Effect of Three Nanoparticles (Se, Si and Cu) on the Bioactive Compounds of Bell Pepper Fruits under Saline Stress
Abstract
:1. Introduction
2. Results
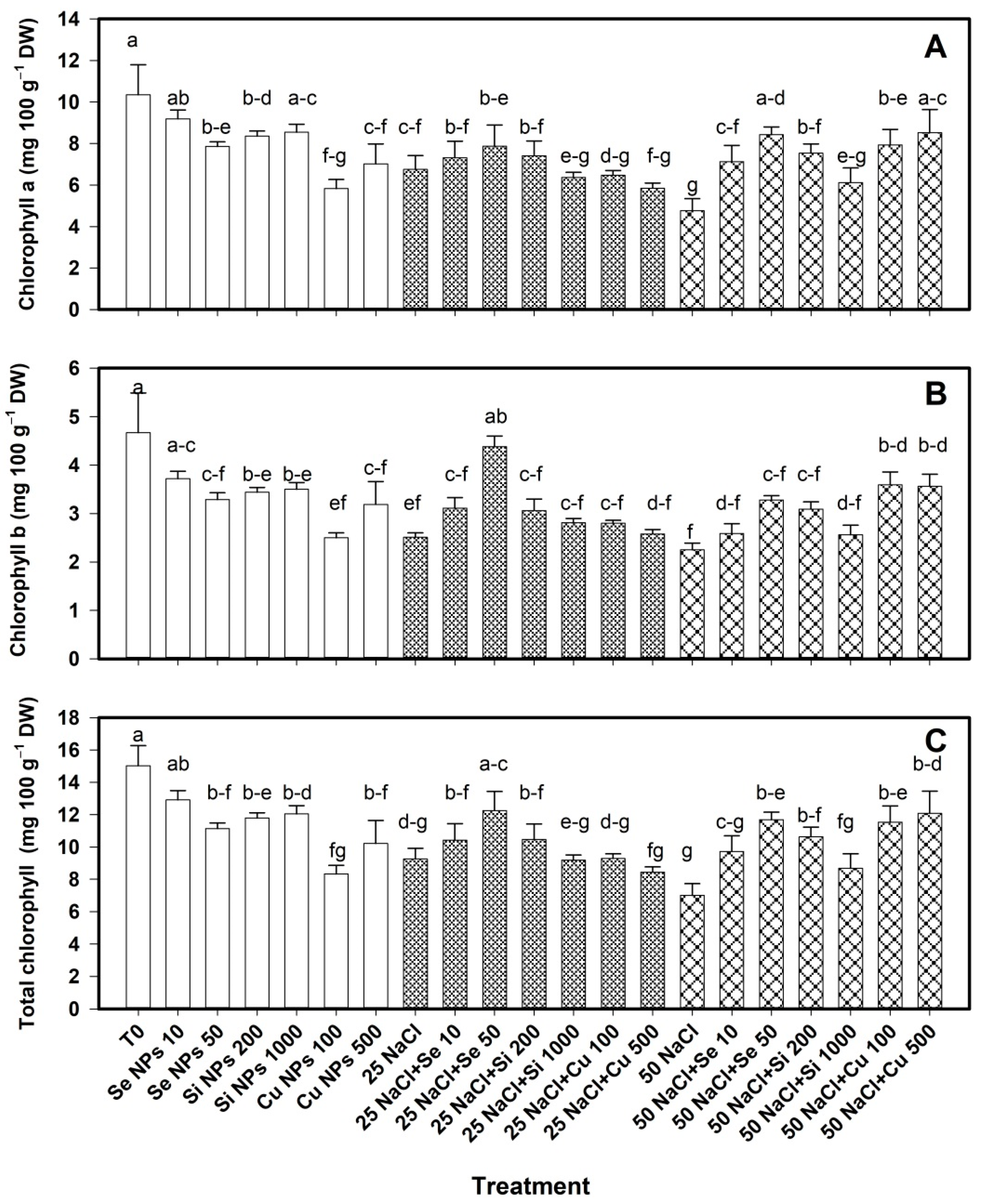
2.1. Photosynthetic Pigments
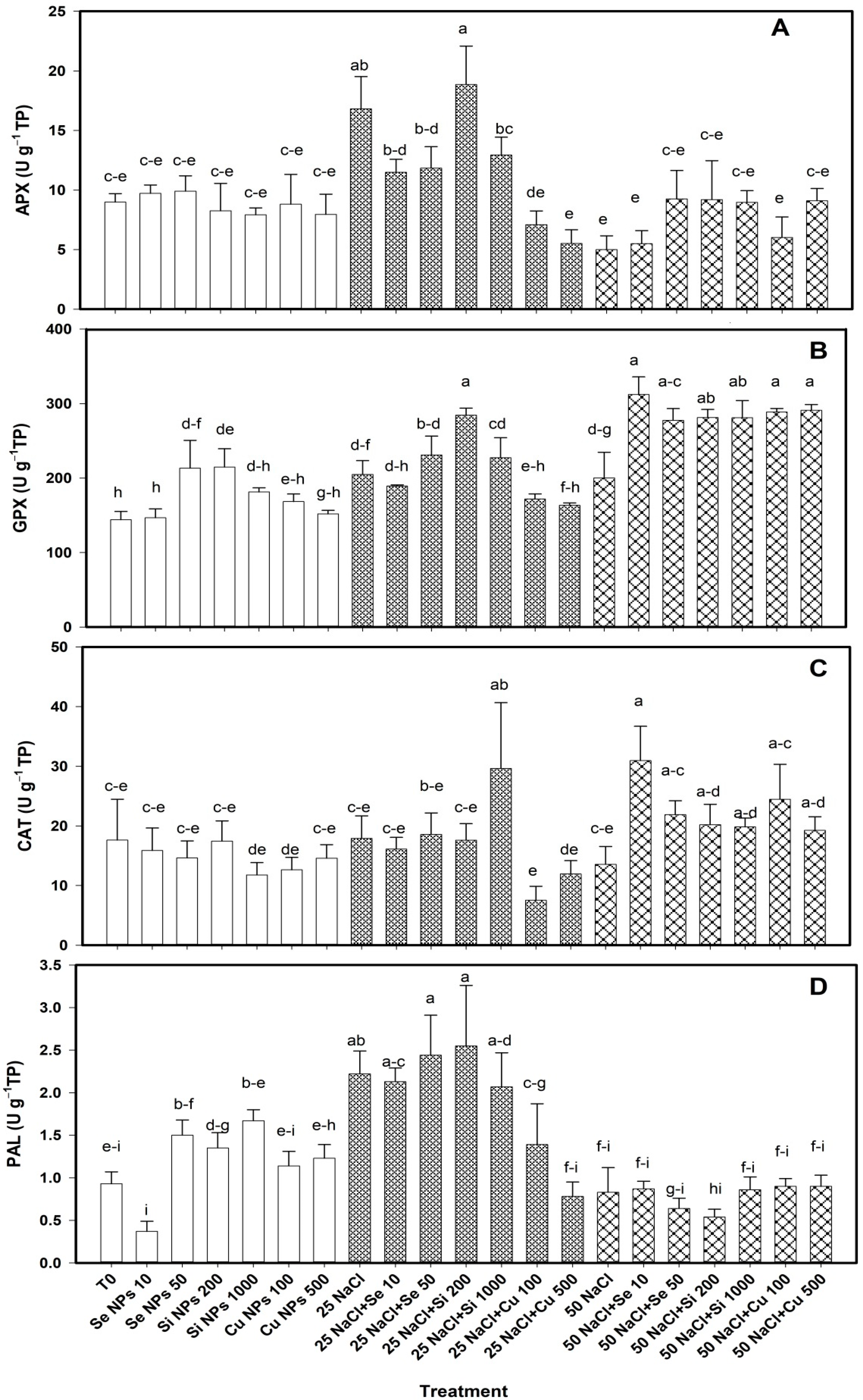
2.2. Antioxidant Activity in Leaves
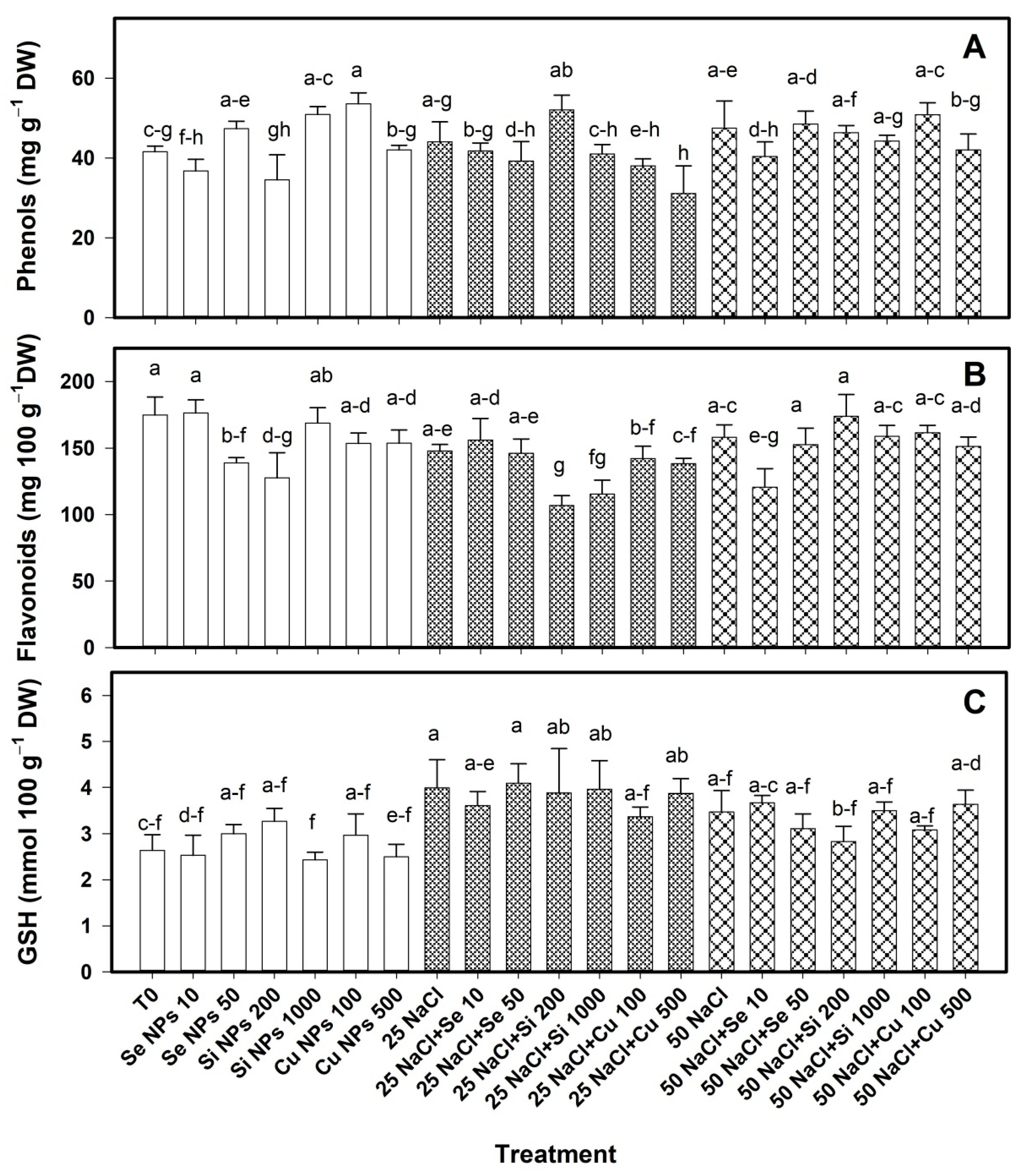
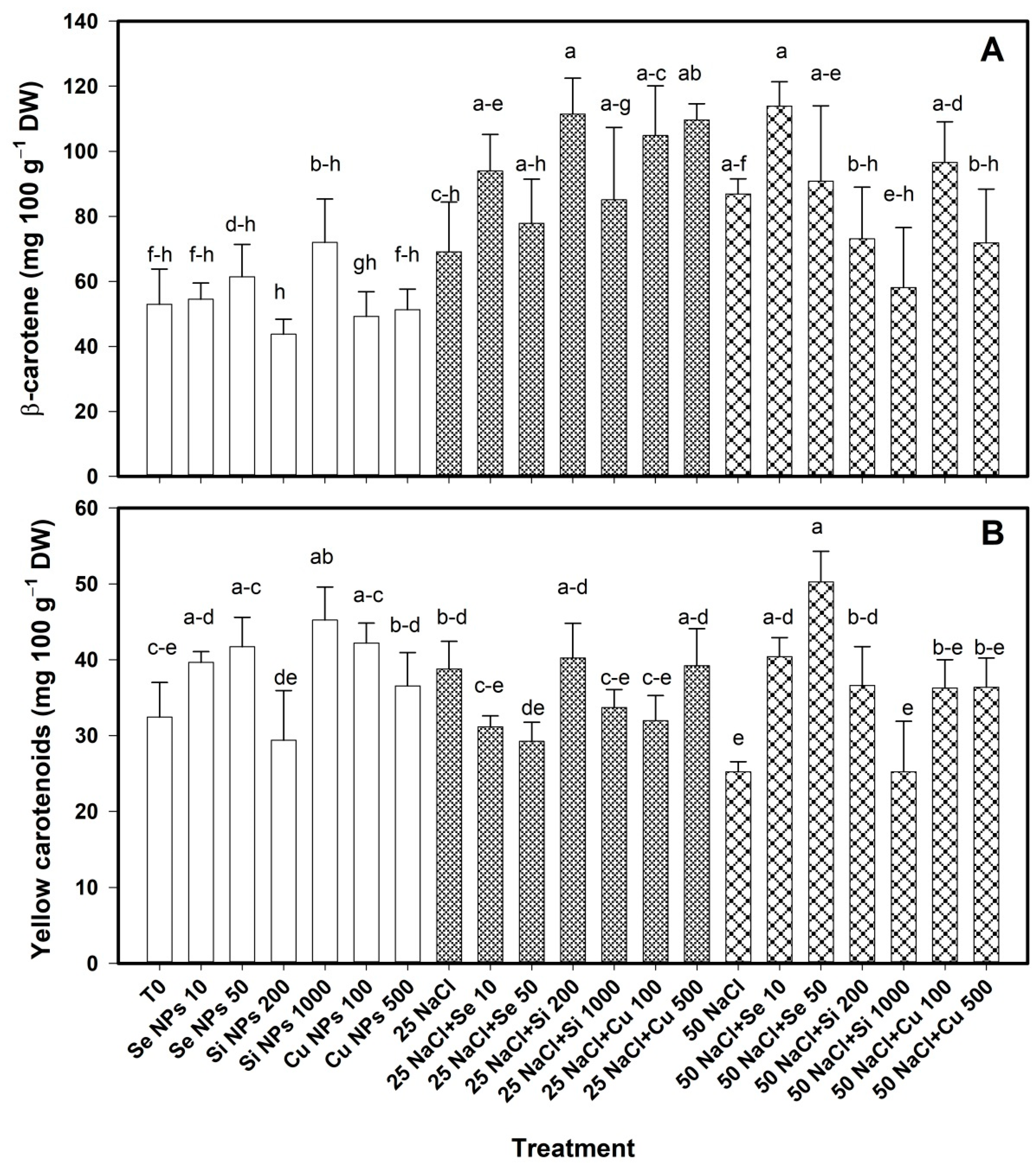
2.3. Bioactive Compounds in Pepper Fruits
3. Discussion
4. Materials and Methods
4.1. Establishment of the Experiment
4.2. Application of Treatments
4.3. Biochemical Analysis
4.3.1. Photosynthetic Pigments
4.3.2. Enzymatic Activity
4.3.3. Non-Enzymatic Antioxidant Compounds
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Z.; Jia, J.; Bai, L.; Liu, H.; Shen, S.; Yan, H. Newly designed molecularly imprinted 3-aminophenol-glyoxal-urea resin as hydrophilic solid-phase extraction sorbent for specific simultaneous determination of three plant growth regulators in green bell peppers. Food Chem. 2020, 311. [Google Scholar] [CrossRef]
- Estrada, C.C.E.; Velázquez, G.T.; Revilla, O.G.; Pérez, C.E.; Márquez, M.O.G.; Cortez, L.M.S.; Martínez, H.D.M. Prediction of total phenolics, ascorbic acid, antioxidant capacities, and total soluble solids of Capsicum annuum L. (bell pepper) juice by FT-MIR and multivariate analysis. LWT 2020, 126. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Menichini, F.; Tundis, R. Evaluation of chemical profile and antioxidant activity of twenty cultivars from Capsicum annuum, Capsicum baccatum, Capsicum chacoense and Capsicum chinense: A comparison between fresh and processed peppers. LWT Food Sci. Technol. 2015, 64, 623–631. [Google Scholar] [CrossRef]
- Minhas, P.S.; Ramos, T.B.; Gal, B.A.; Pereira, L.S. Coping with salinity in irrigated agriculture: Crop evapotranspiration and water management issues. Agric. Water Manag. 2020, 227. [Google Scholar] [CrossRef]
- Wang, F.; Shi, Z.; Biswas, A.; Yang, S.; Ding, J. Multi-algorithm comparison for predicting soil salinity. Geoderma 2020, 365. [Google Scholar] [CrossRef]
- Kamanga, R.M.; Echigo, K.; Yodoya, K.; Mohammad, A.; Mekawy, M.; Ueda, A. Salinity acclimation ameliorates salt stress in tomato (Solanum lycopersicum L.) seedlings by triggering a cascade of physiological processes in the leaves. Sci. Hortic. 2020, 270, 1–13. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.H.; Whaibi, M.H.; Faisal, M.; Sahli, A.A. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014, 33, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Tohidfar, M.; Alizadeh, M.; Daneshvar, M.H. Effects of sodium nitroprusside on callus browning of Ficus religiosa: An important medicinal plant. J. For. Res. 2020, 31, 789–796. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Rehman, Z.M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2016, 322, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.A.; Darwesh, O.M.; Mekki, B.B.; Hallouty, S.M. Evaluation of cytotoxicity, biochemical profile and yield components of groundnut plants treated with nano-selenium. Biotechnol. Rep. 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.; Darwesh, O.M.; Mekki, B.B. Environmentally friendly nano-selenium to improve antioxidant system and growth of groundnut cultivars under sandy soil conditions. Biocatal. Agric. Biotechnol. 2019, 18. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Abdelrahman, M.; Hosseini, M.S.; Hoveizeh, N.F.; Tran, L.S.P. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ. Pollut. 2019, 253, 246–258. [Google Scholar] [CrossRef]
- Chandra, J.; Chauhan, R.; Korram, J.; Satnami, M.L.; Keshavkant, S. Silica nanoparticle minimizes aluminium imposed injuries by impeding cytotoxic agents and over expressing protective genes in Cicer arietinum. Sci. Hortic. 2020, 260. [Google Scholar] [CrossRef]
- Abriz, F.S.; Torabian, S. Nano-silicon alters antioxidant activities of soybean seedlings under salt toxicity. Protoplasma 2018, 255, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Yu, S.H.I.; Jun, H.G.; Liang, H.Z.; Li, H.L.I.; Hong, Y.H.U.; Chao, Y.W. Beneficial effects of silicon on photosynthesis of tomato seedlings under water stress. J. Integr. Agric. 2018, 17, 2151–2159. [Google Scholar] [CrossRef] [Green Version]
- Le, V.N.; Rui, Y.; Gui, X.; Li, X.; Liu, S.; Han, Y. Uptake, transport, distribution and Bio-effects of SiO2 nanoparticles in Bt-transgenic cotton. J. Nanobiotechnol. 2014, 12, 50. [Google Scholar] [CrossRef] [Green Version]
- Hernández, H.H.; Morales, G.S.; Mendoza, B.A.; Ortiz, O.H.; Pliego, C.G.; Maldonado, J.A. Effects of Chitosan–PVA and Cu Nanoparticles on the Growth and Antioxidant Capacity of Tomato under Saline Stress. Molecules 2018, 23, 178. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Singh, N.B.; Hussain, I.; Singh, H. Effect of biologically synthesized copper oxide nanoparticles on metabolism and antioxidant activity to the crop plants Solanum lycopersicum and Brassica oleracea var. botrytis. J. Biotechnol. 2017, 262, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Sairam, R.K.; Tyagi, A. Physiology and molecular biology of stress tolerance in plants. Curr. Sci. 2004, 86, 407–421. [Google Scholar]
- Houimli, S.I.M.; Denden, M.; Mouhandes, B.D. Effects of 24-epibrassinolide on growth, chlorophyll, electrolyte leakage and proline by pepper plants under NaCl-stress. Eur. Asian J. Biosci. 2010, 96–104. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2020. [Google Scholar] [CrossRef] [PubMed]
- Motos, A.J.; Ortuño, M.; Vicente, B.A.; Vivancos, D.P.; Blanco, S.M.; Hernandez, J. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Espinoza, M.M.C.; Pliego, C.G.; Alvarez, P.M.; Fuentes, H.A.D.; Fuente, C.M.; Mendoza, B.A.; Reyna, V.J.; Maldonado, J.A. Se Nanoparticles Induce Changes in the Growth, Antioxidant Responses, and Fruit Quality of Tomato Developed under NaCl Stress. Molecules 2019, 24, 3030. [Google Scholar] [CrossRef] [Green Version]
- Avestan, S.; Ghasemnezhad, M.; Esfahani, M.; Byrt, C.S. Application of Nano-Silicon Dioxide Improves Salt Stress Tolerance in Strawberry Plants. Agronomy 2019, 9, 246. [Google Scholar] [CrossRef] [Green Version]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, M.V.J.; Sharma, P.K. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 2016, 54, 110–119. [Google Scholar] [CrossRef]
- Akbar Mozafari, A.; Ghaderi, N. Iron nanoparticles and potassium silicate interaction effect on salt-stressed grape cuttings under in vitro conditions: A morphophysiological and biochemical evaluation. Vitr. Cell. Dev. Biol. Plant 2019, 55, 510–518. [Google Scholar] [CrossRef]
- Hossain, Z.; Yasmeen, F.; Komatsu, S. Nanoparticles: Synthesis, Morphophysiological Effects, and Proteomic Responses of Crop Plants. Int. J. Mol. Sci. 2020, 21, 3056. [Google Scholar] [CrossRef]
- Mateus, M.P.B.; Tavanti, R.F.R.; Tavanti, T.R.; Santos, E.F.; Jalal, A.; Reis, A.R. Selenium biofortification enhances ROS scavenge system increasing yield of coffee plants. Ecotoxicol. Environ. Saf. 2021, 209. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.A.; Ortiz, O.H.; Morales, G.S.; Moreno, M.Á.; Fuente, C.M.; Rangel, S.A.; Pliego, C.G.; Mendoza, B.A. Nanoparticles and Nanomaterials as Plant Biostimulants. Int. J. Mol. Sci. 2019, 20, 162. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, J.A.; Tortella, G.; Rubilar, O.; Fincheira, P.; Mendoza, B.A. Biostimulation and toxicity: The magnitude of the impact of nanomaterials in microorganisms and plants. J. Adv. Res. 2021. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Mestre, T.C.; Sanchez, G.F.; Rubio, F.; Martinez, V.; Rivero, R.M. Glutathione homeostasis as an important and novel factor controlling blossom-end rot development in calcium-deficient tomato fruits. J. Plant Physiol. 2012, 169, 1719–1727. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Hedbavny, J.; Štork, F.; Bačkor, M. Comparison of cadmium and copper effect on phenolic metabolism, mineral nutrients and stress-related parameters in Matricaria chamomilla plants. Plant Soil 2009, 320, 231–242. [Google Scholar] [CrossRef]
- Nájera, C.C.F.; Morales, G.S.; Ortíz, O.H.; Pliego, C.G.; Mendoza, B.A.; Maldonado, J.A. The application of copper nanoparticles and potassium silicate stimulate the tolerance to Clavibacter michiganensis in tomato plants. Sci. Hortic. 2019, 245, 82–89. [Google Scholar] [CrossRef]
- Fuentes, H.A.; Vargas, L.E.; Espinoza, P.J.; Montiel, C.R.; Reyna, V.J.; Maldonado, J.A. Postharvest Behavior of Bioactive Compounds in Tomato Fruits Treated with Cu Nanoparticles and NaCl Stress. Appl. Sci. 2017, 7, 980. [Google Scholar] [CrossRef] [Green Version]
- Labrada, P.F.; Vargas, L.E.R.; Ortiz, O.H.; Pliego, C.G.; Mendoza, B.A.; Maldonado, J.A. Responses of Tomato Plants under Saline Stress to Foliar Application of Copper Nanoparticles. Plants 2019, 8, 151. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Cano, A.; Acosta, M. Analytical, Nutritional and Clinical Methods Section The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Woodill, H.M.; Flanagan, J.A.; Deemer, E.K. Development and Validation of Oxygen Radical Absorbance Capacity Assay for Lipophilic Antioxidants Using Randomly Methylated β-Cyclodextrin as the Solubility Enhancer. J. Agric. Food Chem. 2002, 50, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Pandhair, V.; Sekhon, B.S. Reactive oxygen species and antioxidants in plants: An overview. J. Plant Biochem. Biotechnol. 2006, 15, 71–78. [Google Scholar] [CrossRef]
- Jamet, C.J.; Martínez, S.R.; Gálvez, V.A.; Stoll, A.; Uribe, E.; Goñi, M.G. Biochemical composition as a function of fruit maturity stage of bell pepper (Capsicum annum) inoculated with Bacillus amyloliquefaciens. Sci. Hortic. 2020, 263. [Google Scholar] [CrossRef]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, Q.T.; Ortiz, O.H.; Pliego, C.G.; Fuentes, H.A.D.; Rangel, S.A.; Mendoza, B.A.; Fuente, C.M.; Maldonado, J.A. The Application of Selenium and Copper Nanoparticles Modifies the Biochemical Responses of Tomato Plants under Stress by Alternaria solani. Int. J. Mol. Sci. 2019, 20, 1950. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, P.Z.H.; Pliego, C.G.; Ortiz, O.H.; Morales, G.S.; Mendoza, B.A.; Reyna, V.J.; Maldonado, J.A. Form of silica improves yield, fruit quality and antioxidant defense system of tomato plants under salt stress. Agriculture 2020, 10, 367. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. J. Jpn. Soc. Food Sci. Technol. Shokuhin Kagaku Kogaku Kaishi 1992, 39, 925–928. [Google Scholar] [CrossRef] [Green Version]
- Méndez, H.D.; Mosquera, M.M.I. Rapid spectrophotometric determination of red and yellow isochromic carotenoid fractions in paprika and red pepper oleoresins. J. Agric. Food Chem. 2001, 49, 3584–3588. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar] [CrossRef]
- Xue, T.; Hartikainen, H.; Piironen, V. Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil 2001, 237, 55–61. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Dhindsa, P.P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Baranek, S.K.; Pietrosiuk, A.; Naliwajski, M.R.; Kawiak, A.; Jeziorek, M.; Wyderska, S.; Łojkowska, E.; Chinou, I. Effect of l-phenylalanine on PAL activity and production of naphthoquinone pigments in suspension cultures of Arnebia euchroma (Royle) Johnst. Vitr. Cell. Dev. Biol. Plant 2012, 48, 555–564. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Raventos, L.R.M. Analisys of total phenols and other oxidation sobstrates and antioxidants by means of Folin Ciocalteau reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Grand, A.A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of a propolis extract and identification of the main constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar]
- Jafari, M.; Shahsavar, A. The application of artificial neural networks in modeling and predicting the effects of melatonin on morphological responses of citrus to drought stress. PLoS ONE 2020, 15. [Google Scholar] [CrossRef]
- Salehi, F. Recent Advances in the Modeling and Predicting Quality Parameters of Fruits and Vegetables during Postharvest Storage: A Review. Int. J. Fruit Sci. 2020, 20, 506–520. [Google Scholar] [CrossRef]
- Wakamori, K.; Mizuno, R.; Nakanishi, G.; Mineno, H. Multimodal neural network with clustering-based drop for estimating plant water stress. Comput. Electron. Agric. 2020, 168. [Google Scholar] [CrossRef]
- Gilandeh, A.Y.; Sabzi, S.; Benmouna, B.; Mateos, G.G.; Hernández, H.J.L.; Martínez, M.J.M. Estimation of the Constituent Properties of Red Delicious Apples Using a Hybrid of Artificial Neural Networks and Artificial Bee Colony Algorithm. Agronomy 2020, 10, 267. [Google Scholar] [CrossRef] [Green Version]
- Gabriëls, S.H.E.J.; Mishra, P.; Mensink, M.G.J.; Spoelstra, P.; Woltering, E.J. Non-destructive measurement of internal browning in mangoes using visible and near-infrared spectroscopy supported by artificial neural network analysis. Postharvest Biol. Technol. 2020, 166. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-García, Y.; Cárdenas-Álvarez, C.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Cabrera-de-la-Fuente, M.; Sandoval-Rangel, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Effect of Three Nanoparticles (Se, Si and Cu) on the Bioactive Compounds of Bell Pepper Fruits under Saline Stress. Plants 2021, 10, 217. https://doi.org/10.3390/plants10020217
González-García Y, Cárdenas-Álvarez C, Cadenas-Pliego G, Benavides-Mendoza A, Cabrera-de-la-Fuente M, Sandoval-Rangel A, Valdés-Reyna J, Juárez-Maldonado A. Effect of Three Nanoparticles (Se, Si and Cu) on the Bioactive Compounds of Bell Pepper Fruits under Saline Stress. Plants. 2021; 10(2):217. https://doi.org/10.3390/plants10020217
Chicago/Turabian StyleGonzález-García, Yolanda, Claribel Cárdenas-Álvarez, Gregorio Cadenas-Pliego, Adalberto Benavides-Mendoza, Marcelino Cabrera-de-la-Fuente, Alberto Sandoval-Rangel, Jesús Valdés-Reyna, and Antonio Juárez-Maldonado. 2021. "Effect of Three Nanoparticles (Se, Si and Cu) on the Bioactive Compounds of Bell Pepper Fruits under Saline Stress" Plants 10, no. 2: 217. https://doi.org/10.3390/plants10020217
APA StyleGonzález-García, Y., Cárdenas-Álvarez, C., Cadenas-Pliego, G., Benavides-Mendoza, A., Cabrera-de-la-Fuente, M., Sandoval-Rangel, A., Valdés-Reyna, J., & Juárez-Maldonado, A. (2021). Effect of Three Nanoparticles (Se, Si and Cu) on the Bioactive Compounds of Bell Pepper Fruits under Saline Stress. Plants, 10(2), 217. https://doi.org/10.3390/plants10020217








