Dynamic Responses of Ascorbate Pool and Metabolism in Lettuce to Light Intensity at Night Time under Continuous Light Provided by Red and Blue LEDs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Condition
2.2. Light Treatments and Sampling Time
2.3. Measurement Methods
2.3.1. Measurement of Growth Parameters
2.3.2. Measurement of Chlorophyll Fluorescence Parameters
2.3.3. Measurement of AsA Content
2.3.4. Measurement of AsA Metabolism-Related Enzyme Activity
2.3.5. Measurement of H2O2 Content
3. Results
3.1. Effect of Light Intensities at Night Time on Growth of Lettuce under Red and Blue Continuous Light
3.2. Effect of Light Intensities at Night Time on AsA Content of Lettuce under Red and Blue Continuous Light
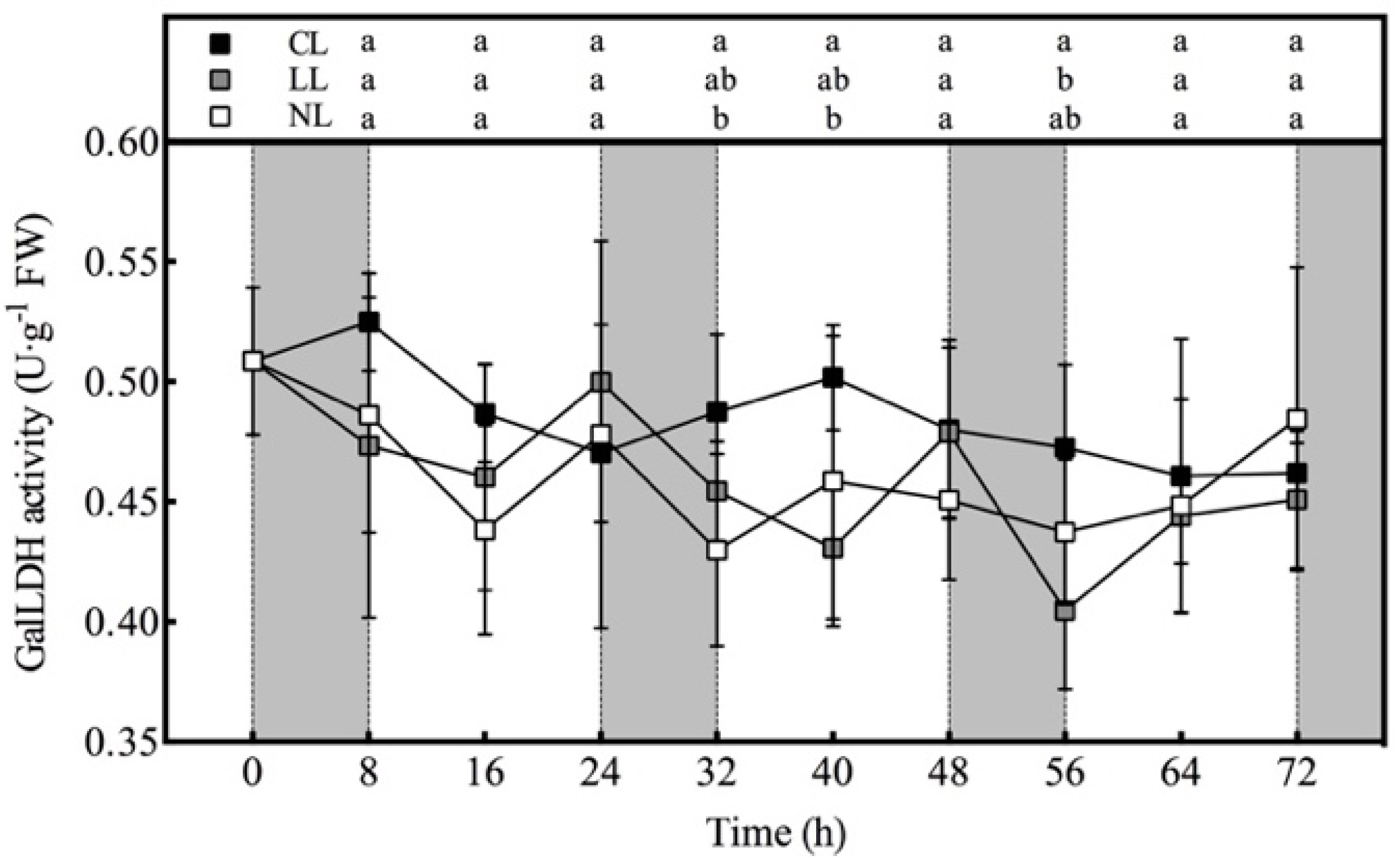
3.3. Effect of Light Intensities at Night Time on AsA Synthase Activity of Lettuce under Red and Blue Continuous Light
3.4. Effect of Light Intensities at Night Time on AsA-GSH Cycle Related Enzyme Activity of Lettuce under Red and Blue Continuous Light
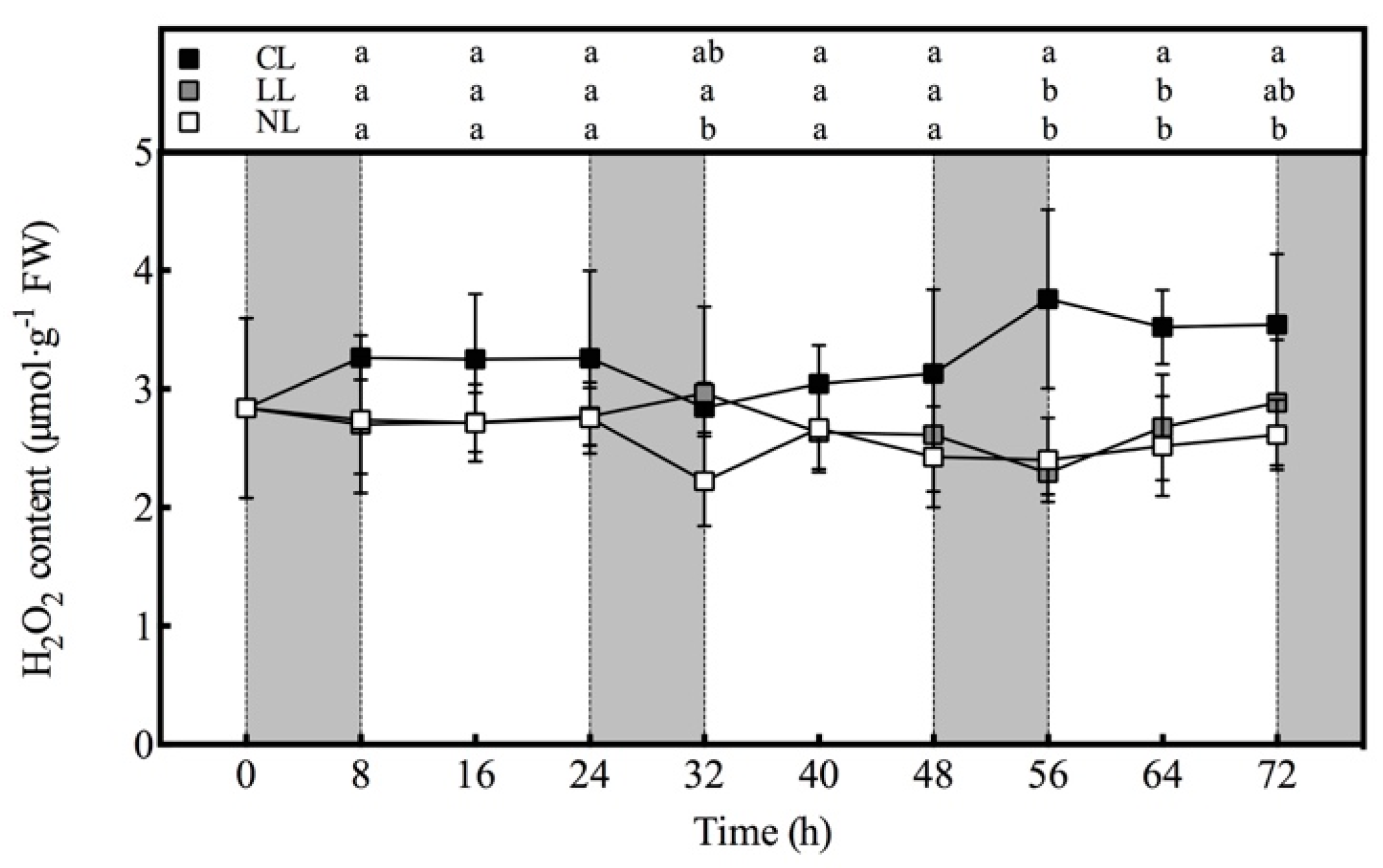
3.5. Effect of Light Intensities at Night Time on H2O2 content of Lettuce under Red and Blue Continuous Light
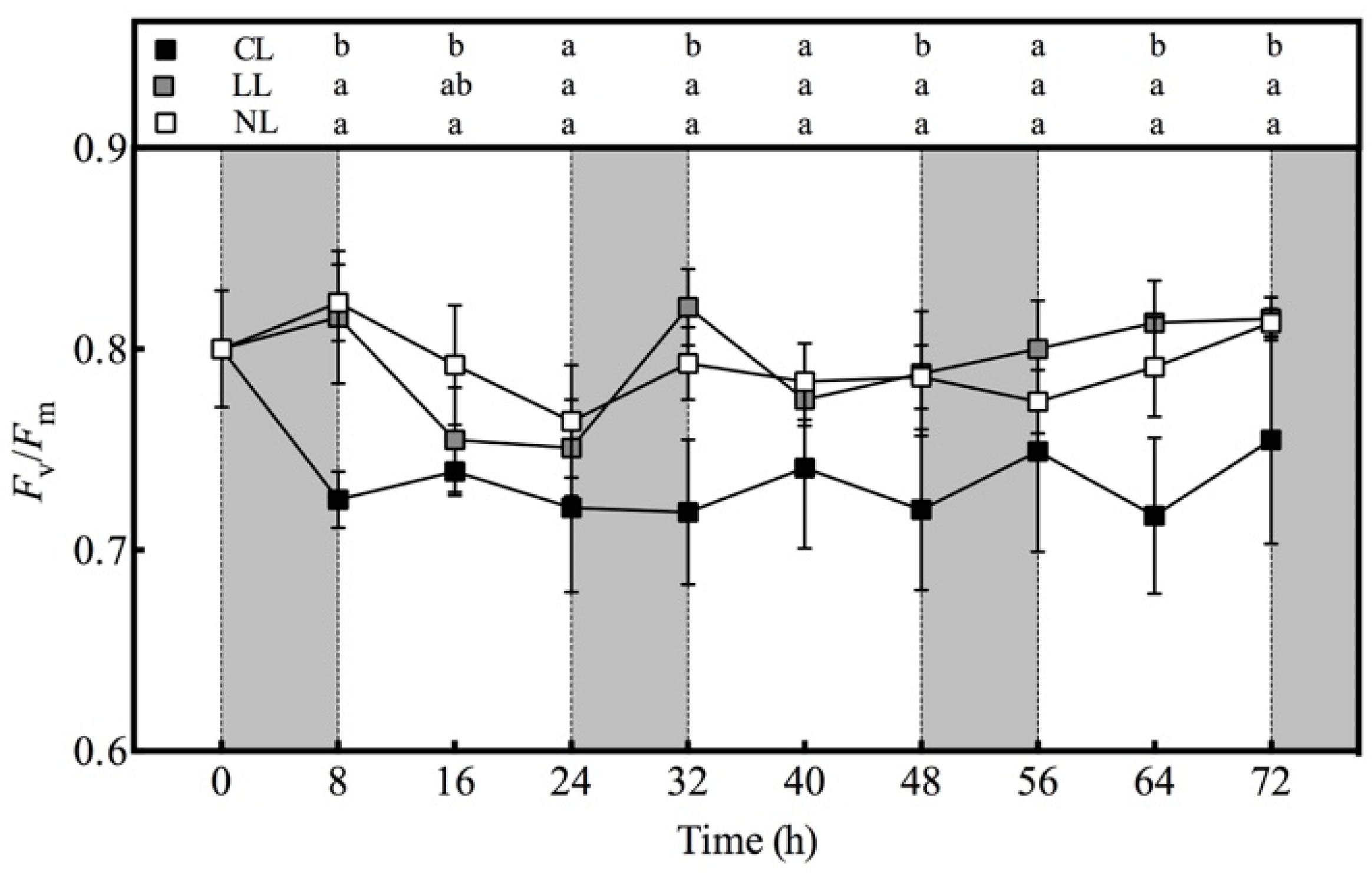
3.6. Effect of Light Intensities at Night Time on Fv/Fm value of Lettuce under Red and Blue Continuous Light
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Massot, C.; Génard, M.; Stevens, R.; Gautier, H. Fluctuations in sugar content are not determinant in explaining variations in vitamin C in tomato fruit. Plant Physiol. Biochem. 2010, 48, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Smirnoff, N.; Wheeler, G.L. Ascorbic acid in plants: Biosynthesis and function. Crc Crit. Rev. Biochem. 2000, 19, 267–290. [Google Scholar]
- Plumb, W.; Townsend, A.J.; Rasool, B.; Alomrani, S.; Razak, N.; Karpinska, B.; Ruban, A.V.; Foyer, C.H. Ascorbate-mediated regulation of growth, photoprotection, and photoinhibition in Arabidopsis thaliana. J. Exp. Bot. 2018, 69, 2823–2835. [Google Scholar] [CrossRef]
- Ntagkas, N.; Woltering, E.J.; Marcelis, L.F.M. Light regulates ascorbate in plants: An integrated view on physiology and biochemistry. Environ. Exp. Bot. 2018, 147, 271–280. [Google Scholar] [CrossRef]
- Minor, E.A.; Court, B.L.; Young, J.I.; Wang, G. Ascorbate Induces Ten-Eleven Translocation (Tet) Methylcytosine Dioxygenase-mediated Generation of 5-Hydroxymethylcytosine. J. Biol. Chem. 2013, 288, 13669–13674. [Google Scholar] [CrossRef] [Green Version]
- Asensi-Fabado, M.A.; Munné-Bosch, S. Vitamins in plants: Occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 2010, 15, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, I.B. Evolution and the Biosynthesis of Ascorbic Acid. Science (Am. Assoc. Adv. Sci.) 1973, 182, 1271–1272. [Google Scholar] [CrossRef] [PubMed]
- Lachapelle, M.Y.; Drouin, G. Inactivation dates of the human and guinea pig vitamin C genes. Genetica 2011, 139, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Komoda, H.; Uchida, T.; Node, K. Tropical fruit camu-camu (Myrciaria dubia) has anti-oxidative and anti-inflammatory properties. J. Cardiol. 2008, 52, 127–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzpatrick, T.B.; Basset, G.J.C.; Borel, P.; Carrari, F.; DellaPenna, D.; Fraser, P.D.; Hellmann, H.; Osorio, S.; Rothan, C.; Valpuesta, V.; et al. Vitamin Deficiencies in Humans: Can Plant Science Help? Plant Cell 2012, 24, 395–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smirnoff, N. Ascorbate biosynthesis and function in photoprotection. Philos. Trans. R. Soc. Lond. Ser. B: Biol. Sci. 2000, 355, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Prescott, A.G.; John, P. DIOXYGENASES: Molecular Structure and Role in Plant Metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, A.; Pinto, M.C.D.; Locato, V.; Gara, L.D. Galactone-γ-lactone-dependent ascorbate biosynthesis alters wheat kernel maturation. Plant Biol. 2012, 14, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.P.; Baek, K.H.; Lee, H.S.; Kwak, S.S.; Kwon, S.Y. Tobacco seeds simultaneously over-expressing Cu/Zn-superoxide dismutase and ascorbate peroxidase display enhanced seed longevity and germination rates under stress conditions. J. Exp. Bot. 2010, 61, 2499–2506. [Google Scholar] [CrossRef] [Green Version]
- Kotchoni, S.O.; Larrimore, K.E.; Mukherjee, M.; Kempinski, C.F.; Barth, C. Alterations in the Endogenous Ascorbic Acid Content Affect Flowering Time in Arabidopsis. Plant Physiol. 2009, 149, 803–815. [Google Scholar] [CrossRef] [Green Version]
- Massa, G.D.; Kim, H.; Wheeler, R.M.; Mitchell, C.A. Plant Productivity in Response to LED Lighting. Hortscience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Sabzalian, M.R.; Heydarizadeh, P.; Zahedi, M.; Boroomand, A.; Agharokh, M.; Sahba, M.R.; Schoefs, B. High performance of vegetables, flowers, and medicinal plants in a red-blue LED incubator for indoor plant production. Agron. Sustain. Dev. 2014, 34, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Taiz, L.; Zeiger, E. Plant Physiology; Sinauer Associates: Sunderland, MA, USA, 2006. [Google Scholar]
- Tamaoki, M.; Mukai, F.; Asai, N.; Nakajima, N.; Kubo, A.; Aono, M.; Saji, H. Light-controlled expression of a gene encoding l-galactono-γ-lactone dehydrogenase which affects ascorbate pool size in Arabidopsis thaliana. Plant Sci. 2003, 164, 1111–1117. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; van Ieperen, W.; Vreugdenhil, D.; Millenaar, F.F. Plants under continuous light. Trends Plant Sci. 2011, 16, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Yabuta, Y.; Mieda, T.; Rapolu, M.; Nakamura, A.; Motoki, T.; Maruta, T.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J. Exp. Bot. 2007, 58, 2661–2671. [Google Scholar] [CrossRef] [Green Version]
- Bartoli, C.G. Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J. Exp. Bot. 2006, 57, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Dowdle, J.; Ishikawa, T.; Gatzek, S.; Rolinski, S.; Smirnoff, N. Two genes in Arabidopsis thaliana encoding GDP-l-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 2007, 52, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, K.; Fujikawa, Y.; Esaka, M. Light Regulation of Ascorbic Acid Biosynthesis in Ricevia Light Responsivecis Elements in Genes Encoding Ascorbic Acid Biosynthetic Enzymes. Biosci. Biotechnol. Biochem. 2014, 74, 888–891. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Moscatello, S.; Leccese, A.; Colla, G.; Battistelli, A. The effect of growing spinach (Sypinacia oleracea L.) at two light intensities on the amounts of oxalate, ascorbate and nitrate in their leaves. J. Hortic. Sci. Biotechnol. 2004, 79, 606–609. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W.; Zhang, Y.; Zhou, C.; Shao, M. Morphological and Physiological Stress Responses of Lettuce to Different Intensities of Continuous Light. Front. Plant Sci. 2019, 10, 1440. [Google Scholar] [CrossRef]
- Kim, G.N.; Won, K.K. Analysis Growth Performance and Ascorbic Acid Contents of Allium victorialis var. platyphyllum, Ligularia fischeri, and L. stenocephala Under Changing Light Intensity. J. Korean Soc. For. Sci. 2010, 99, 68–74. [Google Scholar]
- Izumi, H.; Ito, T.; Yoshida, Y. Effect of light intensity during the growing period on ascorbic acid content and its histochemical distribution in the leaves and peel, and fruit quality of satsuma mandarin. J. Jpn. Soc. Hortic. Sci. 1992, 61, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Zolotukhin, I.G.; Lisovskii, G.M.; Bayanova, Y.I. Effect of light of different spectral composition and intensity on biosynthesis of ascorbic acid in plants. Fiziologiya i Biokhimiya Kul’turnykh Rastenii 1979, 11, 141–146. [Google Scholar]
- Zushi, K.; Suehara, C.; Shirai, M. Effect of light intensity and wavelengths on ascorbic acid content and the antioxidant system in tomato fruit grown in vitro. Sci. Hortic.-Amst. 2020, 274. [Google Scholar]
- Bartoli, C.G.; Tambussi, E.A.; Diego, F.; Foyer, C.H. Control of ascorbic acid synthesis and accumulation and glutathione by the incident light red/far red ratio in Phaseolus vulgaris leaves. FEBS Lett. 2009, 583, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Mastropasqua, L.; Borraccino, G.; Bianco, L.; Paciolla, C. Light qualities and dose influence ascorbate pool size in detached oat leaves. Plant Sci. 2012, 183, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.; Lee, D.; Harris, H.; Raab, A.; Feldmann, J.; Meharg, A.; Kumabe, B.; Komives, E.A.; Schroeder, J.I. Identification of an arsenic tolerant double mutant with a thiol-mediated component and increased arsenic tolerance in phyA mutants. Plant J. 2007, 49, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.M.; Chun, C. Growth and leaf injury in tomato plants under continuous light at different settings of constant and diurnally varied photosynthetic photon flux densities. Sci. Hortic.-Amst. 2020, 269, 109347. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Ainsworth, E.A. Measurement of reduced, oxidized and total ascorbate content in plants. Nat. Protoc. 2007, 2, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyuki, N.; Kozi, A. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Ma, F.W.; Cheng, L.L. Exposure of the shaded side of apple fruit to full sun leads to up-regulation of both the xanthophyll cycle and the ascorbate-glutathione cycle. Plant Sci. 2004, 166, 1479–1486. [Google Scholar] [CrossRef]
- Brennan, T.; Frenkel, C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977, 59, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngoc, N.P.; Thi, B.T.; Vinh, T.L.; Ngoc, L.P.; Sa, N.T.T.; Bich, T.V.T. Effects of color led light intensities and different photoperiod regimes on growth of hydroponic lettuce (Latuca sativa L.). Can Tho Univ. J. Sci. 2016, 2, 1–7. [Google Scholar]
- Zha, L.; Zhang, Y.; Liu, W. Dynamic Responses of Ascorbate Pool and Metabolism in Lettuce to Long-term Continuous Light Provided by Red and Blue LEDs. Environ. Exp. Bot. 2019, 163, 15–23. [Google Scholar] [CrossRef]
- Arve, L.E.; Terfa, M.T.; Gislerød, H.R.; Olsen, J.E.; Torre, S. High relative air humidity and continuous light reduce stomata functionality by affecting the ABA regulation in rose leaves. Plant Cell Environ. 2013, 36, 382–392. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W.; Yang, Q.; Zhang, Y.; Zhou, C.; Shao, M. Regulation of Ascorbate Accumulation and Metabolism in Lettuce by the Red:Blue Ratio of Continuous Light Using LEDs. Front. Plant Sci. 2020, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Rébeillé, F.; Douce, R. Biosynthesis of Vitamins in Plants Part B Volume 59 || Vitamin C. Advances in Botanical Research 2011, 59, 107–177. [Google Scholar]
- Dodd, A.N. Plant Circadian Clocks Increase Photosynthesis, Growth, Survival, and Competitive Advantage. Science 2005, 309, 630–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, R.M. Circadian Rhythms Confer a Higher Level of Fitness to Arabidopsis Plants. Plant Physiol. 2002, 129, 576–584. [Google Scholar] [CrossRef] [Green Version]
- Yan, O.Y.; Andersson, C.R.; Kondo, T.; Golden, S.S.; Johnson, C.H. Resonating circadian clocks enhance fitness in cyanobacteria. Proc. Natl. Acad. Sci. USA 1998, 95, 8660–8664. [Google Scholar]


| Treatments | Light Period (6:00–22:00) | Dark Period (22:00–6:00) |
|---|---|---|
| NL | 200 μmol·m−2·s−1 | 0 μmol·m−2·s−1 |
| LL | 200 μmol·m−2·s−1 | 20 μmol·m−2·s−1 |
| CL | 200 μmol·m−2·s−1 | 200 μmol·m−2·s−1 |
| Treatments | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Shoot Dry Weight (g) | Root Dry Weight (g) | Root/Shoot Ratio | Dry Matter Content (%) | Specific Leaf Weight (g/dm2) | Leaf Area (dm2) |
|---|---|---|---|---|---|---|---|---|
| NL | 35.0 ± 2.1b | 7.3 ± 0.7ab | 1.94 ± 0.13b | 0.36 ± 0.02b | 0.186 ± 0.010a | 5.5 ± 0.25b | 3.59 ± 0.05b | 6.08 ± 0.65a |
| LL | 37.0 ± 1.9b | 6.0 ± 1.0b | 2.11 ± 0.11b | 0.34 ± 0.03b | 0.161 ± 0.007b | 5.8 ± 0.15b | 3.26 ± 0.16c | 6.62 ± 0.47a |
| CL | 43.8 ± 3.7a | 7.8 ± 0.8a | 2.61 ± 0.33a | 0.47 ± 0.06a | 0.182 ± 0.006a | 6.5 ± 0.28a | 3.82 ± 0.05a | 6.24 ± 0.97a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Zha, L.; Liu, W. Dynamic Responses of Ascorbate Pool and Metabolism in Lettuce to Light Intensity at Night Time under Continuous Light Provided by Red and Blue LEDs. Plants 2021, 10, 214. https://doi.org/10.3390/plants10020214
Wen Y, Zha L, Liu W. Dynamic Responses of Ascorbate Pool and Metabolism in Lettuce to Light Intensity at Night Time under Continuous Light Provided by Red and Blue LEDs. Plants. 2021; 10(2):214. https://doi.org/10.3390/plants10020214
Chicago/Turabian StyleWen, Yuan, Lingyan Zha, and Wenke Liu. 2021. "Dynamic Responses of Ascorbate Pool and Metabolism in Lettuce to Light Intensity at Night Time under Continuous Light Provided by Red and Blue LEDs" Plants 10, no. 2: 214. https://doi.org/10.3390/plants10020214
APA StyleWen, Y., Zha, L., & Liu, W. (2021). Dynamic Responses of Ascorbate Pool and Metabolism in Lettuce to Light Intensity at Night Time under Continuous Light Provided by Red and Blue LEDs. Plants, 10(2), 214. https://doi.org/10.3390/plants10020214





