Iodine and Selenium Biofortification of Chervil Plants Treated with Silicon Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biometrical Parameters, Dry Matter and Nitrates
2.2. Photosynthetic Pigments
2.3. Total Antioxidant Activity and Phenolic Content
2.4. Mineral Composition
2.4.1. Se, I and Si
2.4.2. Macro-Elements
2.4.3. Micro-Elements
2.4.4. As, Al, Cd, Cr, Ni, Pb, Sr and V
2.4.5. Correlations
3. Materials and Methods
3.1. Growing Conditions and Experimental Protocol
3.2. Colloidal Solution of Silicon Nanoparticles
3.2.1. Laser Ablation in Liquid (LAL)
3.2.2. Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES)
3.2.3. Dynamic Light Scattering (DLS) and Z-Potential
3.3. Biochemical Analyses and Elemental Composition of Plants
3.3.1. Dry Matter
3.3.2. Photosynthetic Pigments
3.3.3. Ascorbic Acid
3.3.4. Preparation of Ethanolic Extracts
3.3.5. Total Polyphenols (TP)
3.3.6. Antioxidant Activity (AOA)
3.3.7. Nitrates
3.3.8. Element Composition
3.3.9. Determination of Iodine
3.3.10. Determination of Selenium
3.3.11. Biofortification Values
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Golubkina, N.; Moldovan, A.; Kekina, H.; Kharchenko, V.; Sekara, A.; Vasileva, V.; Skrypnik, L.; Tallarita, A.; Caruso, G. Joint Biofortification of Plants with Iodine and Selenium: A New Field of Discoveries. Plants 2021, 10, 1352. [Google Scholar] [CrossRef]
- Golubkina, N.; Papazyan, T. Selenium in Nutrition. Plants, Animals, Human beings-Moscow; Pechatny Gorod: Yekaterinburg, Russia, 2006. (In Russian) [Google Scholar]
- Rayman, M.P. The importance of Se to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Zimmermann, M.B. The role of iodine in human growth and development. Semin. Cell Dev. Biol. 2011, 22, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.Y.; Dambaeva, S.; Kwak-Kim, J.; Gilman-Sachs, A.; Beaman, K.D. A Role for Iodide and Thyroglobulin in Modulating the Function of Human Immune Cells. Front. Immunol. 2017, 8, 1573. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Vitamin and Mineral Requirements in Human Nutrition; Report of a Joint FAO/WHO Expert Consultation; WHO: Bangkok, Tailand; Geneva, Switzerland, 2004. [Google Scholar]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminen, P.; Hietaniemi, V.; Aspila, P.; et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 2015, 31, 142–147. [Google Scholar] [CrossRef]
- Smolen, S.; Kowalska, I.; Halka, M.; Ledwozyw-Smolen, I.; Grzanka, M.; Skoczylas, Ł.; Czernicka, M.; Pitala, J. Selected Aspects of Iodate and Iodosalicylate Metabolism in Lettuce Including the Activity of Vanadium Dependent Haloperoxidases as Affected by Exogenous Vanadium. Agronomy 2020, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Golubkina, N.; Gomez, L.; Kekina, H.; Hallam, R.; Tallarita, A.; Cozzolino, E.; Torino, V.; Koshevarov, A.; Cuciniello, A.; Maiello, R.; et al. Joint Selenium-iodine Supply and Arbuscular Mycorrhizal Fungi Inoculation Affect Yield and Quality of Chickpea Seeds and Residual Biomass. Plants 2020, 9, 804. [Google Scholar] [CrossRef]
- Smolen, S.; Wierzbínska, J.; Sady, W.; Kołton, A.; Wiszniewska, A.; Liszka-Skoczylas, M. Iodine biofortification with additional application of salicylic acid affects yield and selected parameters of chemical composition of tomato fruits (Solanum lycopersicum L.). Sci. Hortic. 2015, 188, 89–96. [Google Scholar] [CrossRef]
- Badawy, S.A.; Zayed, B.A.; Bassiouni, S.M.A.; Mahdi, A.H.A.; Majrashi, A.; Ali, E.F.; Seleiman, M.F. Influence of Nano Silicon and Nano Selenium on Root Characters, Growth, Ion Selectivity, Yield, and Yield Components of Rice (Oryza sativa L.) under Salinity Conditions. Plants 2021, 10, 1657. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Feizi, M.; Kumari, A.; Khan, M.; Mandzhieva, S.; Sushkova, S.; El-Ramady, H.; Verma, K.K.; Singh, A.; et al. Effects of Silicon and Silicon-Based Nanoparticles on Rhizosphere Microbiome, Plant Stress and Growth. Biology 2021, 10, 791. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.-P.; Li, D.-M.; Singh, M.; Rajput, V.D.; Malviya, M.K.; Minkina, T.; Singh, R.K.; Singh, P.; Li, Y.-R. Interactive Role of Silicon and Plant–Rhizobacteria Mitigating Abiotic Stresses: A New Approach for Sustainable Agriculture and Climate Change. Plants 2020, 9, 1055. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, S.; Yin, L.; Deng, X. How Does Silicon Mediate Plant Water Uptake and Loss Under Water Deficiency? Front. Plant Sci. 2018, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Kleiber, T.; Borowiak, K.; Kosiada, T.; Breś, W.; Ławniczak, B. Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species. Open Chem. 2020, 18, 1468–1480. [Google Scholar] [CrossRef]
- Shalaby, T.A.; Abd-Alkarim, E.; El-Aidy, F.; El-Samahy, H.; Sharaf-Eldin, M.; Taha, N.; El-Ramady, H.; Bayoumi, Y.; Reis, A.R. Nano-selenium, silicon and H2O2 boost growth and productivity of cucumber under combined salinity and heat stress. Ecotoxicol. Environ. Saf. 2021, 212, 111962. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Moharrami, F.; Sarikhani, S.; Padervand, M. Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci. Rep. 2021, 10, 17672. [Google Scholar] [CrossRef]
- Guo, K.; Hu, A.; Wang, K.; Wang, L.; Fu, D.; Hao, Y.; Wang, Y.; Ali, A.; Adeel, M.; Rui, Y.; et al. Effects of spraying nano-materials on the absorption of metal(loid)s in cucumber. IET Nanobiotechnol. 2019, 13, 712–719. [Google Scholar] [CrossRef]
- Le, V.N.; Rui, Y.; Gui, X.; Li, X.; Liu, S.; Han, Y. Uptake, transport, distribution and Bio-effects of SiO2 nanoparticles in Bt-transgenic cotton. J. Nanobiotechnol. 2014, 12, 50. [Google Scholar] [CrossRef] [Green Version]
- Attia, E.A.; Elhawat, N. Combined foliar and soil application of silica nanoparticles enhances the growth, flowering period and flower characteristics of marigold (Tagetes erecta L.). Sci. Hortic. 2021, 282, 110015. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Zivcak, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of Silicon with Essential and Beneficial Elements in Plants. Front. Plant Sci. 2021, 12, 697592. [Google Scholar] [CrossRef]
- Kharchenko, V.A.; Moldovan, A.I.; Golubkina, N.A.; Gins, M.S.; Shafigullin, D.R. Comparative evaluation of several biologically active compounds content in Anthriscus sylvestris (L.) Hoffm. and Anthriscus cerefolium (L.) Hoffm. Veg. Crop. Russ. 2020, 5, 81–87. (In Russian) [Google Scholar] [CrossRef]
- Ligowe, I.S.; Bailey, E.H.; Young, S.D.; Ander, E.L.; Kabambe, V.; Chilimba, A.D.; Lark, R.M.; Nalivata, P.C. Agronomic iodine biofortification of leafy vegetables grown in Vertisols, Oxisols and Alfisols. Environ. Geochem. Health 2021, 43, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Nardi, S.; Dalla Vecchia, F.; Ertani, A. Selenium biofortification in the 21st century: Status and challenges for healthy human nutrition. Plant Soil 2020, 453, 245–270. [Google Scholar] [CrossRef]
- Kharchenko, V.A.; Golubkina, N.A.; Moldovan, A.I.; Caruso, G. Biofortification of chervil with selenium. Veg. Crop. Russ. 2021, 1, 3–7. (In Russian) [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef] [Green Version]
- Kiferle, C.; Martinelli, M.; Salzano, A.M.; Gonzali, S.; Beltrami, S.; Salvadori, P.A.; Hora, K.; Holwerda, H.T.; Scaloni, A.; Perata, P. Evidences for a Nutritional Role of Iodine in Plants. Front. Plant Sci. 2021, 12, 616868. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Belanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef]
- Greger, M.; Landberg, T.; Vaculík, M. Silicon influences soil availability and accumulation of mineral nutrients in various plant species. Plants 2018, 7, 41. [Google Scholar] [CrossRef] [Green Version]
- Janmohammadi, M.; Amanzadeh, T.; Sabaghnia, N.; Ion, V. Effect of nano-silicon foliar application on safflower growth under organic and inorganic fertilizer regimes. Bot. Lith. 2016, 22, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Strzetelski, P.; Smolen, S.; Rozek, S.; Sady, W. The effect of diverse iodine fertilization on nitrate accumulation and content of selected compounds in radish plants (Raphanus sativus L.). Acta Sci. Pol. Hortorum Cultus 2010, 9, 65–73. [Google Scholar]
- Rios, J.J.; Blasco, B.; Rosales, M.A.; Sanchez-Rodriguez, E.; Leyva, L.; Cervilla, L.M.; Romera, L.; Ruiz, J.M. Response of nitrogen metabolism in lettuce plants subjected to different doses and forms of selenium. J. Sci. Food Agric. 2010, 90, 1914–1919. [Google Scholar] [CrossRef]
- Detmann, K.C.; Araujo, W.L.; Martins, S.C.; Sanglard, L.M.; Reis, J.V.; Detmann, E.; Rodrigues, F.Á.; Nunes-Nesi, A.; Fernie, A.R.; DaMatta, F.M. Silicon nutrition increases grain yield, which, in turn, exerts a feed-forward stimulation of photosynthetic rates via enhanced mesophyll conductance and alters primary metabolism in rice. New Phytol. 2012, 196, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Yu, Y.; Baerson, S.R.; Song, Y.; Liang, G.; Ding, C.; Niu, J.; Pan, Z.; Zeng, R. Interactions between nitrogen and silicon in rice and their effects on resistance toward the brown planthopper Nilaparvata lugens. Front. Plant Sci. 2017, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Haddad, C.; Arkoun, M.; Jamois, F.; Schwarzenberg, A.; Yvin, J.C.; Etienne, P.; Laîné, P. Silicon promotes growth of Brassica napus L. and delays leaf senescence induced by nitrogen starvation. Front. Plant Sci. 2018, 9, 516. [Google Scholar] [CrossRef]
- Gou, T.; Yang, L.; Hu, W.; Chen, X.; Zhu, Y.; Guo, J.; Gong, H. Silicon improves the growth of cucumber under excess nitrate stress by enhancing nitrogen assimilation and chlorophyll synthesis. Plant Physiol. Biochem. 2020, 152, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and Plants: Current Knowledge and Technological Perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Dobosy, P.; Vetési, V.; Sandil, S.; Endrédi, A.; Kröpfl, K.; Óvári, M.; Takács, T.; Rékási, M.; Záray, G. Effect of Irrigation Water Containing Iodine on Plant Physiological Processes and Elemental Concentrations of Cabbage (Brassica oleracea L. var. capitata L.) and Tomato (Solanum lycopersicum L.) Cultivated in Different Soils. Agronomy 2020, 10, 720. [Google Scholar] [CrossRef]
- Incrocci, L.; Carmassi, G.; Maggini, R.; Poli, C.; Saidov, D.; Tamburini, C.; Kiferle, C.; Perata, P.; Pardossi, A. Iodine Accumulation and Tolerance in Sweet Basil (Ocimum basilicum L.) with Green or Purple Leaves Grown in Floating System Technique. Front. Plant Sci. 2019, 10, 1494. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.; Kekina, H.; Caruso, G. Yield, quality and antioxidant properties of Indian mustard (Brassica juncea L.) in response to foliar biofortification with selenium and iodine. Plants 2018, 7, 80. [Google Scholar] [CrossRef] [Green Version]
- Bhat, J.A.; Rajora, N.; Raturi, G.; Sharma, S.; Dhiman, P.; Sanand, S.; Shivaraj, S.M.; Sonah, H.; Deshmukh, R. Silicon nanoparticles (SiNPs) in sustainable agriculture: Major emphasis on the practicality, efficacy and concerns. Nanoscale Adv. 2021, 3, 4019–4028. [Google Scholar] [CrossRef]
- Krzepiłko, A.; Zych-Wężyk, I.; Święciło, A.; Molas, J.; Skwaryło-Bednarz, B. Effect of iodine biofortification of lettuce seedlings on their mineral composition and biological quality. J. Elem. 2016, 21, 1071–1080. [Google Scholar] [CrossRef]
- Sattara, A.; Cheema, M.A.; Abbas, T.; Sher, A.; Ijaz, M.; Hussain, M. Separate and Combined Effects of Silicon and Selenium on Salt Tolerance of Wheat Plants. Russ. J. Plant Physiol. 2017, 64, 341–348. [Google Scholar] [CrossRef]
- Macías, J.M.; Caltzontzit, M.G.L.; Martínez, E.N.R.; Ortiz, W.A.N.; Mendoza, A.B.; Lagunes, P.M. Enhancement to Salt Stress Tolerance in Strawberry Plants by Iodine Products Application. Agronomy 2021, 11, 602. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Faisal, M.; Al Sahli, A.A. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014, 33, 2429–2437. [Google Scholar] [CrossRef]
- Abdel-Haliem, M.E.F.; Hegazy, H.S.; Hassan, N.S.; Naguib, D.M. Effect of silica ions and nano silica on rice plants under salinity stress. Ecol. Eng. 2017, 99, 282–289. [Google Scholar] [CrossRef]
- Zhu, Y.-X.; Gong, H.-J.; Yin, J.-L. Role of Silicon in Mediating Salt Tolerance in Plants: A Review. Plants 2019, 8, 147. [Google Scholar] [CrossRef] [Green Version]
- Medrano-Macías, J.; Leija-Martínez, P.; González-Morales, S.; Juárez-Maldonado, A.; Benavides-Mendoza, A. Use of Iodine to Biofortify and Promote Growth and Stress Tolerance in Crops. Front. Plant Sci. 2016, 7, 1146. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium Biofortification and Interaction with other elements in plants: A Review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.; Zamana, S.; Seredin, T.; Poluboyarinov, P.; Sokolov, S.; Baranova, H.; Krivenkov, L.; Pietrantonio, L.; Caruso, G. Effect of selenium biofortification and beneficial microorganism inoculation on yield, quality and antioxidant properties of shallot bulbs. Plants 2019, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Golubkina, N.; Amagova, Z.; Matsadze, V.; Caruso, G. Effects of arbuscular mycorrhizal fungi on yield, biochemical characteristics and elemental composition of garlic and onion under selenium supply. Plants 2020, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, F.Y.; Gao, S. Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environ. Sci. Pollut. Res. 2014, 22, 2837–2845. [Google Scholar] [CrossRef]
- Feng, R.W.; Zhao, P.P.; Zhu, Y.M.; Yang, J.G.; Wei, X.Q.; Yang, L.; Yan, H.; Rensing, C.; Ding, Y.Z. Application of inorganic selenium to reduce accumulation and toxicity of heavy metals (metalloids) in plants: The main mechanisms, concerns, and risks. Sci. Total Environ. 2021, 771, 144776. [Google Scholar] [CrossRef]
- Zhou, P.; Adeel, M.; Shakoor, N.; Guo, M.; Hao, Y.; Azeem, I.; Li, M.; Liu, M.; Rui, Y. Application of Nanoparticles Alleviates Heavy Metals Stress and Promotes Plant Growth: An Overview. Nanomaterials 2021, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Golubkina, N.A.; Kekina, H.G.; Molchanova, A.V.; Antoshkina, M.S.; Nadezhkin, S.M.; Soldatenko, A.V. Plants Antioxidants and Methods of Their Determination. Infra-M Mosc. Russ. 2020, 181. [Google Scholar] [CrossRef]
- Baranov, V.I.; Soldatenkova, N.A.; Kekina, H.G. Iodine determination in marine products using inversion voltamperometry. Hyg. Sanit. 2008, 3, 35–37. (In Russian) [Google Scholar]
- Skalny, A.V.; Lakarova, H.V.; Kuznetsov, V.V.; Skalnaya, M.G. Analytical Methods in Bioelementology; Saint Petersburg-Science: St. Petersburg, Russia, 2009. (In Russian) [Google Scholar]
- Alfthan, G.V. A micromethod for the determination of selenium in tissues and biological fluids by single-test-tube fluorimetry. Anal. Chim. Acta 1984, 165, 187–194. [Google Scholar] [CrossRef]
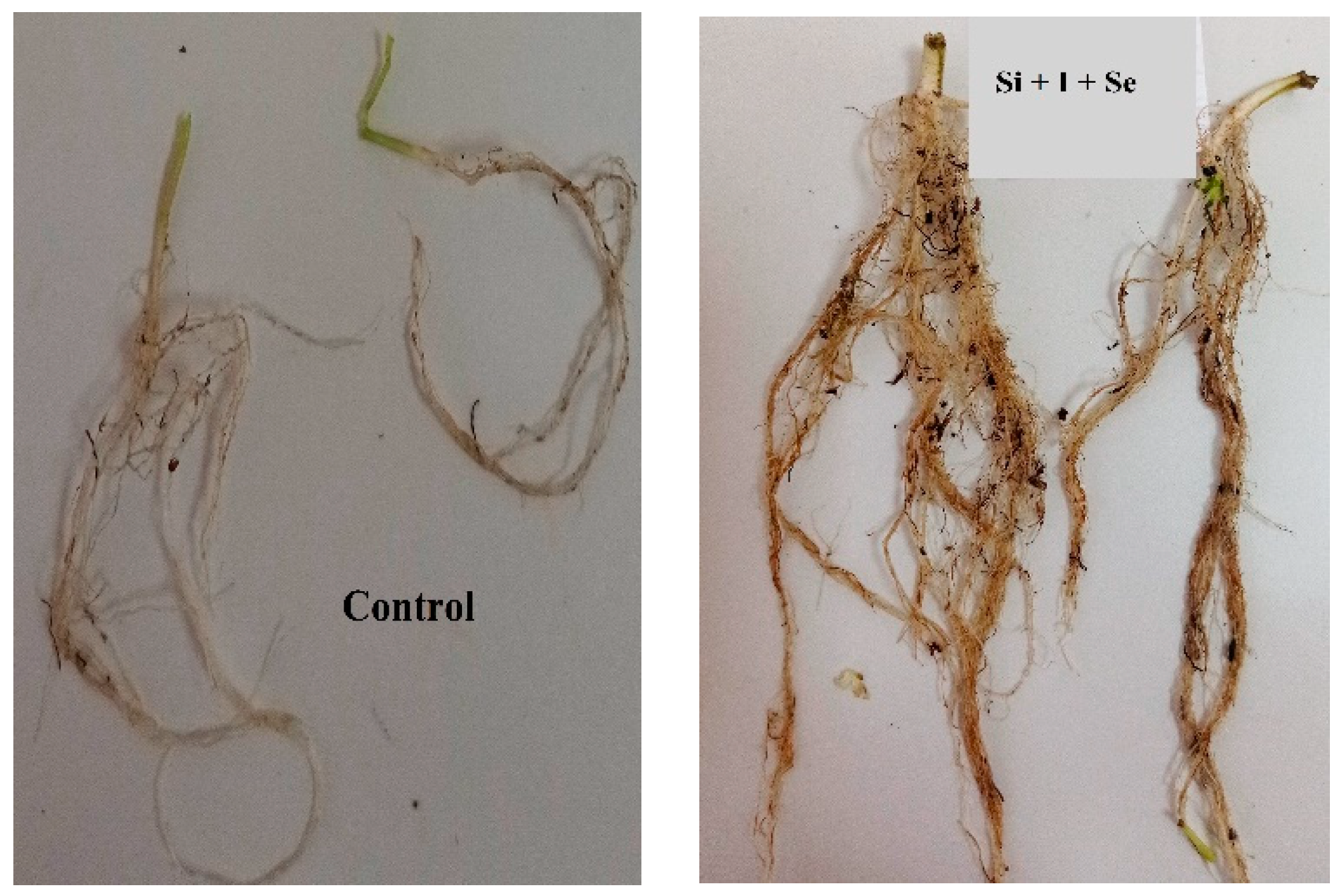





| Treatment | Length (cm) | Yield (g·m−2) | Dry Matter (%) | |||
|---|---|---|---|---|---|---|
| Shoots | Roots | Shoots | Roots | Shoots | Roots | |
| Control | 18 ± 2 c | 12 ± 1 b | 470.4 ± 47.0 e | 33.6 ± 3.4 g | 7.6 ± 0.8 b | 7.3 ± 0.7 c |
| Se | 18 ± 2 c | 12 ± 1 b | 1283.2 ± 128.3 d | 54.4 ± 5.4 f | 8.8 ± 0.9 ab | 10.9 ± 1.1 ab |
| I | 20 ± 2 bc | 13 ± 1 b | 1622.4 ± 162.1 c | 105.6 ± 10.6 e | 8.3 ± 0.8 ab | 7.4 ± 0.7 c |
| Se + I | 21 ± 2 abc | 17 ± 2 a | 1672.0 ± 167.0 c | 288.0 ± 28.8 c | 9.6 ± 0.9 a | 10.0 ± 1.0 ab |
| Si | 20 ± 2 bc | 17 ± 2 a | 2278.4 ± 228.0 b | 192.0 ± 19.2 d | 7.8 ± 0.8 b | 8.5 ± 0.9 bc |
| Si + Se | 22 ± 2 ab | 19 ± 2 a | 2622.4 ± 262.0 ab | 448.0 ± 44.8 a | 8.9 ± 0.9 ab | 9.5 ± 0.9 b |
| Si + I | 25 ± 3 a | 20 ± 2 a | 2928.0 ± 292.8 a | 404.8 ± 40.4 ab | 8.6 ± 0.8 ab | 9.3 ± 0.9 b |
| Si + I + Se | 22 ± 2 ab | 21 ± 2 a | 1851.2 ± 185.0 c | 336.0 ± 33.6 bc | 10.3 ± 1.0 a | 11.7 ± 1.2 a |
| Treatment | Chlorophyll a (mg g−1 Fresh Weight) | Chlorophyll b (mg g−1 Fresh Weight) | Total Chlorophyll (mg g−1 Fresh Weight) | Chl b/a Ratio | Carotene (mg g−1 Fresh Weight) | Carotene:Chlorophyll Ratio |
|---|---|---|---|---|---|---|
| Control | 0.70 ± 0.07 b | 1.77 ± 0.18 b | 2.47 ± 0.24 b | 2.53 a | 0.38 ± 0.04 b | 1:6.66 |
| Se | 0.72 ± 0.07 b | 1.98 ± 0.19 ab | 2.70 ± 0.27 b | 2.75 a | 0.43 ± 0.04 ab | 1:6.58 |
| I | 0.71 ± 0.07 b | 1.87 ± 0.19 b | 2.58 ± 0.25 b | 2.63 a | 0.40 ± 0.04 ab | 1:6.58 |
| Se + I | 0.90 ± 0.09 a | 2.18 ± 0.21 ab | 3.08 ± 0.31 ab | 2.42 a | 0.46 ± 0.04 a | 1:5.26 |
| Si | 0.88 ± 0.08 a | 2.23 ± 0.22 a | 3.11 ± 0.31 a | 2.53 a | 0.48 ± 0.05 a | 1:5.27 |
| Si + Se | 0.96 ± 0.09 a | 2.34 ± 0.23 a | 3.30 ± 0.33 a | 2.44 a | 0.48 ± 0.05 a | 1:5.08 |
| Si + I | 0.83 ± 0.08 ab | 2.39 ± 0.23 a | 3.22 ± 0.32 a | 2.88 a | 0.43 ± 0.04 ab | 1:6.70 |
| Si + Se + I | 0.83 ± 0.08 ab | 2.29 ± 0.22 a | 3.12 ± 0.31 a | 2.76 a | 0.49 ± 0.05 a | 1:5.63 |
| Treatment | Ascorbic Acid (mg 100 g−1 Fresh Weight) | Antioxidant Activity-AOA (mg GAE g−1 Dry Weight) | Total Polyphenols-TP (mg GAE g−1 Dry Weight) |
|---|---|---|---|
| Control | 30.0 ± 3.1 b | 20.2 ± 2.0 bc | 7.2 ± 0.71 c |
| Se | 34.2 ± 3.4 b | 24.6 ± 2.4 b | 9.8 ± 0.9 ab |
| I | 29.8 ± 3.0 b | 24.0 ± 2.3 b | 9.5 ± 0.9 ab |
| Se + I | 30.4 ± 3.0 b | 22.3 ± 2.2 b | 10.3 ± 1.0 a |
| Si | 31.4 ± 3.1 b | 23.7 ± 2.3 b | 8.4± 0.8 bc |
| Si + Se | 28.5 ± 2.8 b | 23.7 ± 2.3 b | 9.4 ± 0.9 ab |
| Si + I | 39.0 ± 3.8 a | 19.1 ± 1.9 cd | 8.9 ± 0.9 ab |
| Si + Se + I | 25.7 ± 2.6 c | 30.0 ± 3.0 a | 11.3 ± 1.0 a |
| Treatment | Se | I | Si |
|---|---|---|---|
| Control | 0.23 ± 0.02 f | 0.20 ± 0.02 e | 20.59 ± 2.05 ab |
| Se | 3.75 ± 0.32 ab | 2.50 ± 0.21 c | 23.81 ± 2.34 a |
| I | 0.80 ± 0.07 c | 16.00 ± 1.59 b | 21.97 ± 2.20 a |
| Se + I | 4.36 ± 0.41 a | 15.00 ± 1.51 b | 17.22 ± 1.71 b |
| Si | 0.45 ± 0.04 e | 1.50 ± 0.15 d | 24.10 ± 2.40 a |
| Si + Se | 3.73 ± 0.35 ab | 1.60 ± 0.15 d | 20.14 ± 2.00 ab |
| Si + I | 0.62 ± 0.06 d | 34.00 ± 3.38 a | 19.66 ± 2.00 ab |
| Si + Se + I | 3.07 ± 030 b | 35.00 ± 3.51 a | 17.16 ± 1.70 b |
| Treatment | Ca | K | Mg | Na | P |
|---|---|---|---|---|---|
| Control | 22.45 ± 2.22 bc | 97.24 ± 9.71 a | 4.35 ± 0.43 a | 1.17 ± 0.11 a | 7.86 ± 0.78 a |
| Se | 20.09 ± 20.00 c | 108.78 ± 10.80 a | 3.76 ± 0.37 a | 0.94 ± 0.09 a | 8.36 ± 0.83 a |
| I | 19.03 ± 19.0 c | 91.07 ± 9.10 ab | 3.51 ± 0.35 a | 0.53 ± 0.05 d | 6.70 ± 0.67 a |
| Se + I | 25.22 ± 2.52 ab | 88.22 ± 8.82 b | 4.01 ± 0.40 a | 0.76 ± 0.08 b | 6.24 ± 0.62 a |
| Si | 22.35 ± 2.23 bc | 83.17 ± 8.31 ba | 4.11 ± 0.40 a | 0.72 ± 0.07 bc | 6.95 ± 0.69 a |
| Si + Se | 22.15 ± 2.21 bc | 88.16 ± 8,80 ba | 3.66 ± 0.36 a | 0.44 ± 0.04 d | 6.37 ± 0.63 a |
| Si + I | 27.42 ± 2.74 a | 100.78 ± 10.11 a | 4.31 ± 0.43 a | 0.71 ± 0.07 bc | 7.37 ± 0.74 a |
| Si + Se + I | 25.01 ± 2.50 ab | 96.80 ± 9.66 a | 3.84 ± 0.38 a | 0.62 ± 0.06 c | 7.21 ± −0.71 a |
| Treatment | B | Co | Cu | Fe | Li | Mn | Mo | Zn |
|---|---|---|---|---|---|---|---|---|
| Control | 32.18 ± 3.21 ab | 0.42 ± 0.04 bc | 21.33 ± 2.11 a | 1323 ± 132 b | 0.93 ± 0.09 ab | 61.82 ± 6.18 b | 1.85 ± 0.18 a | 244 ± 24 a |
| Se | 33.77 ± 3.37 ab | 0.33 ± 0.03 d | 18.85 ± 1.88 ab | 776 ± 77 d | 0.76 ± 0.07 bc | 43.61 ± 4.36 c | 1.43 ± 0.14 b | 143 ± 14 bc |
| I | 27.55 ± 2.75 b | 0.33 ± 0.03 d | 15.57 ± 1.55 c | 753 ± 75 d | 0.65 ± 0.06 c | 42.94 ± 4.29 c | 1.46 ± 0.15 b | 121 ± 12 c |
| Se + I | 33.29 ± 3.33 ab | 0.52 ± 0.05 b | 16.78 ± 1.67 c | 1486 ± 148 b | 1.06 ± 0.11 a | 66.54 ± 6.65 b | 2.06 ± 0.20 a | 202 ± 20 a |
| Si | 31.34 ± 3.13 ab | 0.77 ± 0.07 a | 16.76 ± 1.67 c | 1880 ± 188 a | 1.01 ± 0.10 a | 93.71 ± 9.37 a | 1.69 ± 0.17 a | 166 ± 16 b |
| Si + Se | 35.25 ± 3.52 a | 0.39 ± 0.04 cd | 14.16 ± 1.41 c | 1237 ± 124 bc | 0.87 ± 0.09 b | 46.10 ± 4.60 c | 1.80 ± 0.18 a | 118 ± 12 c |
| Si + I | 34.07 ± 3.40 a | 0.49 ± 0.05 b | 17.47 ± 1.75 a | 1161 ± 116 c | 0.92 ± 0.09 ab | 62.30 ± 6.22 b | 1.90 ± 0.19 a | 145 ± 15 bc |
| Si + Se + I | 36.42 ± 3.62 a | 0.51 ± 0.05 b | 17.14 ± 1.71 bc | 1306 ± 130 bc | 1.09 ± 0.11 a | 63.81 ± 6.38 b | 1.95 ± 0.19 a | 161 ± 16 b |
| Treatment | Al | As | Cd | Cr | Ni | Pb | Sr | V |
|---|---|---|---|---|---|---|---|---|
| Control | 364 ± 36 de | 0.28 ± 0.03 d | 1.09 ± 0.11 b | 13.39 ± 1.3 bc | 3.51 ± 0.35 ab | 3.59 ± 0.36 a | 105.0 ± 10.0 a | 1.33 ± 0.13 cd |
| Se | 350 ± 35 e | 0.23 ± 0.02 e | 1.15 ± 0.11 ab | 9.14 ± 0.91 d | 3.33 ± 0.33 bc | 2.55 ± 0.25 b | 81.5 ± 8.1 b | 1.04 ± 0.10 e |
| I | 305 ± 30 e | 0.27 ± 0.03 de | 1.06 ± 0.10 b | 8.29 ± 0.82 d | 3.02 ± 0.30 c | 2.67 ± 0.26 b | 87.5 ± 8.7 ab | 1.12 ± 0.11 de |
| Se + I | 568 ± ab | 0.41 ± 0.04 b | 1.04 ± 0.10 b | 14.75 ± 1.43 ab | 3.68 ± 0.36 ab | 3.53 ± 0.25 a | 112.0 ± 11.1 a | 1.79 ± 0.18 b |
| Si | 513 ± 51 bc | 0.71 ± 0.07 a | 1.17 ± 0.11 ab | 17.70 ± 1.76 a | 3.43 ± 0.34 ab | 3.55 ± 0.25 a | 109.0 ± 10.7 a | 2.32 ± 0.23 a |
| Si + Se | 504 ± 50 bc | 0.32 ± 0.03 cd | 1.03 ± 0.10 b | 12.81 ± 1.26 c | 3.20 ± 0.32 cb | 2.41 ± 0.24 b | 82.8 ± 8.2 b | 1.52 ± 0.15 c |
| Si + I | 431 ± 43 cd | 0.32 ± 0.03 cd | 1.30 ± 0.13 a | 12.91 ± 1.29 c | 4.26 ± 0.42 a | 2.93 ± 0.29 b | 94.1 ± 9.4 ab | 1.49 ± 0.15 c |
| Si + Se + I | 660 ± 66 a | 0.34 ± 0.03 bc | 1.35 ± 0.13 a | 14.01 ± 1.40 bc | 3.93 ± 0.39 a | 3.78 ± 0.38 a | 99.4 ± 9.9 ab | 1.95 ± 0.19 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubkina, N.; Moldovan, A.; Fedotov, M.; Kekina, H.; Kharchenko, V.; Folmanis, G.; Alpatov, A.; Caruso, G. Iodine and Selenium Biofortification of Chervil Plants Treated with Silicon Nanoparticles. Plants 2021, 10, 2528. https://doi.org/10.3390/plants10112528
Golubkina N, Moldovan A, Fedotov M, Kekina H, Kharchenko V, Folmanis G, Alpatov A, Caruso G. Iodine and Selenium Biofortification of Chervil Plants Treated with Silicon Nanoparticles. Plants. 2021; 10(11):2528. https://doi.org/10.3390/plants10112528
Chicago/Turabian StyleGolubkina, Nadezhda, Anastasia Moldovan, Mikhail Fedotov, Helene Kekina, Viktor Kharchenko, Gundar Folmanis, Andrey Alpatov, and Gianluca Caruso. 2021. "Iodine and Selenium Biofortification of Chervil Plants Treated with Silicon Nanoparticles" Plants 10, no. 11: 2528. https://doi.org/10.3390/plants10112528
APA StyleGolubkina, N., Moldovan, A., Fedotov, M., Kekina, H., Kharchenko, V., Folmanis, G., Alpatov, A., & Caruso, G. (2021). Iodine and Selenium Biofortification of Chervil Plants Treated with Silicon Nanoparticles. Plants, 10(11), 2528. https://doi.org/10.3390/plants10112528







