Stories from the Greenhouse—A Brief on Cotton Seed Germination
Abstract
:1. Introduction
1.1. Cotton
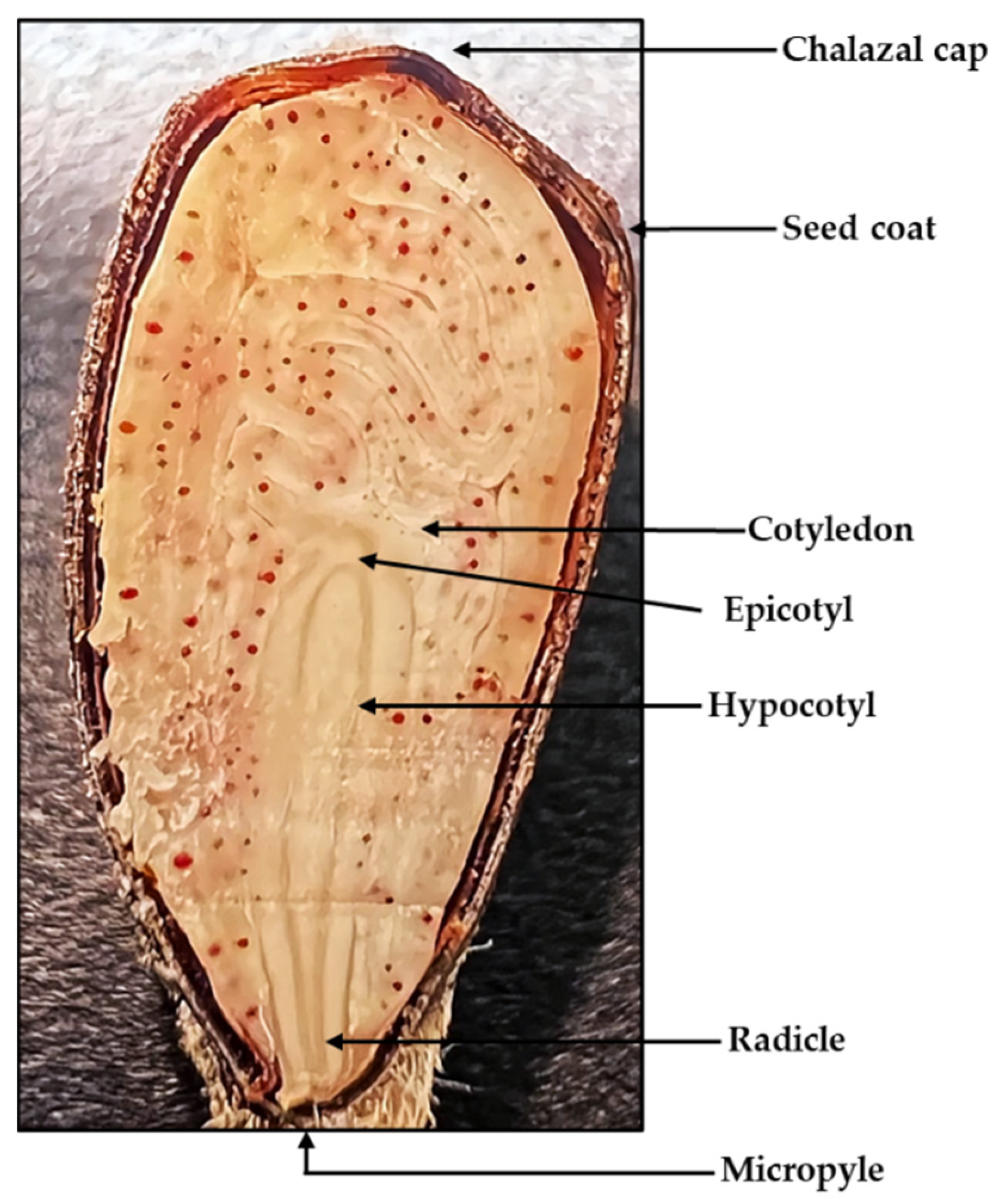
1.2. Cotton Seed Anatomy and Physiology
1.3. Factors Affecting Cotton Seed Germination
1.3.1. Seed Coat
1.3.2. Drought
1.3.3. Temperature
1.3.4. Seed Storage
1.3.5. Dormancy
2. Our Program
2.1. Seed Germination In-House Protocols
2.1.1. Seed Coat Mechanical Scarification
2.1.2. Complete Removal of Seed Coat
2.1.3. Petri Dish with Water Agar
2.1.4. Gibberellic Acid
2.1.5. Smoke
2.1.6. Seed Storage
2.1.7. Photoperiodism
3. Appendix-Stories
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef] [Green Version]
- Donohue, K.; Rubio de Casas, R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, postgermination adaptation, and species ecological ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Klupczyńska, E.A.; Pawłowski, T.A. Regulation of Seed Dormancy and Germination Mechanisms in a Changing Environment. Int. J. Mol. Sci. 2021, 22, 1357. [Google Scholar] [CrossRef] [PubMed]
- Donohue, K. Germination timing influences natural selection on life-history characters in Arabidopsis thaliana. Ecology 2002, 83, 1006–1016. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and, Evolution of Dormancy and Germination; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Footitt, S.; Huang, Z.; Clay, H.A.; Mead, A.; Finch-Savage, W.E. Temperature, light and nitrate sensing coordinate Arabidopsis seed dormancy cycling, resulting in winter and summer annual phenotypes. Plant J. 2013, 74, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C.; Li, X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biol. 2000, 15, 139–152. [Google Scholar] [CrossRef]
- Penfield, S. Seed dormancy and germination. Curr. Biol. 2017, 27, R874–R878. [Google Scholar] [CrossRef] [Green Version]
- Poehlman, J.M. Breeding field crops; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Han, Z.-G.; Guo, W.-Z.; Song, X.-L.; Zhang, T.-Z. Genetic mapping of EST-derived microsatellites from the diploid Gossypium arboreum in allotetraploid cotton. Mol. Genet. Genom. 2004, 272, 308–327. [Google Scholar] [CrossRef]
- Maeda, A.B.V. Expression Analysis and Mapping of the Semigamy Gene in Cotton (Gossypium barbadense L.). Master’s Thesis, Texas A&M University, College Station, TX, USA, 2015. [Google Scholar]
- Ruan, Y.-L. Recent advances in understanding cotton fibre and seed development. Seed Sci. Res. 2005, 15, 269–280. [Google Scholar] [CrossRef]
- Basra, A.S.; Malik, C. Development of the cotton fiber. Int. Rev. Cytol. 1984, 89, 65–113. [Google Scholar]
- Smith, C.W.; Cothren, J.T. Cotton: Origin, History, Technology, and Production; John Wiley & Sons: Hoboken, NJ, USA, 1999; Volume 4. [Google Scholar]
- Gordon, S.; Hsieh, Y.-L. Cotton: Science and Technology; Woodhead Publishing: Cambridge, UK, 2006. [Google Scholar]
- Cothren, J. Physiology of the cotton plant. In Cotton: Origin, History, Technology, and Production; John Wiley & Sons: Hoboken, NJ, USA, 1999; pp. 207–268. [Google Scholar]
- Oosterhuis, D.M.; Jernstedt, J. Morphology and anatomy of the cotton plant. In Cotton: Origin, History, Technology, and Production; John Wiley & Sons: Hoboken, NJ, USA, 1999; pp. 175–206. [Google Scholar]
- Ritchie, G.L.; Bednarz, C.W.; Jost, P.H.; Brown, S.M. Cotton Growth and Development; Bulletin 1252, Cooperative Extension Service, University of Georgia: Athens, GA, USA, 2007. [Google Scholar]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Hopper, N.W.; McDaniel, R.G. The cotton seed. In Cotton: Origin, History, Technology, and Production; John Wiley & Sons: Hoboken, NJ, USA, 1999; pp. 289–318. [Google Scholar]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fryxell, P.A. The Natural History of the Cotton Tribe (Malvaceae, Tribe Gossypieae); Texas A & M University Press: College Station, TX, USA, 1979. [Google Scholar]
- Turner, J.A. The Cotton Planter’s Manual: Being a Compilation of Facts from the Best Authorities on the Culture of Cotton; Its Natural History, Chemical Analysis, Trade, and Consumption; and Embracing a History of Cotton and the Cotton Gin; CM Saxton and Company: New York, NY, USA, 1857. [Google Scholar]
- Cunningham, E.B.; Thiele, F.C. Process of Delinting Cotton-Seed; United States Patent Office: Alexandria, VA, USA, 1899. [Google Scholar]
- Brown, J.G.; Gibson, F. A Machine for Treating Cotton Seed with Sulphuric Acid; College of Agriculture, University of Arizona: Tucson, AZ, USA, 1925. [Google Scholar]
- Simpson, D.M.; Adams, C.L.; Stone, G.M. Anatomical Structure of the Cottonseed Coat as Related to Problems of Germination; US Department of Agriculture: Washington, DC, USA, 1940. [Google Scholar]
- Oosterhuis, D.M. Growth and development of a cotton plant. In Nitrogen Nutrition of Cotton: Practical Issues; ASA-CSSA-SSSA; American Society of Agronomy: Madison, WI, USA, 1990; pp. 1–24. [Google Scholar]
- Toole, E.H.; Drummond, P.L. The germination of cottonseed. J. Agric. Res. 1925, 28, 285–295. [Google Scholar]
- Christiansen, M.N.; Moore, R. Seed Coat Structural Differences that Influence Water Uptake and Seed Quality in Hard Seed Cotton 1. Agron. J. 1959, 51, 582–584. [Google Scholar] [CrossRef]
- Rolston, M.P. Water impermeable seed dormancy. Bot. Rev. 1978, 44, 365–396. [Google Scholar] [CrossRef]
- Barton, L.V. Dormancy in seeds imposed by the seed coat. In Differenzierung und Entwicklung/Differentiation and Development; Springer: Berlin/Heidelberg, Germany, 1965; pp. 2374–2392. [Google Scholar]
- Paulsen, T.R.; Colville, L.; Kranner, I.; Daws, M.I.; Högstedt, G.; Vandvik, V.; Thompson, K. Physical dormancy in seeds: A game of hide and seek? New Phytol. 2013, 198, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Hudson, A.R.; Ayre, D.J.; Ooi, M.K. Physical dormancy in a changing climate. Seed Sci. Res. 2015, 25, 66–81. [Google Scholar] [CrossRef] [Green Version]
- Walhood, V. A Method of Reducing the Hard Seed Problem in Cotton 1. Agron. J. 1956, 48, 141–142. [Google Scholar] [CrossRef]
- Kimura, E.; Islam, M. Seed scarification methods and their use in forage legumes. Res. J. Seed Sci. 2012, 5, 38–50. [Google Scholar]
- Carleton, A.E.; Austin, R.; Stroh, J.; Wiesner, L.; Scheetz, J. Cicer milkvetch (Astragalus cicer L.): Seed germination, scarification and field emergence studies. Mont. Agr. Exp. Sta. Bull 1971, 655, 1–21. [Google Scholar]
- Stephens, S. Salt water tolerance of seeds of Gossypium species as a possible factor in seed dispersal. Am. Nat. 1958, 92, 83–92. [Google Scholar] [CrossRef]
- McDonald, M.B., Jr.; Khan, A.A. Acid Scarification and Protein Synthesis during Seed Germination 1. Agron. J. 1983, 75, 111–114. [Google Scholar] [CrossRef]
- Marani, A.; Amirav, A. Effect of Delinting and of Genetical Factors on the Germination of Cotton Seeds at Low Temperatures 1. Crop. Sci. 1970, 10, 509–511. [Google Scholar] [CrossRef]
- Bai, Y.; Xiao, S.; Zhang, Z.; Zhang, Y.; Sun, H.; Zhang, K.; Wang, X.; Bai, Z.; Li, C.; Liu, L. Melatonin improves the germination rate of cotton seeds under drought stress by opening pores in the seed coat. PeerJ 2020, 8, e9450. [Google Scholar] [CrossRef]
- de Groot, G.J.; Hundt, A.; Murphy, A.B.; Bange, M.P.; Mai-Prochnow, A. Cold plasma treatment for cotton seed germination improvement. Sci. Rep. 2018, 8, 1–10. [Google Scholar]
- Wang, X.-Q.; Zhou, R.-W.; de Groot, G.; Bazaka, K.; Murphy, A.B.; Ostrikov, K.K. Spectral characteristics of cotton seeds treated by a dielectric barrier discharge plasma. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S. Plant drought stress: Effects, mechanisms and management. In Agronomy for Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2009; Volume 29, pp. 185–212. [Google Scholar]
- Wanjura, D.; Buxton, D. Water Uptake and Radicle Emergence of Cottonseed as Affected by Soil Moisture and Temperature 1. Agron. J. 1972, 64, 427–431. [Google Scholar] [CrossRef]
- Cole, D.; Christiansen, M. Effect of Chilling Duration on Germination of Cottonseed 1. Crop. Sci. 1975, 15, 410–412. [Google Scholar] [CrossRef]
- Stiles, I.E. Relation of water to the germination of corn and cotton seeds. Plant Physiol. 1948, 23, 201–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christiansen, M. Influence of chilling upon seedling development of cotton. Plant Physiol. 1963, 38, 520–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christiansen, M. Periods of sensitivity to chilling in germinating cotton. Plant Physiol. 1967, 42, 431–433. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, M.; Thomas, R. Season-long effects of chilling treatments applied to germinating cottonseed. Crop. Sci. 1969, 9, 672–673. [Google Scholar] [CrossRef]
- Hake, K.; McCarty, W.; Hopper, N.; Jividen, G. Seed quality and germination. In Cotton Physiology Today–Newsletter of the Cotton Physiology Education Program; National Cotton Council: Cordova, TN, USA, 1990; Volume 1, p. 4. [Google Scholar]
- Krzyzanowski, F.C.; Delouche, J.C. Germination of cotton seed in relation to temperature. Rev. Bras. De Sementes 2011, 33, 543–548. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, C. The germination of cotton seed at low temperature. J. Agric. Res. 1932, 44, 367–380. [Google Scholar]
- Joao Abba, E.; Lovato, A. Effect of seed storage temperature and relative humidity on maize (Zea mays L.) seed viability and vigour. Seed Sci. Technol. 1999, 27, 101–114. [Google Scholar]
- Harman, G.; Mattick, L. Association of lipid oxidation with seed ageing and death. Nature 1976, 260, 323–324. [Google Scholar] [CrossRef]
- Simpson, D. Factors affecting the longevity of cottonseed. J. Agric. Res 1942, 64, 407–419. [Google Scholar]
- Priestley, D.A.; Leopold, A.C. Absence of lipid oxidation during accelerated aging of soybean seeds. Plant Physiol. 1979, 63, 726–729. [Google Scholar] [CrossRef] [Green Version]
- Priestley, D.A.; Leopold, A.C. Lipid changes during natural aging of soybean seeds. Plant Physiol. 1983, 59, 467–470. [Google Scholar] [CrossRef]
- McDonald, M. Seed deterioration: Physiology, repair and assessment. Seed Sci. Technol. 1999, 27, 177–237. [Google Scholar]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Hilhorst, H.W. A critical update on seed dormancy. I. Primary dormancy1. Seed Sci. Res. 1995, 5, 61–73. [Google Scholar] [CrossRef]
- Ellis, R.H.; Hong, T.; Roberts, E.H. Handbook of Seed Technology for Genebanks: Volume II: Compendium of Specific Germination Information and Test Recommendation; International Board for Plant Genetic Resources: Rome, Italy, 1985. [Google Scholar]
- Taylor, R.; Lankford, M. Secondary Dormancy in Cotton 1. Crop. Sci. 1972, 12, 195–196. [Google Scholar] [CrossRef]
- Simpson, D. Dormancy and maturity of cottonseed. J. Agric. Res 1935, 50, 429–434. [Google Scholar]
- Dever, J.; Sheehan, M. Update of cotton race-stock screening and phenotypic char-acterization. In Proceedings of the Beltwide Cotton Conferences, Atlanta, GA, USA, 4–7 January 2011; pp. 718–719. [Google Scholar]
- Rao, N.K.; Hanson, J.; Dulloo, M.E.; Ghosh, K.; Nowell, A. Manual of Seed Handling in Genebanks; Bioversity International: Rome, Italy, 2006. [Google Scholar]
- Sackville Hamilton, N.; Chorlton, K. Regeneration of Accessions in Seed Collections: A Decision Guide; International Board for Plant Genetic Resources: Rome, Italy, 1997. [Google Scholar]
- Percy, R.G.; Frelichowski, J.E.; Arnold, M.D.; Campbell, T.B.; Dever, J.K.; Fang, D.D.; Hinze, L.L.; Main, D.; Scheffler, J.; Sheehan, M.A. The US National Cotton Germplasm Collection–Its contents, preservation, characterization, and evaluation. In World Cotton Germplasm Resources; Abdurakhmonov, I., Ed.; InTech: Rijeka, Croatia, 2014; pp. 167–201. [Google Scholar]
- Mehanna, H.T.; Martin, G.C. Effect of seed coat on peach seed germination. Sci. Hortic. 1985, 25, 247–254. [Google Scholar] [CrossRef]
- Muralidhara, B.; Reddy, Y.; Srilatha, V.; Akshitha, H. Effect of seed coat removal treatments on seed germination and seedling attributes in mango varieties. Int. J. Fruit Sci. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Eggers, E. Effect of the removal of the seed coats on avocado seed germination. In California Avocado Association Yearbook; California Avocado Society: Los Angeles, CA, USA, 1942; Volume 27, pp. 41–43. [Google Scholar]
- Crocker, W. Role of seed coats in delayed germination. Bot. Gaz. 1906, 42, 265–291. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yang, X.; Gao, Y.; Zhang, X.; Hu, W.; Zhou, Z.; Meng, Y. Investigating seed dormancy in cotton (Gossypium hirsutum L.): Understanding the physiological changes in embryo during after-ripening and germination. Plant Biol. 2019, 21, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Taiz, L.; Zeiger, E. Gibberellins: Regulators of plant height and seed germination. In Plant Physiology; Sinauer Associates, Inc.: Sunderland, MA, USA, 2010; pp. 583–619. [Google Scholar]
- White, C.N.; Proebsting, W.M.; Hedden, P.; Rivin, C.J. Gibberellins and seed development in maize. I. Evidence that gibberellin/abscisic acid balance governs germination versus maturation pathways. Plant Physiol. 2000, 122, 1081–1088. [Google Scholar] [CrossRef] [Green Version]
- Dixon, K.W.; Roche, S.; Pate, J.S. The promotive effect of smoke derived from burnt native vegetation on seed germination of Western Australian plants. Oecologia 1995, 101, 185–192. [Google Scholar] [CrossRef]
- Tieu, A.; Dixon, K.; Meney, K.; Sivasithamparam, K. The interaction of heat and smoke in the release of seed dormancy in seven species from southwestern Western Australia. Ann. Bot. 2001, 88, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Dixon, K. The role of combustion products (smoke) in stimulating ex-situ and in-situ germination of Western Australian plants. Proc. Int. Plant Propagators Soc. 1995, 45, 53–56. [Google Scholar]
- Brown, N.; Van Staden, J. Smoke as a germination cue: A review. Plant Growth Regul. 1997, 22, 115–124. [Google Scholar] [CrossRef]
- Drewes, F.; Smith, M.; Van Staden, J. The effect of a plant-derived smoke extract on the germination of light-sensitive lettuce seed. Plant Growth Regul. 1995, 16, 205–209. [Google Scholar] [CrossRef]
- Thomas, T.; Van Staden, J. Dormancy break of celery (Apium graveolens L.) seeds by plant derived smoke extract. Plant Growth Regul. 1995, 17, 195–198. [Google Scholar] [CrossRef]
- Dixon, K.; Merritt, D.; Flematti, G.; Ghisalberti, E. Karrikinolide—A phytoreactive compound derived from smoke with applications in horticulture, ecological restoration and agriculture. Acta Hortic. 2009, 813, 155–170. [Google Scholar] [CrossRef]
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D. A compound from smoke that promotes seed germination. Science 2004, 305, 977. [Google Scholar] [CrossRef]
- Stephens, S. The origin of Sea Island cotton. Agric. Hist. 1976, 50, 391–399. [Google Scholar]
- Fryxell, P.A. A redefinition of the tribe Gossypieae. Bot. Gaz. 1968, 129, 296–308. [Google Scholar] [CrossRef]
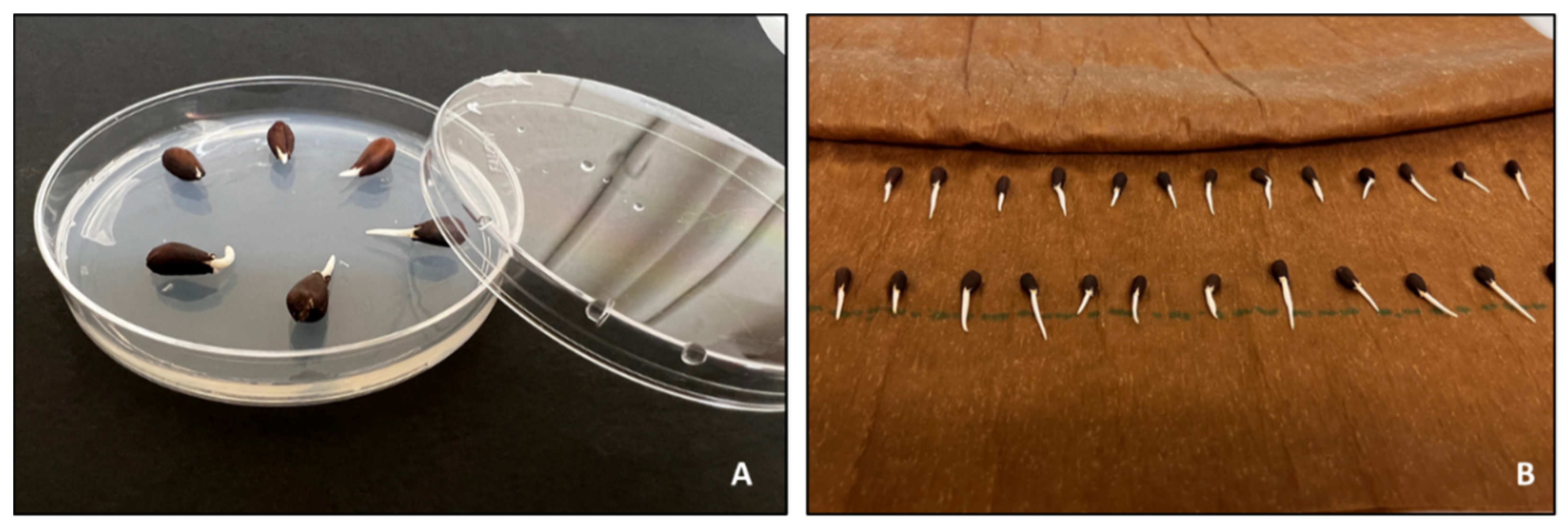

| Steps | Description |
|---|---|
| 1 | Fuzzy cotton seeds are placed in a labeled perforated plastic container. |
| 2 | The container is submerged in a bucket with 93% sulfuric acid and seeds are stirred for 2–3 min using a wood stake. The friction while stirring the seeds facilitates the delinting process. |
| 3 | Seeds are rinsed with tap water to remove the excess sulfuric acid. |
| 4 | The container is submerged in a sodium carbonate solution to neutralize the acid. |
| 5 | Seeds are rinsed with tap water. |
| 6 | Seeds are submerged in methyl alcohol for a few seconds. This step helps with the drying process. |
| 7 | Seeds are spread in a screened tray and are placed in the drier. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, A.B.; Wells, L.W.; Sheehan, M.A.; Dever, J.K. Stories from the Greenhouse—A Brief on Cotton Seed Germination. Plants 2021, 10, 2807. https://doi.org/10.3390/plants10122807
Maeda AB, Wells LW, Sheehan MA, Dever JK. Stories from the Greenhouse—A Brief on Cotton Seed Germination. Plants. 2021; 10(12):2807. https://doi.org/10.3390/plants10122807
Chicago/Turabian StyleMaeda, Andrea B., Leslie W. Wells, Monica A. Sheehan, and Jane K. Dever. 2021. "Stories from the Greenhouse—A Brief on Cotton Seed Germination" Plants 10, no. 12: 2807. https://doi.org/10.3390/plants10122807
APA StyleMaeda, A. B., Wells, L. W., Sheehan, M. A., & Dever, J. K. (2021). Stories from the Greenhouse—A Brief on Cotton Seed Germination. Plants, 10(12), 2807. https://doi.org/10.3390/plants10122807







