Production of Gynogenic Plants of Red Beet (Beta vulgaris L.) in Unpollinated Ovule Culture In Vitro
Abstract
:1. Introduction
2. Results
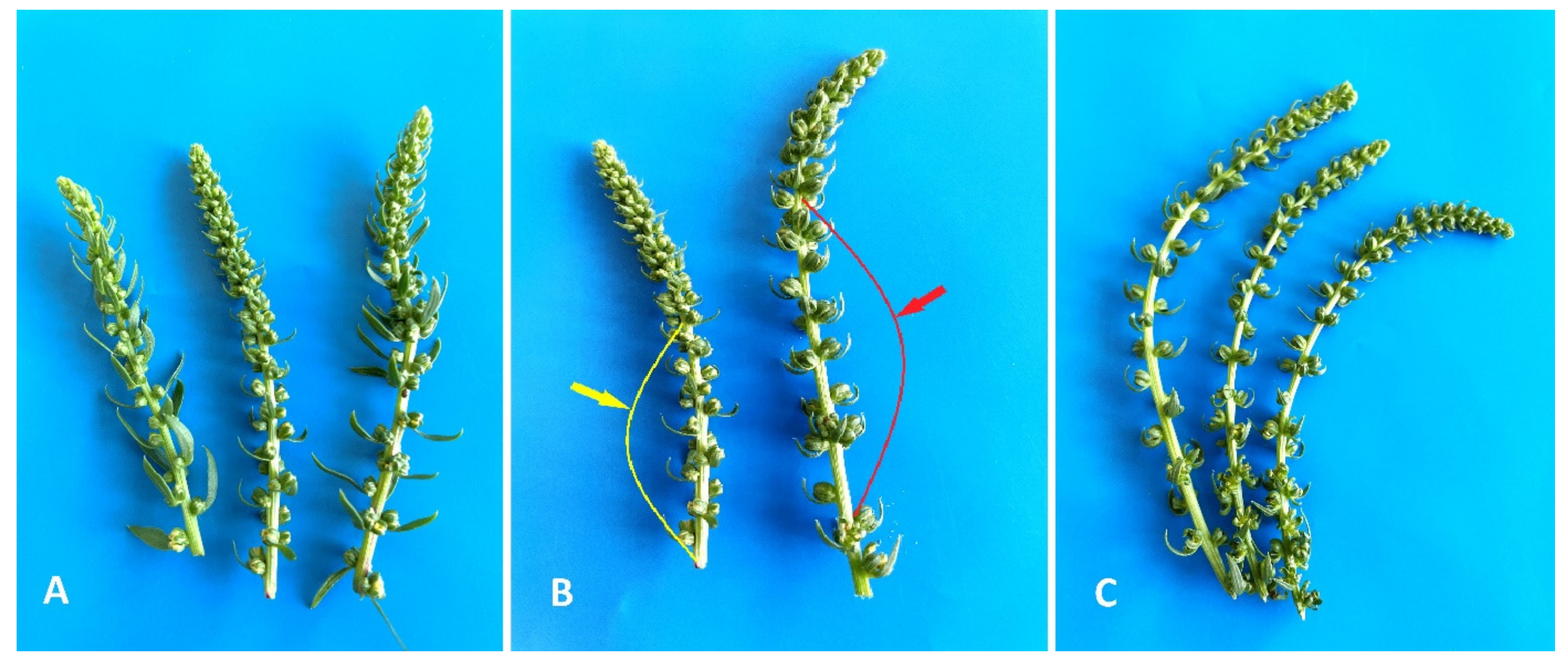
2.1. Induction of Gynogenesis in an Unpollinated Ovule Culture of Red Beet In Vitro
2.2. Effect of Silver Nitrate on Induction Capacity of Red Beet in an Unpollinated Ovule Culture In Vitro
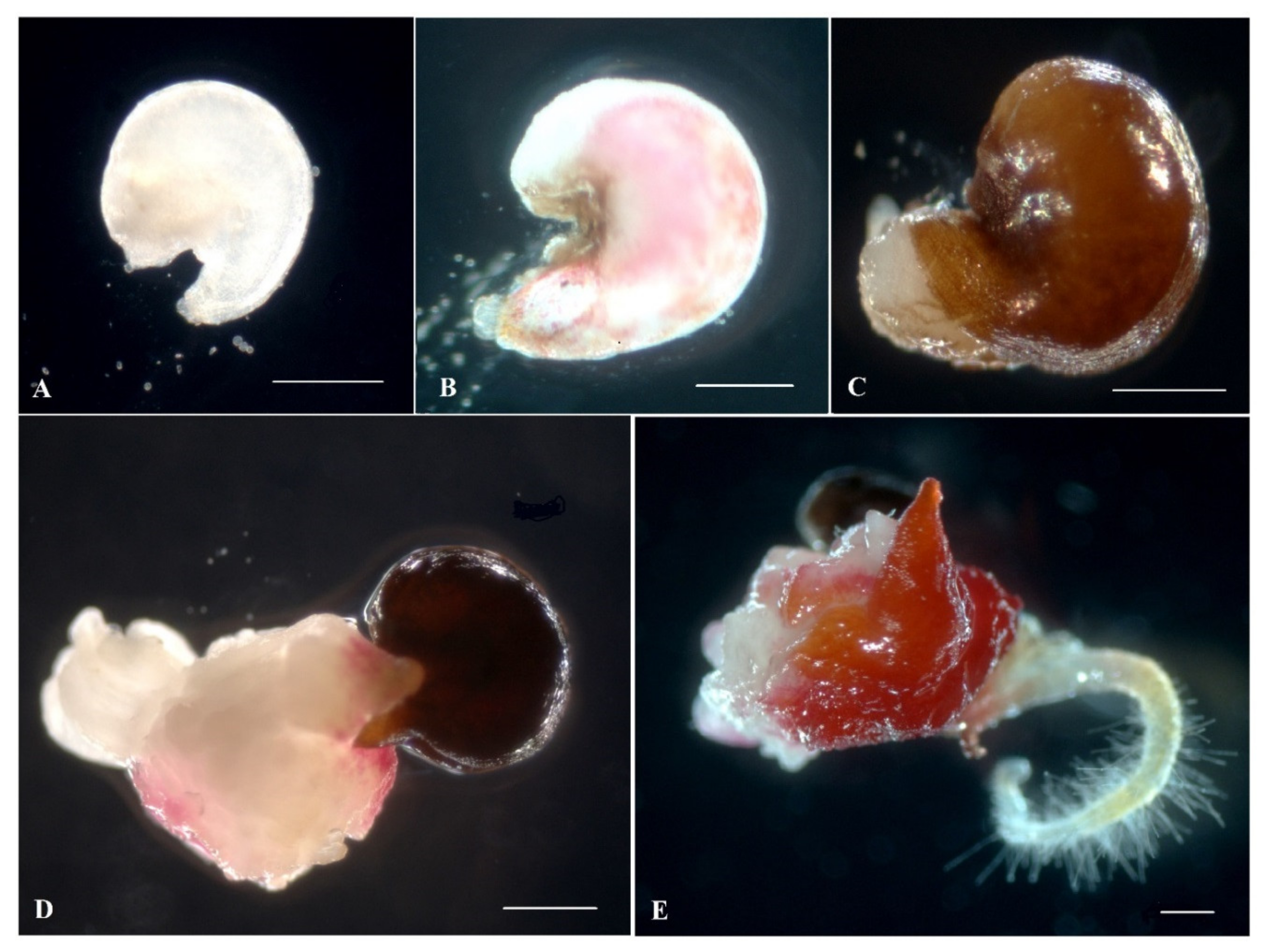
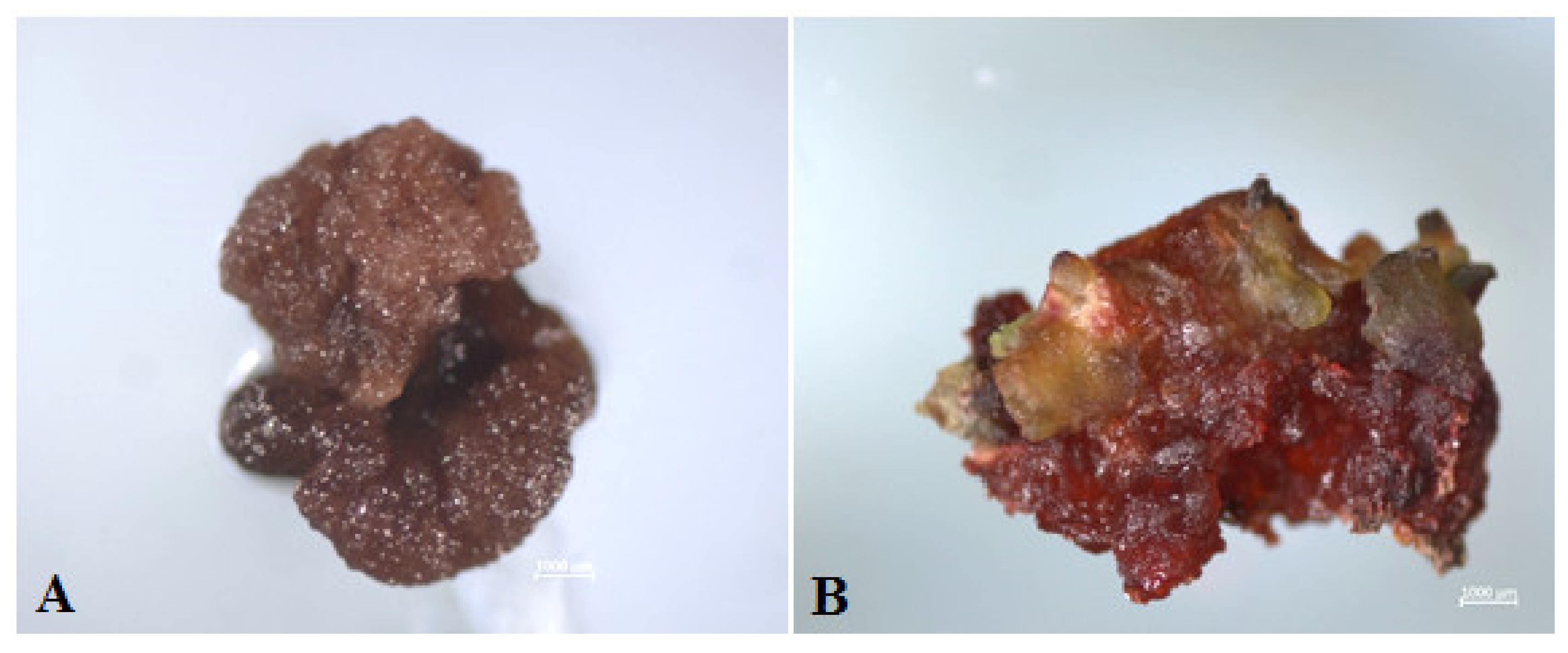
2.3. Plant Regeneration
2.4. Rooting of Regenerant Plants, Derived from Unpollinated Ovule Culture In Vitro
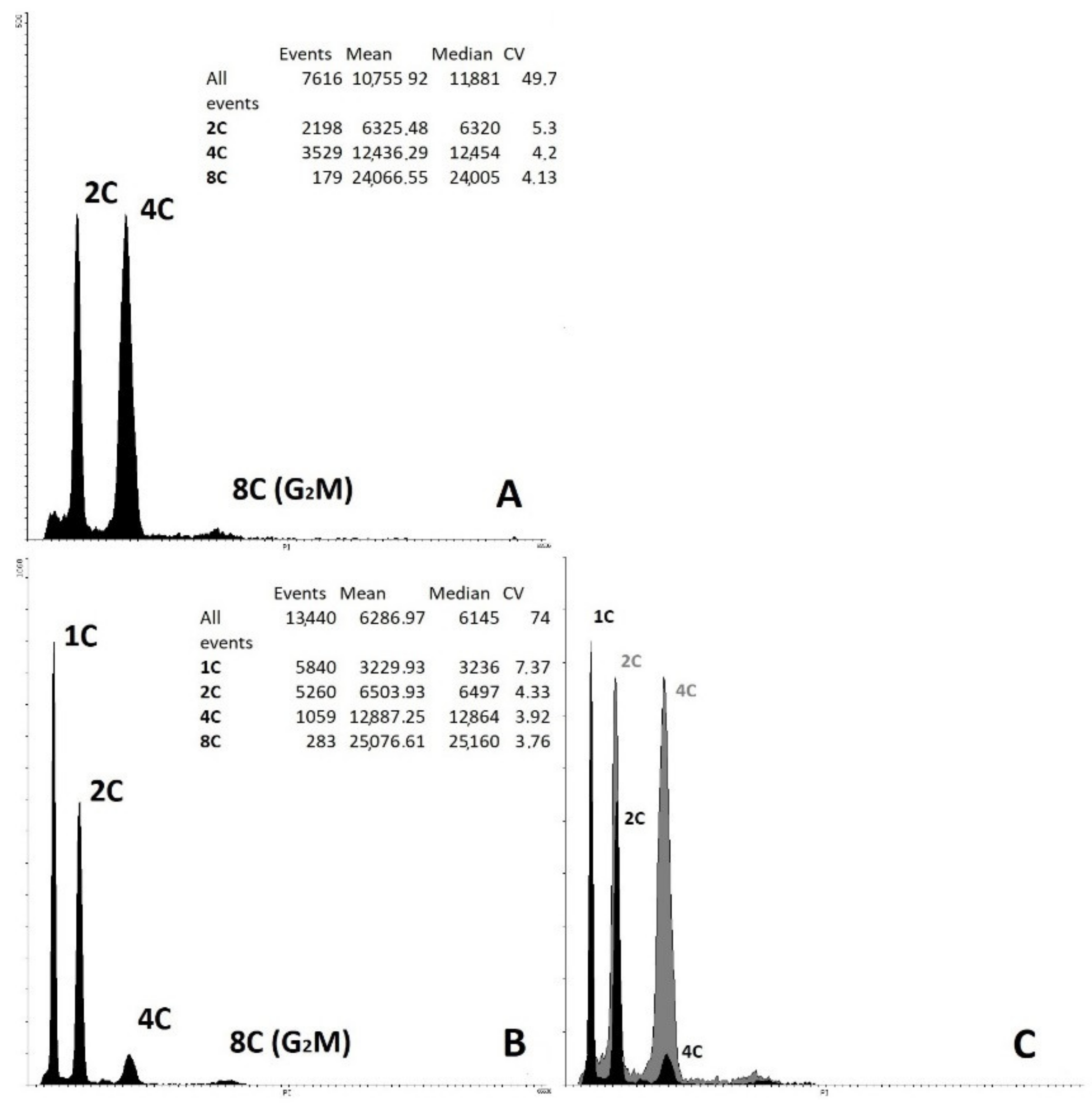
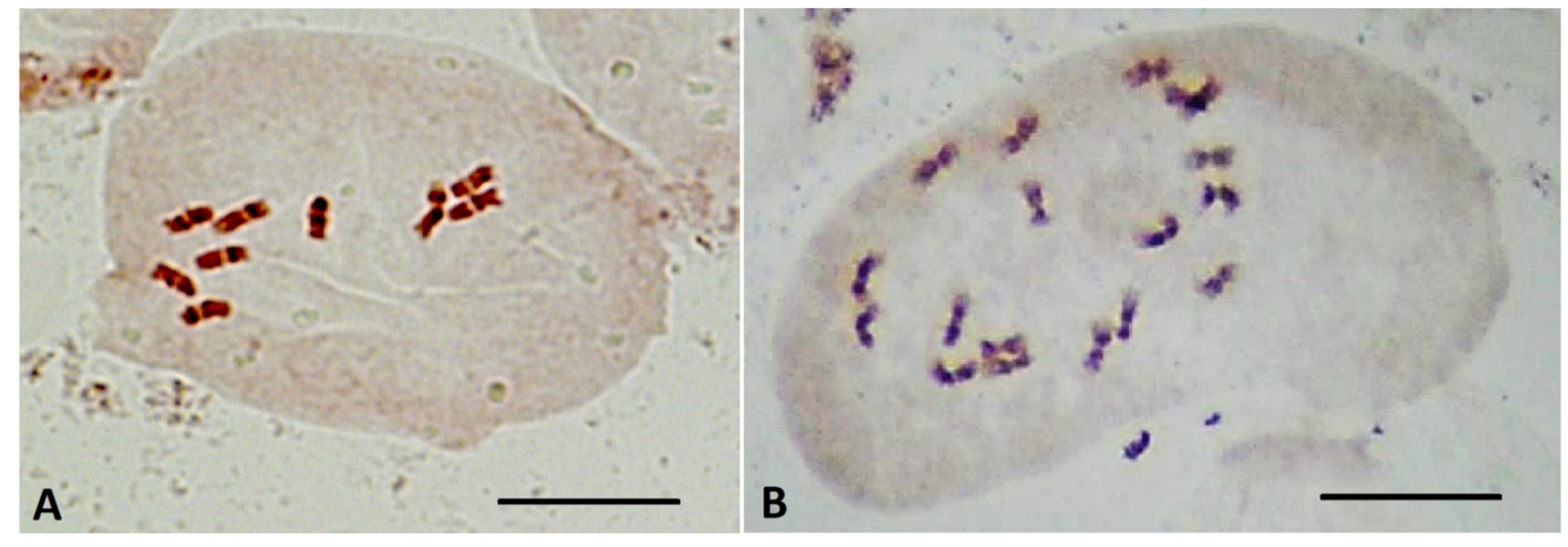
2.5. Determination of Ploidy of Regenerant Plants, Obtained in Unpollinated Ovule Culture
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growing Conditions
4.2. Sterilization of Explants
4.3. Cultivation of Unpollinated Ovules In Vitro
4.4. Plant Regeneration
4.5. Rooting of Regenerant Shoots
- (1)
- MS media without plant growth regulators supplemented with 2% sucrose, 3 g/L phytogel;
- (2)
- ½ IMB supplemented with 2 mg/L IBA, 2% sucrose, 3 g/L phytogel;
- (3)
- ½ IMB without plant growth regulators, supplemented with 2% sucrose and 3 g/L phytogel (basal part of microrosettes was placed in sterile concentrated IBA solution (50 mg/L) for 10–15 s before putting them on the nutrient medium). The second immersion in concentrated IBA solution was performed after two weeks. Transplantation to fresh nutrient medium was performed every two weeks. For rooting microrosettes obtained from cv. Dobrynya and b.a. 128, only a variant of nutrient medium № 3 was used.
4.6. Cultivation of Regenerating Plants
4.7. Ploidy-Level Determination by Flow Cytometry
4.8. Ploidy-Level Determination by Chromosome Counting
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devadiga, D.; Ahipa, T.N. Betanin: A Red-Violet Pigment—Chemistry and Applications. In Chemistry and Technology of Natural and Synthetic Dyes and Pigments; Samanta, A.K., Awwad, N., Algarni, H.M., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Nowacki, L.; Vigneron, P.; Rotellini, L.; Cazzola, H.; Merlier, F.; Prost, E.; Ralanairina, R.; Gadonna, J.P.; Rossi, C.; Vayssade, M. Betanin-Enriched Red Beetroot (Beta vulgaris L.) Extract Induces Apoptosis and Autophagic Cell Death in MCF-7 Cells. Phytother. Res. 2015, 12, 1964–1973. [Google Scholar] [CrossRef]
- Romero, S.A.; Pavan, I.C.B.; Morelli, A.P.; Mancini, M.C.S.; da Silva, L.G.S.; Fagundes, I.; Silva, C.H.R.; Ponte, L.G.S.; Rostagno, M.A.; Bezerra, R.M.N.; et al. Anticancer effects of root and beet leaf extracts (Beta vulgaris L.) in cervical cancer cells (HeLa). Phytother. Res. 2021, 35, 6191–6203. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef] [PubMed]
- Madadi, E.; Mazloum-Ravasan, S.; Yu, J.S.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Therapeutic Application of Betalains: A Review. Plants 2020, 9, 1219. [Google Scholar] [CrossRef]
- Vieira Teixeira da Silva, D.; dos Santos Baião, D.; de Oliveira Silva, F.; Alves, G.; Perrone, D.; Mere Del Aguila, E.M.; Flosi Paschoalin, V. Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments. Molecules 2019, 24, 458. [Google Scholar] [CrossRef] [Green Version]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food). Scientific opinion on the revaluation of beetroot red (E 162) as a food additive. EFSA J. 2015, 13, 4318. [Google Scholar]
- Nemzer, B.; Pietrzkowski, Z.; Spórna, A.; Stalica, P.; Thresher, W.; Michałowski, T.; Wybraniec, S. Betalainic and nutritional profiles of pigment-enriched red beet root (Beta vulgaris L.) dried extracts. Food Chem. 2011, 127, 42–53. [Google Scholar] [CrossRef]
- Pivovarov, V.F. Vegetables of Russia; VNIISSOK: Moscow, Russia, 2006; pp. 239–243. [Google Scholar]
- Fedorova, M.I.; Vetrova, S.A.; Zayachkovsky, V.A.; Stepanov, V.A. Red beet varieties selected by the VNIISSOK. Veg. Crop. Russ. 2016, 2, 56–59. [Google Scholar] [CrossRef] [Green Version]
- Burenin, V.I.; Sokolova, D.V.; Piskunova, T.M. Gene Pool for Red Beet Breeding (Current Aspects of Research and Use). Proc. Appl. Bot. Genet. Breed. 2019, 180, 19–25. [Google Scholar] [CrossRef]
- Available online: https://ab-centre.ru/news/svekla-stolovaya-ploschadi-i-sbory-v-rossii-v-2001-2020-gg (accessed on 20 October 2021).
- Biancardi, E.; McGrath, J.M.; Panella, L.W.; Lewellen, R.T.; Stevanato, P. Sugar Beet. In Root and Tuber Crops, Handbook of Plant Breeding; Bradshaw, J.E., Ed.; Springer: New York, NY, USA, 2010; Volume 7, pp. 173–219. [Google Scholar] [CrossRef]
- Kasha, K.J. Chromosome doubling and recovery of doubled haploid plants. In Haploids in Crop Improvement; Palmer, C.E., Keller, W.A., Kasha, K.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 56, pp. 123–152. [Google Scholar]
- Shariatpanahi, M.E.; Niazian, M.; Ahmadi, B. Methods for Chromosome Doubling. Methods Mol. Biol. 2021, 2287, 127–148. [Google Scholar] [CrossRef]
- Levan, A. A haploid sugar beet after colchicine treatment. Hereditas 1945, 31, 399–410. [Google Scholar] [CrossRef]
- Zimmermann, K. Vermendung haploider Pflanzen in der Zuchtung. Ber. Dtsch. Bot. Ges. 1953, 66, 28–30. [Google Scholar]
- Fischer, H.E. Untersuchungen an Zwillingen von Beta vulgaris L. Der Züchter 1956, 26, 136–152. [Google Scholar] [CrossRef]
- Butterfass, T. Ploidie und Chloroplastenzahlen. Ber. Dtsch. Bot. Ges. 1959, 72, 440–451. [Google Scholar]
- Kruse, A. Haploids in polyembryos of beet, Beta vulgaris L. Roy. Vet. Agric. College. Yearbook, Coppenhagen, Denmark. 1961; 87–98. [Google Scholar]
- Dobretsova, T.B.; Lutkov, A.N.; Manzhos, A.M. On the possibility of obtaining polyploid and haploid forms of sugar beet from twin plants. In Polyploidy and Selection; Nauka: Moscow, Russia, 1965; pp. 232–238. [Google Scholar]
- Hammond, B.L. Homozygous diploid sugar beets. J. Am. Soc. Sugar Beet Technol. 1966, 14, 75–78. [Google Scholar] [CrossRef]
- Bosemark, N. Haploids and homozygous diploids, triploids and tetraploids in sugar beet. Hereditas 1971, 69, 193–204. [Google Scholar] [CrossRef]
- Yuce, S. Haploidie bei der Zuckerriibe. Ph.D. Thesis, Justus Liebig University, Giessen, Germany, 1973. [Google Scholar]
- Guha, S.; Maheshwari, S.C. In vitro production of embryos from anthers of Datura. Nature 1964, 204, 497. [Google Scholar] [CrossRef]
- Tulecke, W. A haploid tissue culture from the female gametophyte of Ginkgo biloba L. Nature 1964, 203, 94–95. [Google Scholar] [CrossRef]
- San Noeum, L.H. Haploides d’Hordeum vulgare L. par culture in vitro d’ovaries non fecondes. Ann. Amelior Plant. 1976, 26, 751–754. [Google Scholar]
- Rogozinska, J.H.; Goska, M.; Kuzdowicz, A. Induction of plants from anthers of Beta vulgaris cultured in vitro. Acta Soc. Bot. Pol. 1977, 46, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Speckmann, G.J.; Van Geyt, J.P.C.; Jacobs, M. The induction of haploids of sugar beet (Beta vulgaris L.) using anther and free pollen culture or ovule and ovary culture. In Genetic Manipulation in Plant Breeding; Horn, W., Jensen, C.J., Odenbach, W., Schieder, O., Eds.; De Gruyter: Berlin, Germany, 1986; pp. 351–353. [Google Scholar]
- Hosemans, D.; Bossoutrot, D. Induction of haploid plant from in vitro culture of unpollinated beet ovules (Beta vulgaris L.). J. Plant Breed. 1983, 91, 74–77. [Google Scholar]
- D’Halluin, K.; Keimer, B. Production of haploid sugar beets (Beta vulgaris L.) by ovule culture. In Genetic Manipulation in Plant Breeding; Horn, W., Jensen, C.J., Odenbach, W., Schieder, O., Eds.; De Gruyter: Berlin, Germany, 1986; pp. 307–309. [Google Scholar]
- Van Geyt, J.; Speckmann, G.J.; D’Halluin, K.; Jacobs, M. In vitro induction of haploid plants from unpollinated ovules and ovaries of the sugarbeet (Beta vulgaris L.). Theor. Appl. Genet. 1987, 73, 920–925. [Google Scholar] [CrossRef]
- Zhuzhzhalova, T.P.; Kolesnikova, E.O.; Vasilchenko, E.N.; Cherkasova, N.N. Biotechnological methods as a tool for efficient sugar beet breeding. Vavilov J. Genet. Breed. 2020, 24, 40–47. [Google Scholar] [CrossRef]
- Mikami, T.; Sudoh, R.; Nagao, E.; Kinoshita, T. Genotypic variation in the in vitro morphogenesis from leaf explants of Beta vulgaris L. and B. maritima L. Euphytica 1989, 40, 271–273. [Google Scholar] [CrossRef]
- Zhang, C.L.; Chen, D.F.; Elliott, M.C.; Slater, A. Efficient procedures for callus induction and adventitious shoot organogenesis in sugar beet (Beta vulgaris L.) breeding lines. In Vitro Cell. Dev. Biol. Plant. 2004, 40, 475–481. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Kadłuczka, D.; Wȩdzony, M.; Dedicova, B.; Grzebelus, E. Refinement of a clearing protocol to study crassinucellate ovules of the sugar beet (Beta vulgaris L., Amaranthaceae). Plant Methods 2019, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Karimi-Ashtiyani, R.; Ishii, T.; Niessen, M.; Stein, N.; Heckmann, S.; Gurushidze, M.; Banaei-Moghaddam, A.M.; Fuchs, J.; Schubert, V.; Koch, K.; et al. Point mutation impairs centromeric CENH3 loading and induces haploid plants. Proc. Natl. Acad. Sci. USA 2015, 112, 11211–11216. [Google Scholar] [CrossRef] [Green Version]
- Lux, H.; Herrmann, L.; Wetzel, C. Production on haploid sugar beet (Beta vulgaris L.) by culturing unpollinated ovules. Plant Breed. 1990, 104, 177–183. [Google Scholar] [CrossRef]
- Weich, E.W.; Levall, M.W. Doubled haploid production of sugar beet (Beta vulgaris L.): Published protocols for other crop plant species. In Doubled Haploid Production in Crop Plants: A Manual; Maluszynski, M., Kasha, K.J., Forster, B.P., Szareiko, J., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 255–263. [Google Scholar] [CrossRef]
- Svirshchevskaya, A.; Dolezel, J. Production and Performance of Gynogenetic Sugarbeet Lines. J. Sugar Beet Res. 2000, 37, 117–133. [Google Scholar] [CrossRef] [Green Version]
- Nagl, N.; Mezei, S.; Kovacev, L.; Vasic, D.; Cacic, N. Induction and micropropagation potential of sugar beet haploids. Genetika 2004, 36, 187–194. [Google Scholar] [CrossRef]
- Hansen, A.L.; Gertz, A.; Joersbo, M.; Andersen, S.B. Short-duration colchicine treatment for in vitro chromosome doubling during ovule culture of Beta vulgaris L. Plant Breed. 1995, 114, 515–519. [Google Scholar] [CrossRef]
- Gurel, S.; Gurel, E.; Kaya, Z. Doubled haploid plant production from unpollinated ovules of sugar beet (Beta vulgaris L.). Plant Cell Rep. 2000, 19, 1155–1159. [Google Scholar] [CrossRef]
- Pazuki, A.; Aflaki, F.; Gürel, E.; Ergül, A.; Gürel, S. Gynogenesis Induction in Sugar Beet (Beta vulgaris) Improved by 6-Benzylaminopurine (BAP) and Synergized with Cold Pretreatment. Sugar Tech. 2017, 20, 69–77. [Google Scholar] [CrossRef]
- Pazuki, A.; Aflaki, F.; Gurel, S.; Ergul, A.; Gurel, E. The effects of proline on in vitro proliferation and propagation of doubled haploid sugar beet (Beta vulgaris). Turk. J. Bot. 2018, 42, 280–288. [Google Scholar]
- Tomaszewska-Sowa, M. Cytometric analyses of sugar beet (Beta vulgaris L.) plants regenerated from unfertilized ovules cultured in vitro. Electron. J. Pol. Agric. Univ. 2010, 13, 13. [Google Scholar]
- Sohrabi, S.; Abdollahi, M.R.; Mirzaie-Asl, A.; Koulaei, H.E.; Aghaeezadeh, M.; Seguí-Simarro, J.M. A refined method for ovule culture in sugar beet (Beta vulgaris L.). Plant Cell Tissue Organ Cult. 2021, 146, 259–267. [Google Scholar] [CrossRef]
- Baranski, R. In vitro gynogenesis in red beet (Beta vulgaris L.): Effects of ovule culture conditions. Acta Soc. Bot. Pol. Tow. Bot. 1996, 65, 57–60. [Google Scholar] [CrossRef] [Green Version]
- Gorecka, K.; Kryzanowska, D.; Kowalska, U.; Kiszczak, W.; Podwyszynska, M. Development of embryoids by microspore and anther cultures of red beet (Beta vulgaris L. subsp. vulgaris). J. Cent. Eur. Agric. 2017, 18, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Kiszczak, W.; Burian, M.; Kowalska, U.; Gorecka, K.; Podwyszynska, M. Production of Homozygous Red Beet (Beta vulgaris L. subsp. vulgaris) Plants by Ovule In Doubled Haploid Technology: Emerging Tools, Cucurbits, Trees, Other Species, Methods in Molecular Biology; Segui-Simarro, J.M., Ed.; Humana: New York, NY, USA, 2021; Volume 3, pp. 301–312. [Google Scholar] [CrossRef]
- Ayed Slama, O.; Slim Amara, H. Unpollinated Ovaries Used to Produce Doubled Haploid Lines in Durum Wheat. Methods Mol. Biol. 2021, 2287, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Bohanec, B.; Jakše, M.; Ihan, A.; Javornik, B. Studies of gynogenesis in onion (Allium cepa L.) induction procedures and genetic analysis of regenerants. Plant. Sci. 1995, 104, 215–224. [Google Scholar] [CrossRef]
- Alan, A.R. Doubled Haploid Onion (Allium cepa L.) Production Via In Vitro Gynogenesis. Methods Mol. Biol. 2021, 2287, 151–169. [Google Scholar] [CrossRef]
- Gémes-Juhász, A.; Balogh, P.; Ferenczy, A.; Kristóf, Z. Effect of optimal stage of female gametophyte and heat treatment on in vitro gynogenesis induction in cucumber (Cucumis sativus L.). Plant Cell Rep. 2002, 21, 105–111. [Google Scholar]
- Kurtar, E.S.; Seymen, M. Gynogenesis in Cucurbita Species. Doubled In Haploid Technology; Humana: New York, NY, USA, 2021; pp. 123–133. [Google Scholar] [CrossRef]
- Tang, F.; Tao, Y.; Zhao, T.; Wang, G. In vitro production of haploid and doubled haploid plants from pollinated ovaries of maize (Zea mays). Plant Cell Tissue Organ Cult. 2006, 84, 233–237. [Google Scholar] [CrossRef]
- Doctrinal, M.; Sangwan, R.S.; Sangwan-Norreel, B.S. In vitro gynogenesis in Beta vulgaris L.: Effects of plant growth regulators, temperature, genotypes and season. Plant Cell Tissue Organ Cult. 1989, 17, 1–12. [Google Scholar] [CrossRef]
- Galatowitsch, M.W.; Smith, G.A. Regeneration from unfertilized ovule callus of sugarbeet (Beta vulgaris L.). Can. J. Plant Sci. 1990, 70, 83–89. [Google Scholar] [CrossRef]
- Svirshchevskaya, A.; Kozurevich, T.P.; Bormotov, V.E. Haploids in the culture of unfertilized ovules in beet. Natl. Acad. Sci. Belarus 1993, 37, 74–76. [Google Scholar]
- Hansen, A.L.; Plever, C.; Pedersen, H.C.; Keimer, B.; Andersen, S.B. Efficient in vitro chromosome doubling during Beta vulgaris ovule culture. Plant Breed. 1994, 112, 89–95. [Google Scholar] [CrossRef]
- Gurel, S.; Pazuki, A.; Aflaki, F.; Gurel, E. Production of Doubled Haploid Sugar Beet (Beta vulgaris L.) Plants Through Gynogenesis. In Doubled Haploid Technology: Emerging Tools, Cucurbits, Trees, Other Species; Methods in Molecular Biology; Segui-Simarro, J.M., Ed.; Humana: New York, NY, USA, 2021; Volume 3, pp. 313–323. [Google Scholar] [CrossRef]
- Ferrant, V.; Bouharmont, J. Origin of gynogenetic embryos of Beta vulgaris L. Sex. Plant Reprod. 1994, 7, 12–16. [Google Scholar] [CrossRef]
- Bossoutrot, D.; Hosemans, D. Gynogenesis in Beta vulgaris L.: From in vitro culture of unpollinated ovules to the production of doubled haploid plants in soil. Plant Cell Rep. 1985, 4, 300–303. [Google Scholar] [CrossRef]
- Aflaki, F.; Pazuki, A.; Gurel, S.; Stevanato, S.; Biancardi, E.; Gurel, E. Doubled haploid sugar beet: An integrated view of factors influencing the processes of gynogenesis and chromosome doubling. Int. Sugar J. 2017, 119, 884–895. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Zhu, Z.C.; Wu, H.S. In vitro induction of haploid plantlets from unpollinated ovaries of Triticum aestivum L. and Nicotiana tabacum L. Acta Genet. Sin. 1979, 6, 181–184. [Google Scholar]
- Chu, C.C.; Wang, C.C.; Sun, C.S.; Hsu, C.; Yin, K.C.; Chu, C.Y.; Bi, F.Y. Anther culture of cereal. Sci. Sinica. 1975, 18, 659–668. [Google Scholar]
- Mok, M.C.; Mok, D.W.S.; Armstrong, D.J. Cytokinin activity of N-phenyl-N’-l,2,3-thiadiazol-5-yl urea (Thidiazuron). Phytochemistry 1982, 21, 1509–1511. [Google Scholar] [CrossRef]
- Shantz, E.M.; Steward, F.C. Coconut milk factor: The growth-promoting substance in coconut milk. J. Amer. Chem. Soc. 1955, 77, 6351–6353. [Google Scholar] [CrossRef]
- Lu, C.Y. The use of thidiazuron in tissue culture. In Vitro Cell. Dev. Biol. Plant 1993, 29, 92–96. [Google Scholar] [CrossRef]
- Arndt, F.; Rusxh, R.; Stilfried, H.V.S.N. 49537 A new cotton defoliant. Plant Physiol. 1976, 57, 99. [Google Scholar]
- Thomas, J.C.; Katterman, F.R. Cytokinin activity induced by thidiazuron. Plant Physiol. 1986, 81, 681–683. [Google Scholar] [CrossRef] [Green Version]
- Domblides, E.A.; Shmykova, N.A.; Belov, S.N.; Korottseva, I.B.; Soldatenko, A.V. DH-plant production in culture of unpollinated ovules of cucumber (Cucumis sativus L.). Veg. Crop. Russ 2019, 7132, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Eujayl, I.; Strausbaugh, C.; Lu, C. Registration of sugarbeet doubled haploid line KDH13 with resistance to beet curly top. J. Plant Regist. 2016, 10, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Hansen, A.L.; Gertz, A.; Joersbo, M.; Andersen, S.B. Antimicrotubule herbicides for in vitro chromosome doubling in Beta vulgaris L. ovule culture. Euphytica 1998, 101, 231–237. [Google Scholar] [CrossRef]
- Polevoy, V.V. Plant Physiology; Textbook for Biological Specialties of Higher Education Institutions; Vysshaya shkola: Moscow, Russia, 1989; pp. 244–379. [Google Scholar]
- Raldugina, G.I.; Sobolkova, G.N. Genotypic differences in the action of abscisic acid on callus cultures of Brassica Napus, L. Plant Physiol. 1994, 41, 702–706. [Google Scholar]
- Chi, G.L.; Pua, E.C.; Goh, C.J. Role ethylene on de novo shoot regeneration from cotyledonary explants of Brassica campestris ssp. pekinensia (lour) Olesson in vitro. Plant Physiol. 1991, 96, 176–183. [Google Scholar] [CrossRef] [Green Version]
- Pua, E.C.; Chi, G.L. De novo shoot morphogenesis and plant growth of mustard (Brassica juncea) in vitro in relation to ethylene. Physiol. Plant 1993, 88, 467–474. [Google Scholar] [CrossRef]
- Pua, E.C.; Sim, G.E.; Chi, G.L.; Kong, L.F. Synergistic effect of inhibitors and putrescine on shoot regeneration from hypocotyls explants of Chinese radish (Raphanus sativus L. var. longipinnatus Bailey) in vitro. Plant Cell Rep. 1996, 15, 685–690. [Google Scholar] [CrossRef]
- Chi, G.L.; Barfield, D.G.; Sim, G.E.; Pua, E.C. Effect of AgNO3 and aminoethoxyvinylglycin on in vitro shoot and root organogenesis from seedlings explants of recalcitrant Brassica genotypes. Plant Cell Rep. 1990, 9, 195–198. [Google Scholar] [CrossRef]
- Burnett, L.; Arnoldo, M.; Yarrow, S.; Huang, B. Enhancements of shoot regeneration from cotyledon explants of Brassica rapa ssp. oleifera through pretreatment with auxin and cytokinin and use of ethylene inhibitors. Plant Cell Tissue Organ Cult. 1994, 37, 253–256. [Google Scholar]
- Songstad, D.D.; Duncan, D.R.; Whdholm, J.M. Effect of I-amino-cyclopropane-I-carboxylic acid, silver nitrate and norbornadiene on plant regeneration from maize callus cultures. Plant Cell Rep. 1988, 7, 262–265. [Google Scholar] [CrossRef]
- Purnhauser, L.; Medvyesy, M.; Czeko, P.J.; Marton, L. Stimulation of shoot regeneration in Triticum aestivum and Nicotiana plumbaginifolia Vivo tissue culture using the ethylene inhibitor AgNO3. from cotyledonary explants. Plant Cell Rep. 1987, 6, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Bais, H.P.; Sudha, G.S.; Suresh, B.; Ravishankar, G.A. AgnO3 influences in vitro root formation in Decalepis hamiltonii Wight. Arn. Current Sci. 2000, 79, 894–898. [Google Scholar]
- Bais, H.P.; Sudha, G.S.; Ravishankar, G.A. Influence of putrescine AgNO3 and polyamine inhibitors on the morphogenetic response in untransformed and transformed tissues of Chichorium intybus and their regenerants. Plant Cell Rep. 2001, 20, 547–555. [Google Scholar] [CrossRef]
- Eapen, S.; Geoorge, L. Plant regeneration from peduncle segments of oil seed brassica species: Influence of AgNO3 and silver thiosulphate. Plant Cell Tissue Organ Cult. 1997, 51, 229–232. [Google Scholar] [CrossRef]
- Theologis, A. One rotten apple spoils the whole bushel: The role of ethylene in fruit ripening. Cell 1992, 70, 181–184. [Google Scholar] [CrossRef]
- Beyer, E.M. A potent inhibitor of ethylene action in plants. Plant Physiol. 1976, 58, 268–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomaszewska-Sowa, M. Effect of growth regulators and other components of culture medium on morphogenesis of sugar beet (Beta vulgaris L.) in unfertilised ovule in vitro cultures. Acta Agrobot. 2012, 65, 91–100. [Google Scholar] [CrossRef]
- Pedersen, H.C.; Keimer, B. Haploidy in sugar beet (Beta vulgaris L.). In In Vitro Haploid Production in Higher Plants; Jain, S.M., Sopory, S.K., Veilleux, R.E., Eds.; Important Selected Plants. Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; Volume 3, pp. 17–36. [Google Scholar] [CrossRef]
- Gurel, E.; Wren, M.J. In vitro development from leaf explants of sugar beet (Beta vulgaris L). Rhizogenesis and the effect of sequential exposure to auxin and cytokinin. Ann. Bot. 1995, 75, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Bhojwani, S.S.; Razdan, M.K. Plant Tissue Culture: Theory and Practice; Elsevier Science Publishing: Amsterdam, The Netherlands, 1983; p. 194. [Google Scholar]
- Goska, M. Monographs and Scientific Dissertations. In Haploids and Sub-Haploids of Sugar Beet (Beta vulgaris L.) and Possibilities of Their Use in Breeding; Institute of Plant Breeding and Acclimatization: Radzików, Poland, 1997. [Google Scholar]
- Jakse, M.; Bohanec, B. Haploid induction in onion via gynogenesis. In Doubled Haploid Production in Crop Plants: A Manual; Maluszynski, M., Kasha, K.J., Forster, B.P., Szarejko, I., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 281–285. [Google Scholar] [CrossRef]
- da Silva Dias, J.C. Protocol for broccoli microspore culture. In Doubled Haploid Production in Crop Plants: A Manual; Maluszynski, M., Kasha, K.J., Forster, B.P., Szarejko, I., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 195–204. [Google Scholar] [CrossRef]
- Touraev, A.; Heberle-Bors, E. Anther and microspore culture in tobacco. In Doubled Haploid Production in Crop Plants: A Manual; Maluszynski, M., Kasha, K.J., Forster, B.P., Szarejko, I., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 223–228. [Google Scholar] [CrossRef]
- Skaptsov, M.V.; Smirnov, S.V.; Kutsev, M.G.; Shmakov, A.I. Problems of a standardization in plant flow cytometry. Turczaninowia 2016, 19, 120–122. [Google Scholar]
- Dospekhov, B.A. Methodology of the Field Experiment (with the Basics of Statistical Processing of Research Results), 5th ed.; Agropromizdat: Moscow, Russia, 1985; p. 351. [Google Scholar]




| Genotype | Average Number of Induced Ovules/Petri Dish | Maximum Number of Induced Ovules/Petri Dish | Average Number of Microshoots In Vitro/Ovule | Number of Plants Acclimatized to Ex Vitro Conditions |
|---|---|---|---|---|
| cv. Nezhnost’ | 7.4 ± 0.23 f | 9 | 4.9 ± 1.8 b | 10 |
| cv. Dobrynya | 5.2 ± 0.17 d | 6 | 3.3 ± 0.8 b | 3 |
| b.a. P-155 | 1.6 ± 0.18 b,c | 3 | 0 a | 0 |
| b.a. B-131 | 6.6 ± 0.18 e | 8 | 2.1 ± 0.5 b | 0 |
| b.a. 135 | 1.2 ± 0.37 b | 2 | 0 a | 0 |
| b.a. 128 | 2.0 ± 0.14 c | 3 | 2.3 ± 0.7 b | 2 |
| b.a. 130 | 0 a | 0 | 0 a | 0 |
| b.a. 132 | 0 a | 0 | 0 a | 0 |
| b.a. 142 | 0 a | 0 | 0 a | 0 |
| b.a. 148 | 0 a | 0 | 0 a | 0 |
| b.a. 138 | 0 a | 0 | 0 a | 0 |
| Genotype | Number of Induced Ovules | Type of Induction, Embryoid/Callus, Number of Ovules | Number Microrosetts Received | Number of Dead Microrosetts | Number of Microshoots Preserved in Culture In Vitro | Number of Microshoots with Roots | |
|---|---|---|---|---|---|---|---|
| cv. Nezhnost’ | 37 | Embryoid | 9 | 19 | 11 | 5 | 3 |
| Callus | 28 | 74 | 33 | 22 | 19 | ||
| cv. Dobrynya | 26 | Embryoid | 6 | 23 | 11 | 8 | 4 |
| Callus | 20 | 48 | 23 | 13 | 12 | ||
| b.a. B-131 | 33 | Embryoid | 5 | 9 | 9 | 0 | 0 |
| Callus | 28 | 15 | 15 | 0 | 0 | ||
| b.a. P-155 | 8 | Embryoid | 0 | 0 | 0 | 0 | 0 |
| Callus | 8 | 0 | 0 | 0 | 0 | ||
| b.a. 128 | 10 | Embryoid | 0 | 0 | 0 | 0 | 0 |
| Callus | 10 | 28 | 14 | 8 | 6 | ||
| b.a. 135 | 6 | Embryoid | 0 | 0 | 0 | 0 | 0 |
| Callus | 6 | 0 | 0 | 0 | 0 | ||
| Variant of Nutrient Medium | Number of Microrosetts | Number of Rooted Microrosetts | Rooted Microrosetts, p ± Sp *, % |
|---|---|---|---|
| MS without plant growth regulators | 20 | 1 | 5.0 ± 4.9 |
| ½ IMB + 2 mg/L IBA | 20 | 5 | 25.0 ± 9.7 |
| ½ IMB without plant growth regulators + IBA (immersion) | 20 | 16 | 80.0 ± 8.9 |
| Nutrient Medium Components | Concentration of Components in the Nutrient Medium (mg/L) | ||
|---|---|---|---|
| Induction Medium IMB (Induction Medium For Beta vulgaris) | Regeneration Medium MS (Murashige, Skoog, 1962) | Rooting Medium 1/2 IMB | |
| NH4NO3 | 825.000 | 1650.0 | 412.500 |
| (NH4)2SO4 | 67.000 | - | 33.500 |
| NaH2PO4 ·2H2O | 84.800 | - | 42.400 |
| KNO3 | 2200.000 | 190.000 | 1100.000 |
| CaCl2 | 222.700 | 332.200 | 111.350 |
| MgSO4·7H2O | 310.000 | 371.000 | 155.000 |
| KH2PO4 | 85.000 | 170.000 | 42.500 |
| Na2EDTA·2H2O | 37.300 | 37.300 | 18.650 |
| FeSO4 7H2O | 27.800 | 27.800 | 13.900 |
| H3BO3 | 4.600 | 6.200 | 2.300 |
| MnSO4·5H2O | 19.200 | 24.100 | 9.600 |
| ZnSO4·7H2O | 6.300 | 10.600 | 3.150 |
| KI | 0.790 | 0.830 | 0.395 |
| Na2MoO4·2H2O | 0.250 | 0.250 | 0.125 |
| CuSO4·5H2O | 0.025 | 0.025 | 0.012 |
| CoCl2·6H2O | 0.025 | 0.025 | 0.012 |
| Thiamine-HCl (B1) | 5.050 | 0.100 | 2.525 |
| Pyridoxine-HCl (B6) | 0.750 | 0.500 | 0.375 |
| Nicotinic acid | 0.750 | 0.500 | 0.375 |
| Glycine | 1.000 | 2.000 | 0.500 |
| Myo-Inositol | 100.000 | 100.000 | 50.000 |
| L-Glutamine | 800.000 | - | - |
| Glutathione | 30.000 | - | - |
| L-Serine | 100.000 | - | - |
| Sucrose | 55,000.000 | 20,000.000 | 20,000.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayachkovskaya, T.; Domblides, E.; Zayachkovsky, V.; Kan, L.; Domblides, A.; Soldatenko, A. Production of Gynogenic Plants of Red Beet (Beta vulgaris L.) in Unpollinated Ovule Culture In Vitro. Plants 2021, 10, 2703. https://doi.org/10.3390/plants10122703
Zayachkovskaya T, Domblides E, Zayachkovsky V, Kan L, Domblides A, Soldatenko A. Production of Gynogenic Plants of Red Beet (Beta vulgaris L.) in Unpollinated Ovule Culture In Vitro. Plants. 2021; 10(12):2703. https://doi.org/10.3390/plants10122703
Chicago/Turabian StyleZayachkovskaya, Tatyina, Elena Domblides, Vladimir Zayachkovsky, Lyudmila Kan, Arthur Domblides, and Alexey Soldatenko. 2021. "Production of Gynogenic Plants of Red Beet (Beta vulgaris L.) in Unpollinated Ovule Culture In Vitro" Plants 10, no. 12: 2703. https://doi.org/10.3390/plants10122703
APA StyleZayachkovskaya, T., Domblides, E., Zayachkovsky, V., Kan, L., Domblides, A., & Soldatenko, A. (2021). Production of Gynogenic Plants of Red Beet (Beta vulgaris L.) in Unpollinated Ovule Culture In Vitro. Plants, 10(12), 2703. https://doi.org/10.3390/plants10122703







