 50 μM | [161] | |
| MCF-7, and MDA-MB-231 | Induces apoptosis | ↓ cancer cell proliferation and augments chemosensitivity of taxol, ↑ cleaved PARP, ↑ Cytochrome c, ↑ Bax, ↓ Bcl-2, ↑ intracellular free calcium concentration | BA - 0.1–50 μMTaxol 0–24 nM | [129] | |
| MCF7 | Induces apoptosis | ↓ cancer cell growth, ↑ DNA fragmentation, | IC50 values of 8.32 | [162] | |
| MCF-7 | Induces apoptosis | ↑ caspase-9 activity, ↑ caspase-3, ↑ Bax, ↑ Bak | 0, 1, 5, 10, 20, 50 and 100 µg/ µl | [55] | |
| Ductal | T47D | Induces apoptosis | ↓ NQO1 protein, ↑ formation of superoxide, ↑ oxidative stress, ↑ TP53 ↑ CDKN1A genes, | 0.1 to 100 μg/mL | [157] |
| Lung | A549, HepG2and 5RP7 | Induces apoptosis | ↑ rate of Apoptosis, caused G1 cell cycle arrest, ↑ cleaved caspase 3 | IC50 values of 207.7, 125.0 and 28.3 μg/mL | [163] |
| HKULC2, H1299, and H23 | Inhibit metastatic ability | ↑ cycle arrest in G1 phase, ↓ migration and invasive potential of cells, ↑ p21, ↑ p53, ↓ CD133, ↓ ALDH, ↓ BCL2, ↓ MCL1, ↓ c-Myc expression, ↓ ABCG1 protein | 10 µM of betulinic acid nanoparticles | [164] | |
| A549 | Induces apoptosis | ↓ histone H3, ↓ NQO1 protein, ↑ formation of superoxide, ↑ oxidative stress, ↑ TP53 ↑ CDKN1A genes, ↓ p53, ↑ BAX/BCL-2 ratio | 0.1 to 100 μg/mL | [157] | |
| NCI-H460 | Antimetastatic and Apoptosis | ↑ caspase-3, 6 and 9), ↑ BAX, ↑ BAK, ↓ BCL-2, ↓ p53, ↓ MMP-2/-9. ↓ Osteopontin | 10, 25, 50, 75, and 100 µM | [165] | |
| A549 | Induces apoptosis | ↑ caspase-9 activity, ↑ caspase-3, ↑ Bax, ↑ Bak | 0, 1, 5, 10, 20, 50 and 100 µg/µL | [55] | |
| A549 | Induced apoptosis | ↓ PCBP1, ↓ isoform 1 of 3-HAD CoA dehydrogenase, ↓ HSP 90-α 2, ↓ ECH | 0, 12.5, 25, 50 and 100 μM | [166] | |
| A549 | Induces apoptosis | ↓ proliferation, migration and invasion by tumor cells, ↓ bcl-2, ↑ bax, ↓ cyclin D1, No change in CREB phosphorylation, | 0, 2.5, 5, 10 and 25 μM | [46] | |
| Gastric | SNU-16 and NCI-N87 | Triggers apoptosis | ↑ cytotoxic and inhibitory effects on cancer cells, ↓ migratory and invasive abilities of cancer cells, ↓ EMT progression, ↓ N-cadherin, ↑ E-cadherin | 0, 2.5, 5, 10, 20, 40 and 80 μM | [166] |
| BGC-823, MNK45 and 293T | Induces apoptosis | ↓ proliferation and migration the cancer cells, ↓ expression of VASP mRNA, ↓ Cyclin D1, ↓ PCNA, ↓ c-Myc, ↓ AKT, ↓ Vimentin, ↓ NF-κB activity, ↓ p-p65 protein | 0–60 μM | [167] | |
| SGC7901 | Induced apoptosis | ↓ cell proliferation, ↑ Caspase- 3 and 9 activities, caspase-8 activity remained unchanged, ↑ PARP cleavage, ↑ Bax,↑ Bak, ↓ Bcl-2, ↓ XIAP, ↑ intracellular ROS level, | 0, 1, 5, 10, 20, 50, 100 µg/ µL | [56] | |
| Bladder | T-24, UMUC-3, and 5637 | Induced apoptosis | ↓ cell proliferation and migration potential of cells, ↓ Cdc25c, loss of mitochondrial membrane potential, ↑ Bax, ↑ cleaved- PARP, ↑ caspase-3, 8, and 9, ↓ wound healing and invasion ability, ↓ Snail, ↓ Slug, ↓ MMP-9 | 0, 10, 15, 20 and 30 µg/ µL | [168] |
| Colon | HCT116 and HT29 | Induced apoptosis | ↓ viability of HCT116 cells, ↑ number of floating cells, ↑ rounding of cells, ↑ emergence of irregular bulges in cell membrane, ↑ condensed chromatin, ↑ micronucleation, | 0, 1, 5, 10, 20, 50 and 100 µg/ µL | [169] |
| HT-29 | Induces apoptosis | ↓ proliferation, migration and invasion by tumor cells, ↓ Bcl-2, ↑ Bax, ↓ cyclin D1, No change in CREB phosphorylation, Amounts of the CREB protein, and ERK1/2, Akt, CaMKII kinases remained unchanged | 0, 2.5, 5, 10 and 25 μM | [46] | |
| HCT116, SW480 and DLD-1 | Promoted apoptosis and inhibited metastasis | ↑ Bax, ↑ caspase-3, ↓ Bcl-2, ↑ ROS, ↓ mitochondrial membrane potential, ↓ migration and invasion of colorectal cancer cells, ↓ MMPs, ↑ MMPs inhibitor (TIMP-2) | 0, 05, 10, 20, 40 and 80 μM | [169] | |
| Pancreatic | Mia PaCa-2 and Panc-1 | Inhibits stemness | ↓ proliferation and tumorsphere formation, ↓ EMT, activates AMPK signaling ↓ mRNA expression levels of Sox2, Oct4, ↓ Nanog and Nanog, ↑ E-cadherin, ↓ vimentin, ↓ effects of gemcitabine on stemness, ↑ sensitivity of pancreatic cancer cells to gemcitabine | 0, 12.5, 25, 50, 100 and 200 µM | [170] |
| Hepatocellular | HepG2, LM3, and MHCC97H | Induces apoptosis | ↓ cell viability and proliferation, ↓ migration and invasion, ↓ adhesive ratios, ↑ condensed nuclei and nuclear fragmentation, ↑ apoptosis rate significantly, ↑ Bax, ↑cleaved caspase-3, ↓ Bcl-2, ↓ ROS level, lost mitochondrial membrane potential, ↓ MMP-2 and MMP-9, ↑ TIMP2 | 2.5–40 μM | [171] |
| HepG2 | Induces apoptosis | ↑ caspase-9 activity, ↑ caspase-3, ↑ Bax, ↑ Bak | 0, 1, 5, 10, 20, 50 and 100 µg/µL | [171] | |
| Renal | 786-O and ACHN | Induces apoptosis | ↓ migrative and invasive capabilities of cancer cells, ↓ Bcl2, ↓ Bcl-2, ↑ Bax, ↑ cleaved caspase-3, ↓ B-cell lymphoma 2, ↑ ROS, ↑ loss of mitochondrial membrane potential, ↓ MMP-2, ↓ MMP9, ↓ Vimentin, ↑ tissue inhibitor of metalloproteinase 2, ↑ E-cadherin | 0, 5, 10 and 20 μg/mL | [172] |
| 786-O and Caki-2 | mTor activation | ↓ colonies of cancer cells, ↓ glucose consumption, ↓ lactate production, ↓ p-S6, p-4EBP1, ↓ aerobic glycolysis | 0, 0.5, 1 and 5 μM | [173] | |
| RCC4 | Induces apoptosis | ↓ cell viability, ↑ caspase-3, 7, 8 and 9, ↑ TRAIL R1/DR4 and R2/DR5, ↑ TNFR1, ↑ cytotoxicity, ↑ cleaved PARP, ↓ protein 1 (MDR1), ↑ t-Bid, ↑ Bax, ↑ PuMA, ↓ Bcl-2, ↓ XIAP | 0, 6.25, 12.5, 25 and 50 μM | [174] | |
| Neuroblastoma | SK-N-AS | Induces apoptosis | ↓ proliferation, migration and invasion by tumor cells, ↓ bcl-2, ↑ bax, ↓ cyclin D1, No change in CREB phosphorylation, | 0, 2.5, 5, 10 and 25 μM | [46] |
| Prostate | LNCaP and PC-3 | Induced apoptosis | ↓ STAT3 (Y727), ↓ c-Jun (S63), ↓ eNOS (S1177), ↓ ap70 S6 kinase (T389), ↓ p53 (S392) ↓ PYK2 (Y402) | 1–90 μM | [175] |
| Ovarian | SKOV3 and SW626 | Inhibited metastasis | ↓ proliferation, ↓ N-cadherin, ↑ E-cadherin, ↓ EMT process | 0, 2.5, 5, 10, 20, 40, and 80 μM | [176] |
| A2780 | Induces apoptosis | ↓ viability of cancer cells, ↑ condensation of nuclei, ↑ caspase-8, 3,9, ↑ Bax, | 25 and 50 µM | [177] | |
| Cervix | HeLa | Suppresses angiogenesis | ↓ hypoxia-induced accumulation of HIF-1α,↓ VEGF, ↓ GLUT1, PDK1, ↑ β1, β 2, and β 5 activities of the proteasome | 3–30 μM | [178] |
| HeLa | Induces apoptosis | ↓ cancer cell growth, ↑ nuclear condensation and fragmentation, | IC50 values of 6.67 | [162] | |
| Equine malignant melanoma | PriFi1, PriFi2, MelDuWi and eRGO1 | Induces apoptosis | ↓ cell proliferation, ↓ cell viability, ↑cell cycle arrest | -- | [179] |
| Canine osteosarcoma | D-17 | Induces apoptosis | ↓ Growth of cancer cells. arrested cell cycle in S phase, ↑ %age of apoptotic cells | 1, 5, 10, 15, 20, 25, 30 and 40 μM | [47] |
| Type of Cancer | Animal Models | Effects | Mechanisms | Dosage | Duration | References |
|---|---|---|---|---|---|---|
| Oral squamous | Balb/c nude mice injected with KB cells (1 × 107 cells per mouse) | Inhibited the increase in tumor volume | ↓ p53 in implanted tumor, ↓ STAT3 signaling, ↓ p- STAT3 in tumor tissues declined | 50, 75 and 150 mg/kg | 21 days | [156] |
| Colorectal | BALB/c nude mice xenografted with HCT116 cells (1 × 107 cells per mouse) | Inhibits metastasis | ↓ MMP-2, ↓ Ki-67, ↑ caspase-3 | 0, 10, and 20 mg/kg | 21 days | [180] |
| Gastric | BALB/c nude mice xenografted with SNU-16 cells (1 × 107 cells/mouse) | Delay tumour growth and inhibit pulmonary metastasis | ↓ tumour weight, ↓ number of metastatic nodules, ↓ Ki-67 ↓ MMP2 | 40 mg/kg | 21 days | [181] |
| Breast | Adult orange zebra danio fishes | Anti-angiogenesis | ↓tail fin regrowth | Betulin suspension (BetS) (5 mg/g of betulin) and Group III – BeTNS (5 mg/g of betulin) | 15 days | [160] |
| Breast | Balb/c-nu/nu mice subcutaneously injecting MDA-MB-231 cells (5 × 106) | Inhibited tumor growth | ↓ Body weight loss, ↑ apoptosis ratio, ↓ Ki67 expression, ↑ expression of GRP78, ↑ CHOP | BA 250 mg/kg + taxol 10 mg/kg | 24 days | [129] |
| Breast | Balb/c nude mice xenografted with MDA-MB-231 cells (2 × 105) | Inhibits metastasis | ↓ MMP-2 & 9, ↓ vimentin, ↑ E-cadherin, ↑ GRP78, ↓ β-catenin, ↓ c-Myc | 125 and 250 mg/kg | 28 days | [161] |
| Hepatocellular | NOD/SCID mice implanted subcutaneously with 100 μL HepG2 cells suspensions (1 × 107 cells/mouse) | Reduces tumour growth | ↓ Ki-67 positive cells, ↓ MMP-2 positive cells, ↓ cancer cell proliferation, ↓ Extents of metastatic nodules, ↓ lung weights | 10 mg/kg | 18 days | [171] |
| Renal | BALB/c nude mice injected with 786-O cells (1 × 106 cells per mouse) | Inhibits metastasis | ↓ Ki67-positive cells, ↓ MMP9-positive cells, | 0, 5, and 10 mg/kg | 15 days | [172] |
| Ovarian | BALB/c nude mice injected with SKOV3 cells (5 × 106 cells) | Inhibits tumor growth and Inhibited metastasis | ↓ EMT process, ↓ Ki-67+ cells, ↓ MMP-2+ cells | 40 mg/kg | 21 days | [176] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuli, H.S.; Sak, K.; Gupta, D.S.; Kaur, G.; Aggarwal, D.; Chaturvedi Parashar, N.; Choudhary, R.; Yerer, M.B.; Kaur, J.; Kumar, M.; et al. Anti-Inflammatory and Anticancer Properties of Birch Bark-Derived Betulin: Recent Developments. Plants 2021, 10, 2663. https://doi.org/10.3390/plants10122663
Tuli HS, Sak K, Gupta DS, Kaur G, Aggarwal D, Chaturvedi Parashar N, Choudhary R, Yerer MB, Kaur J, Kumar M, et al. Anti-Inflammatory and Anticancer Properties of Birch Bark-Derived Betulin: Recent Developments. Plants. 2021; 10(12):2663. https://doi.org/10.3390/plants10122663
Chicago/Turabian StyleTuli, Hardeep Singh, Katrin Sak, Dhruv Sanjay Gupta, Ginpreet Kaur, Diwakar Aggarwal, Nidarshana Chaturvedi Parashar, Renuka Choudhary, Mukerrem Betul Yerer, Jagjit Kaur, Manoj Kumar, and et al. 2021. "Anti-Inflammatory and Anticancer Properties of Birch Bark-Derived Betulin: Recent Developments" Plants 10, no. 12: 2663. https://doi.org/10.3390/plants10122663
APA StyleTuli, H. S., Sak, K., Gupta, D. S., Kaur, G., Aggarwal, D., Chaturvedi Parashar, N., Choudhary, R., Yerer, M. B., Kaur, J., Kumar, M., Garg, V. K., & Sethi, G. (2021). Anti-Inflammatory and Anticancer Properties of Birch Bark-Derived Betulin: Recent Developments. Plants, 10(12), 2663. https://doi.org/10.3390/plants10122663








Manoj_Kumar.jpg)


