Responses to Drought Stress Modulate the Susceptibility to Plasmopara viticola in Vitis vinifera Self-Rooted Cuttings
Abstract
1. Introduction
2. Results
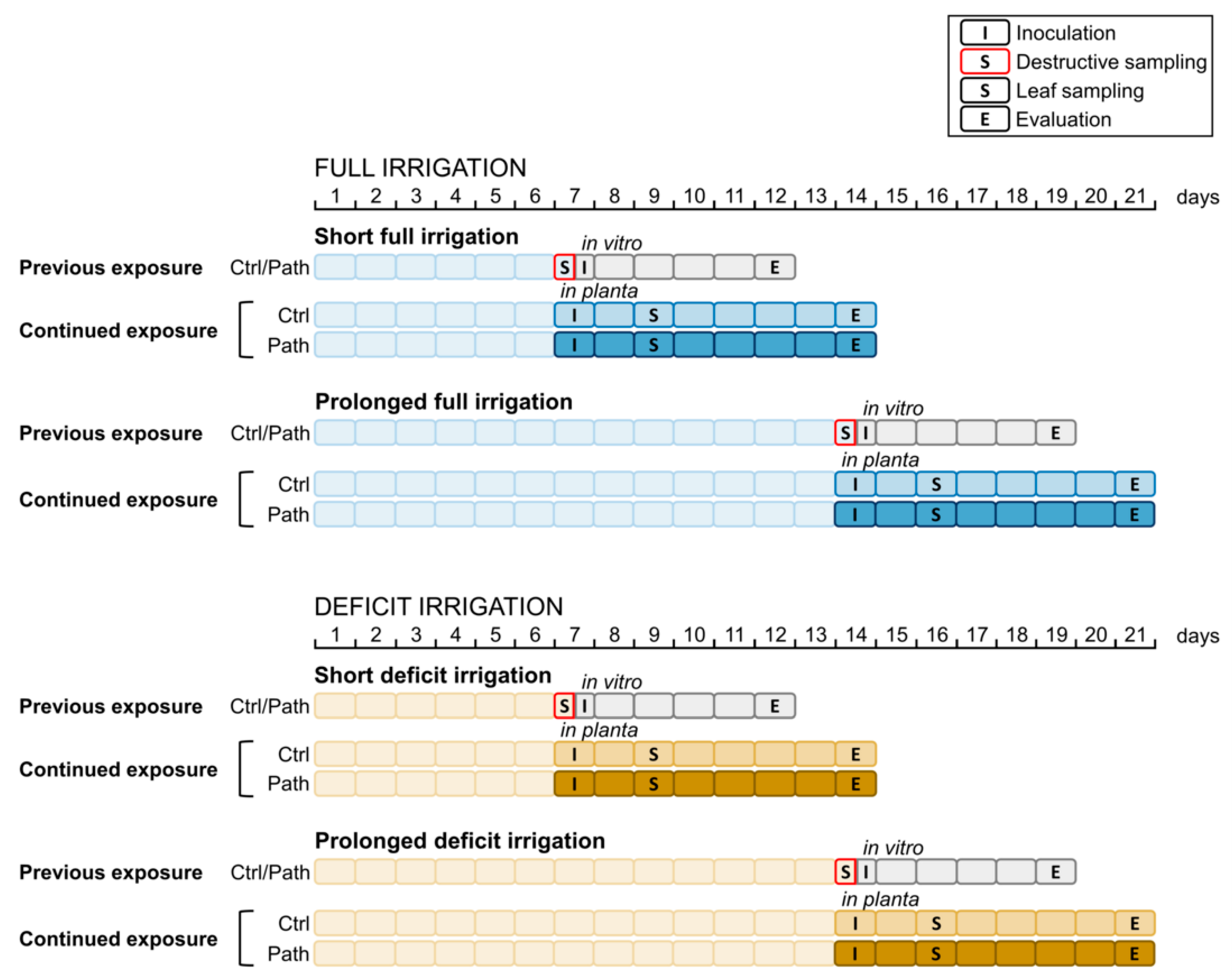
2.1. Experimental Setup
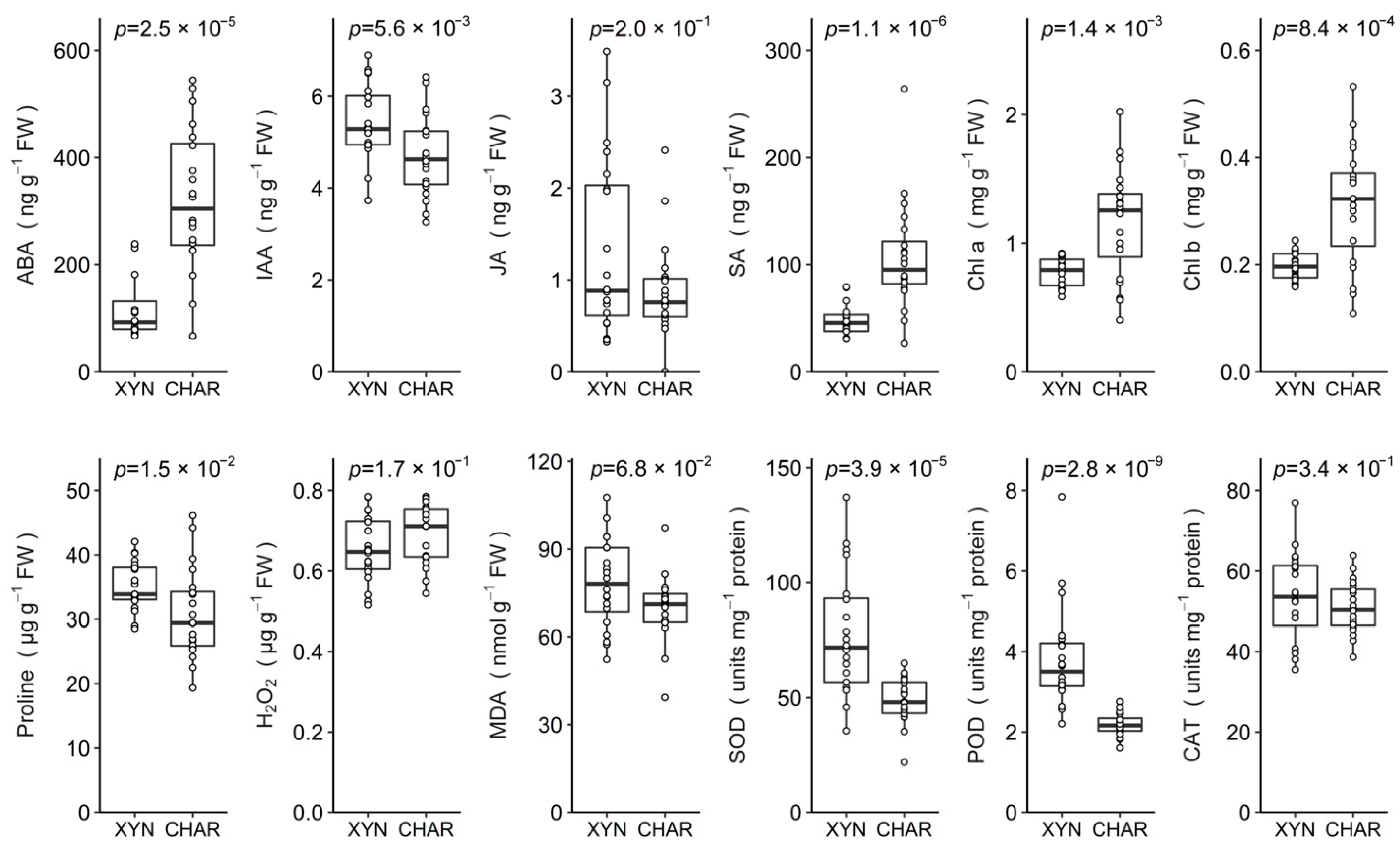
2.2. Basal Differences between Cultivars
2.3. Effect of Drought Stress on Disease Susceptibility
2.3.1. Previous Exposure: In Vitro Inoculation
2.3.2. Continued Exposure: In Planta Inoculation
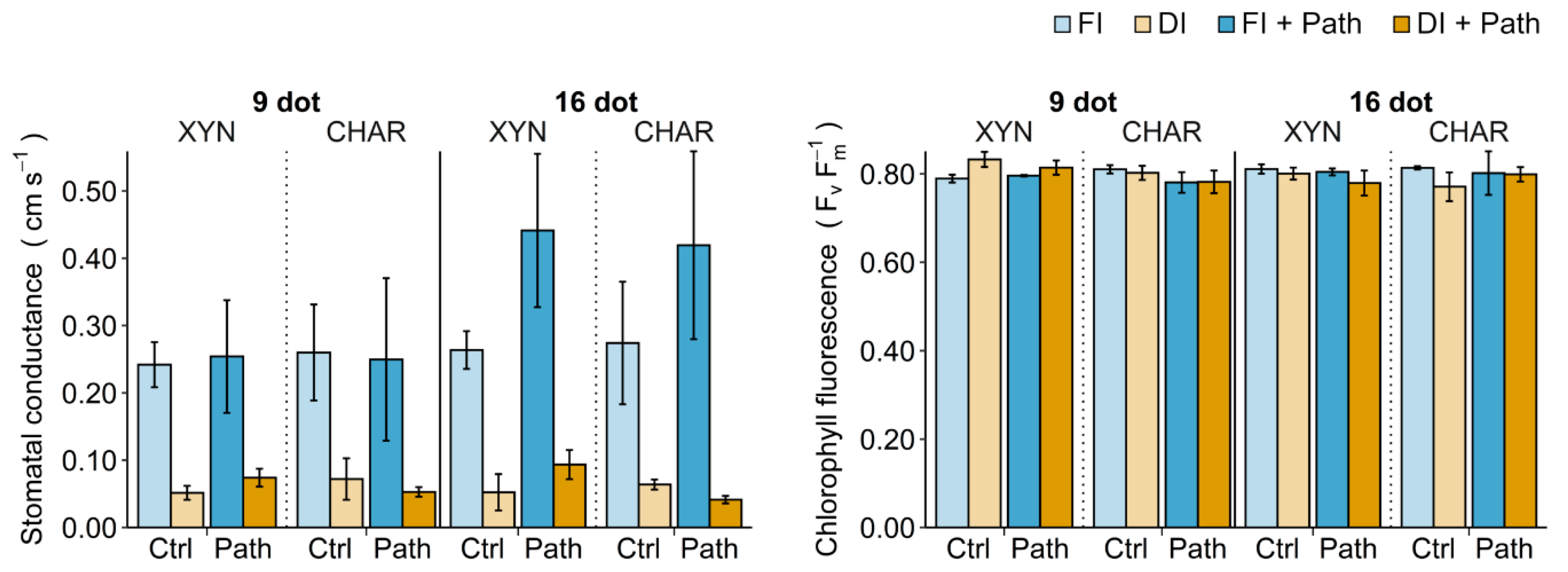
2.4. Field Measurements
2.4.1. Single Drought Stress
2.4.2. Single Pathogen Stress
2.4.3. Combined Stress
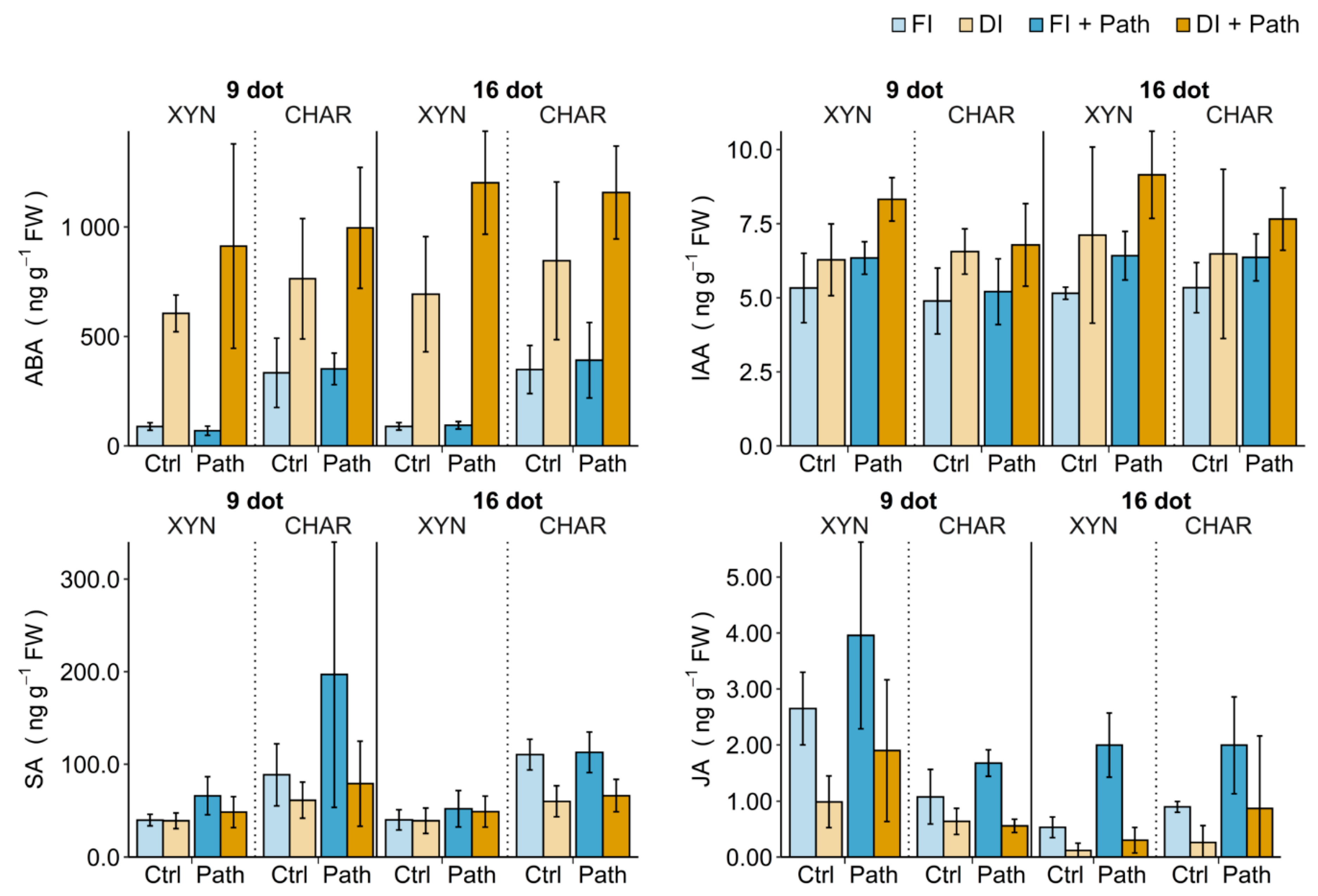
2.5. Phytohormone Balance
2.5.1. Single Drought Stress
2.5.2. Single Pathogen Stress
2.5.3. Combined Stress
2.6. Chlorophylls and Oxidative Balance
2.6.1. Single Drought Stress
2.6.2. Single Pathogen Stress
2.6.3. Combined Stress
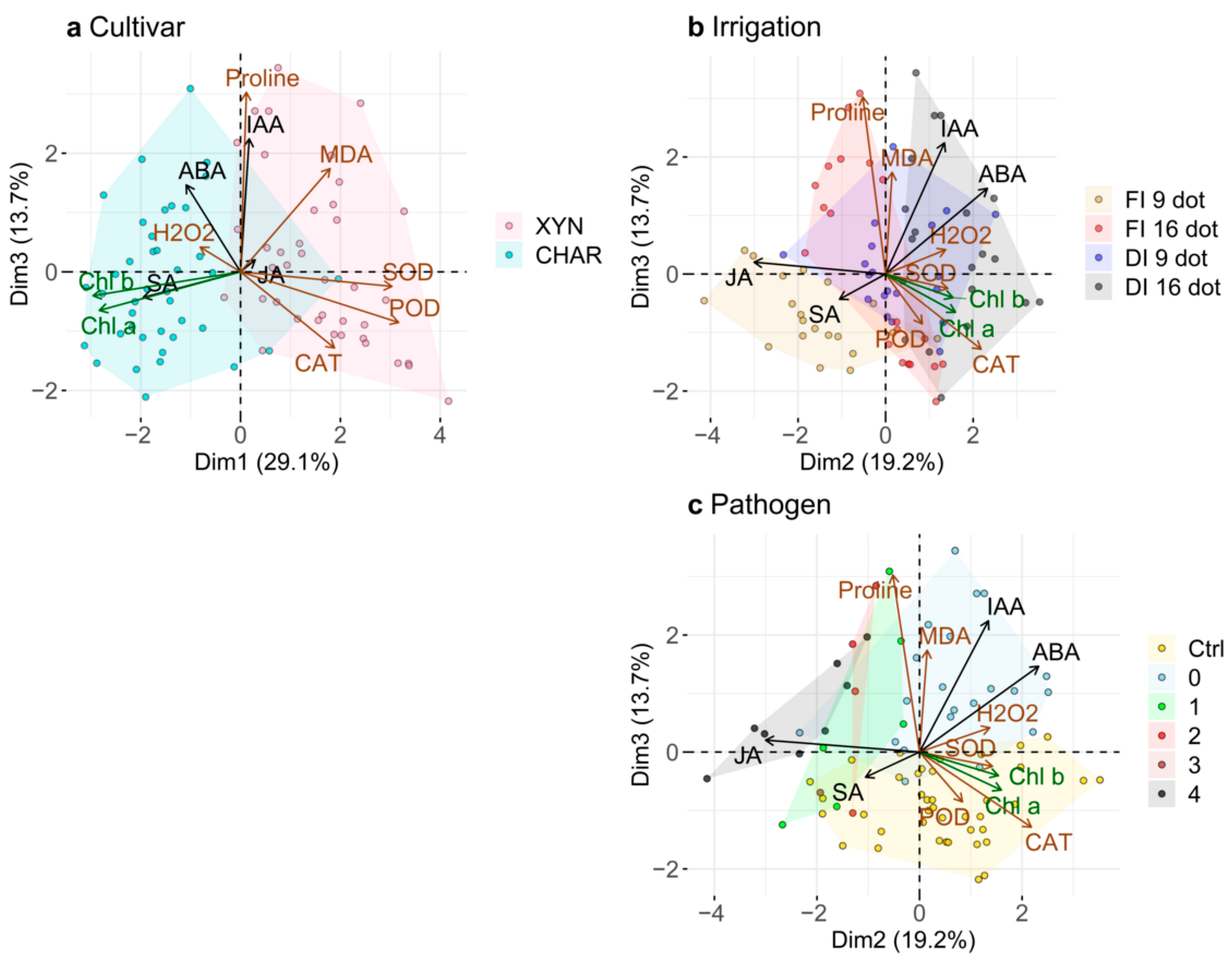
2.7. Principal Component Analysis
3. Discussion
3.1. Single Drought Stress
3.2. Single Pathogen Stress
3.3. Combined Stress
3.4. The Gap between In Vitro and in Planta Experiments
3.5. Concluding Remarks
4. Materials and Methods
4.1. Site Description and Plant Material
4.2. Abiotic Stress
4.3. Biotic Stress
4.4. Field Measurements
4.5. In Vitro Assessment of Disease Susceptibility
4.6. Quantification of Phytohormones
4.7. Quantification of Photosynthetic Pigments
4.8. Quantification of the Hydrogen Peroxide Content, Lipid Peroxidation, and Proline Content
4.9. Quantification of Antioxidant Enzymes
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Predictor Variables | ABA | IAA | JA | SA | H2O2 | CAT | POD | SOD | MDA | Pro | Chl a | Chl b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar | 0.3257 | 0.7236 | 0.2221 | 0.3798 | 0.9512 | 0.0251 ** | 0.0106 ** | 0.0007 ** | 0.0972 * | 0.0658 * | 0.0028 ** | 0.0011 ** |
| Irrigation | 0.0000 ** | 0.0000 ** | 0.0000 ** | 0.0118 ** | 0.9476 | 0.0047 ** | 0.1392 | 0.0333 ** | 0.3146 | 0.1133 | 0.0279 ** | 0.0277 ** |
| Duration | 0.1155 | 0.1011 | 0.0596 * | 0.8415 | 0.3598 | 0.0000 ** | 0.0000 ** | 0.0001 ** | 0.7006 | 0.0350 ** | 0.4966 | 0.3729 |
| Pathogen | 0.0002 ** | 0.1101 | 0.1496 | 0.8354 | 0.6716 | 0.0000 ** | 0.0000 ** | 0.0726 * | 0.1867 | 0.0103 ** | 0.0470 ** | 0.0226 ** |
| Cult:Irrig | 0.0514 * | 0.2309 | 0.0039 ** | 0.0868 * | 0.1119 | 0.1119 | 0.0028 ** | 0.6224 | 0.0221 ** | 0.0457 ** | 0.0356 ** | |
| Cult:Durat | 0.0798 * | 0.6964 | 0.5036 | 0.8031 | 0.1401 | 0.0871 * | 0.3390 | 0.9134 | ||||
| Irrig:Durat | 0.1166 | 0.5044 | 0.6556 | 0.4346 | 0.0001 ** | 0.0709 * | 0.1419 | 0.6275 | 0.0529 * | 0.1446 | 0.1040 | |
| Cult:Pathog | 0.1325 | 0.4623 | 0.7989 | 0.1622 | 0.0080 ** | 0.6546 | 0.0123 ** | 0.1679 | 0.0027 ** | 0.1097 | 0.0956 * | |
| Irrig:Pathog | 0.0002 ** | 0.0105 ** | 0.9742 | 0.0760 * | 0.0323 ** | 0.4419 | 0.8025 | 0.0004 ** | 0.0008 ** | 0.0035 ** | ||
| Durat:Pathog | 0.1203 | 0.1078 | 0.3247 | 0.3329 | 0.0331 ** | 0.0000 ** | 0.0114 ** | 0.5933 | 0.2050 | 0.1565 | 0.1146 | |
| Cult:Irrig:Durat | 0.0033 ** | 0.1068 | 0.0012 ** | 0.0158 ** | 0.0014 ** | 0.2692 | 0.0343 ** | |||||
| Cult:Irrig:Pathog | 0.1453 | 0.1241 | 0.0876 * | 0.0486 ** | 0.0331 ** | 0.0741 * | ||||||
| Cult:Durat:Pathog | 0.1684 | 0.1618 | 0.0404 ** | 0.7027 | 0.0442 ** | 0.0011 ** | ||||||
| Irrigat:Durat:Pathog | 0.1417 | 0.1659 | 0.0030 ** | 0.0141 ** | 0.7374 | 0.0002 ** | 0.0337 ** | 0.0687 * | ||||
| Cult:Irrig:Durat:Pathog | 0.1503 | 0.0438 ** | 0.0197 ** |
| Predictor Variables | ABA | IAA | JA | SA | H2O2 | CAT | POD | SOD | MDA | Pro | Chl a | Chl b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xynisteri | −87.40 | 0.15 | −0.15 | −18.11 | 0.00 | 9.20 ** | 1.60 ** | 25.57 ** | 15.56 * | 7.48 * | −0.33 ** | −0.10 ** |
| Full | −483.00 ** | −1.66 ** | 0.63 ** | 53.06 ** | 0.00 | −11.74 ** | −0.35 * | −14.03 ** | 9.37 | 5.44 * | 0.29 ** | 0.08 ** |
| 9 dot | −141.10 * | −0.50 * | 0.36 * | −4.51 | −0.05 | −26.61 ** | −0.98 ** | −30.10 ** | −3.57 | −6.21 ** | 0.07 | 0.03 |
| Pathogen | 349.77 ** | 0.68 * | 0.53 * | 4.68 | −0.02 | −19.84 ** | −1.01 ** | −10.20 * | 12.33 | 107.46 ** | 0.26 ** | 0.08 ** |
| Xynist:Full | −183.70 * | −0.21 | −54.85 ** | −0.10 * | 7.46* | 1.38 * | 28.35 ** | −6.47 | −11.24 ** | −0.31 ** | −0.09 ** | |
| Xynist:9 dot | 0.56 * | 10.15 | −0.04 | 1.42 | 1.01 * | 15.85 * | −12.59 | −0.45 | ||||
| Full:9 dot | 140.71 * | −0.17 | −11.61 | −0.06 | 24.01 ** | 0.55 * | 13.57 * | −6.37 | −7.35 * | −0.22 * | −0.07 * | |
| Xynist:Pathog | 0.91 * | −0.31 | 6.63 | −0.06 | −12.66 ** | −0.28 | −16.60 ** | −18.23 | 179.03 ** | −0.24 * | −0.07 * | |
| Full:Pathogen | −343.30 ** | 0.65 ** | −0.84 | 0.11 * | 10.13 ** | 0.19 | −3.28 | 288.00 ** | −0.65 ** | −0.15 ** | ||
| 9 dot:Pathogen | −25.24 * | −0.59 * | 31.49 | 0.06 | 12.35 ** | 1.26 ** | 16.80 ** | −7.02 | −53.04 | −0.22 | −0.06 * | |
| Xynist:Full:9 dot | 1.31 ** | 0.14 * | −22.13 ** | −2.37 ** | −43.01 ** | 20.60 | 11.40 ** | |||||
| Xynist:Full:Pathog | 0.55 * | −1.37 * | 32.07 * | −219.50 ** | 0.46 ** | 0.10 * | ||||||
| Xynist:9 dot:Pathog | 0.88 | −51.84 | 13.70 ** | −0.29 | 37.94 ** | −200.70 ** | ||||||
| Full:9 dot:Pathog | 54.49 * | −0.12 | −20.20 ** | −0.80 ** | −6.23 | −311.00 ** | 0.46 ** | 0.11 * | ||||
| Xynist:Full:9 dot:Pathog | 1.57 | −53.75 ** | 263.41 ** |
References
- IPCC. Global Warming of 1.5°C. An IPCC Special Report on the Impacts of Global Warming of 1.5°C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Hannah, L.; Roehrdanz, P.R.; Ikegami, M.; Shepard, A.V.; Shaw, M.R.; Tabor, G.; Zhi, L.; Marquet, P.A.; Hijmans, R.J. Climate change, wine, and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, 6907–6912. [Google Scholar] [CrossRef]
- Bernardo, S.; Dinis, L.-T.; Machado, N.; Moutinho-Pereira, J. Grapevine abiotic stress assessment and search for sustainable adaptation strategies in Mediterranean-like climates. A review. Agron. Sustain. Dev. 2018, 38, 66. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAOSTAT) statistical database: Crop production. Available online: http://www.fao.org/ (accessed on 12 January 2021).
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; ISBN 978-1-107-66182-0. [Google Scholar]
- Chenoweth, J.; Hadjinicolaou, P.; Bruggeman, A.; Lelieveld, J.; Levin, Z.; Lange, M.A.; Xoplaki, E.; Hadjikakou, M. Impact of climate change on the water resources of the eastern Mediterranean and Middle East region: Modeled 21st century changes and implications. Water Resour. Res. 2011, 47, w06506. [Google Scholar] [CrossRef]
- Lentini, A. New archaeobotanical data on the cultivation of Vitis ssp. at Pyrgos—Mavrorachi. In Notes of Kinyras, since 4th Millennium B.C. and Evidence from Erimi; Cyprus Wine Museum and Department of Antiquites Museum: Nicosia, Cyprus, 2009; pp. 56–73. [Google Scholar]
- Cystat Statistical service of Republic of Cyprus: Vineyard surveys, 2010–2014. Available online: https://www.mof.gov.cy/mof/cystat/statistics.nsf/ (accessed on 28 November 2019).
- Litskas, V.D.; Irakleous, T.; Tzortzakis, N.; Stavrinides, M.C. Determining the carbon footprint of indigenous and introduced grape varieties through Life Cycle Assessment using the island of Cyprus as a case study. J. Clean. Prod. 2017, 156, 418–425. [Google Scholar] [CrossRef]
- Garrett, K.A.; Dendy, S.P.; Frank, E.E.; Rouse, M.N.; Travers, S.E. Climate Change Effects on Plant Disease: Genomes to Ecosystems. Annu. Rev. Phytopathol. 2006, 44, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y.; Pertot, I. Climate Change Impacts on Plant Pathogens and Plant Diseases. J. Crop Improv. 2014, 28, 99–139. [Google Scholar] [CrossRef]
- Ramegowda, V.; Senthil-Kumar, M. The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 2015, 176, 47–54. [Google Scholar] [CrossRef]
- Gupta, A.; Senthil-Kumar, M. Concurrent Stresses Are Perceived as New State of Stress by the Plants: Overview of Impact of Abiotic and Biotic Stress Combinations. In Plant Tolerance to Individual and Concurrent Stresses; Springer: New Delhi, India, 2017; pp. 1–15. ISBN 9788132237068. [Google Scholar]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3544. [Google Scholar] [CrossRef]
- Fujita, M.; Fujita, Y.; Noutoshi, Y.; Takahashi, F.; Narusaka, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Crosstalk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 2006, 9, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Kissoudis, C.; van de Wiel, C.; Visser, R.G.F.; van der Linden, G. Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Front. Plant Sci. 2014, 5, 207. [Google Scholar] [CrossRef]
- Choudhary, A.; Pandey, P.; Senthil-Kumar, M. Tailored Responses to simultaneous drought stress and pathogen infection in plants. In Drought Stress Tolerance in Plants; Springer International Publishing: Cham, Switzerland, 2016; Volume 1, pp. 427–438. ISBN 9783319288994. [Google Scholar]
- Zhang, H.; Sonnewald, U. Differences and commonalities of plant responses to single and combined stresses. Plant J. 2017, 90, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Asselbergh, B.; De Vleesschauwer, D.; Höfte, M. Global switches and fine-tuning—ABA modulates plant pathogen defense. Mol. Plant Microbe Interact. 2008, 21, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Gupta, A.; Senthil-Kumar, M. Understanding the Impact of Drought on Foliar and Xylem Invading Bacterial Pathogen Stress in Chickpea. Front. Plant Sci. 2016, 7, 902. [Google Scholar] [CrossRef] [PubMed]
- Dossa, G.S.; Torres, R.; Henry, A.; Oliva, R.; Maiss, E.; Cruz, C.V.; Wydra, K. Rice response to simultaneous bacterial blight and drought stress during compatible and incompatible interactions. Eur. J. Plant Pathol. 2017, 147, 115–127. [Google Scholar] [CrossRef]
- Songy, A.; Fernandez, O.; Clément, C.; Larignon, P.; Fontaine, F. Grapevine trunk diseases under thermal and water stresses. Planta 2019, 249, 1655–1679. [Google Scholar] [CrossRef] [PubMed]
- Mayek-Pérez, N.; García-Espinosa, R.; López-Castañeda, C.; Acosta-Gallegos, J.A.; Simpson, J. Water relations, histopathology and growth of common bean (Phaseolus vulgaris L.) during pathogenesis of Macrophomina phaseolina under drought stress. Physiol. Mol. Plant Pathol. 2002, 60, 185–195. [Google Scholar] [CrossRef]
- Vemanna, R.S.; Bakade, R.; Bharti, P.; Kumar, M.K.P.; Sreeman, S.M.; Senthil-Kumar, M.; Makarla, U. Cross-talk signaling in rice during combined drought and bacterial blight stress. Front. Plant Sci. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Achuo, E.A.; Prinsen, E.; Höfte, M. Influence of drought, salt stress and abscisic acid on the resistance of tomato to Botrytis cinerea and Oidium neolycopersici. Plant Pathol. 2006, 55, 178–186. [Google Scholar] [CrossRef]
- Ramegowda, V.; Senthil-Kumar, M.; Ishiga, Y.; Kaundal, A.; Udayakumar, M.; Mysore, K. Drought Stress Acclimation Imparts Tolerance to Sclerotinia sclerotiorum and Pseudomonas syringae in Nicotiana benthamiana. Int. J. Mol. Sci. 2013, 14, 9497–9513. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The physiology of drought stress in grapevine: Towards an integrative definition of drought tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef]
- Chaves, M.M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under deficit irrigation: Hints from physiological and molecular data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef]
- Armijo, G.; Schlechter, R.; Agurto, M.; Muñoz, D.; Nuñez, C.; Arce-Johnson, P. Grapevine Pathogenic Microorganisms: Understanding Infection Strategies and Host Response Scenarios. Front. Plant Sci. 2016, 7, 1–18. [Google Scholar] [CrossRef]
- Choi, H.-K.; Iandolino, A.; da Silva, F.G.; Cook, D.R. Water deficit modulates the response of Vitis vinifera to the Pierce’s disease pathogen Xylella fastidiosa. Mol. Plant Microbe Interact. 2013, 26, 643–657. [Google Scholar] [CrossRef]
- Roatti, B.; Perazzolli, M.; Gessler, C.; Pertot, I. Abiotic stresses affect Trichoderma harzianum T39-induced resistance to downy mildew in grapevine. Phytopathology 2013, 103, 1227–1234. [Google Scholar] [CrossRef]
- Hatmi, S.; Gruau, C.; Trotel-aziz, P.; Villaume, S.; Rabenoelina, F.; Baillieul, F.; Eullaffroy, P.; Clément, C.; Ferchichi, A.; Aziz, A. Drought stress tolerance in grapevine involves activation of polyamine oxidation contributing to improved immune response and low susceptibility to Botrytis cinerea. J. Exp. Bot. 2015, 66, 775–787. [Google Scholar] [CrossRef]
- Eurostat. Plant Protection in the EU—Consumption of Plant Protection Products in the European Union: Data 1992–1996; Office des Publications Officielles des Communautés Européennes: Luxembourg, 2007; ISBN 92-79-03890-7. [Google Scholar]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop Prot. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Allègre, M.; Daire, X.; Héloir, M.; Trouvelot, S.; Mercier, L.; Adrian, M.; Pugin, A. Stomatal deregulation in Plasmopara viticola-infected grapevine leaves. New Phytol. 2007, 173, 832–840. [Google Scholar] [CrossRef]
- Tiwari, S.; Lata, C.; Chauhan, P.S.; Prasad, V.; Prasad, M. A Functional Genomic Perspective on Drought Signalling and its Crosstalk with Phytohormone-mediated Signalling Pathways in Plants. Curr. Genomics 2017, 18, 469–482. [Google Scholar] [CrossRef]
- Sánchez-Vallet, A.; López, G.; Ramos, B.; Delgado-Cerezo, M.; Riviere, M.-P.; Llorente, F.; Fernández, P.V.; Miedes, E.; Estevez, J.M.; Grant, M.; et al. Disruption of Abscisic Acid Signaling Constitutively Activates Arabidopsis Resistance to the Necrotrophic Fungus Plectosphaerella cucumerina. Plant Physiol. 2012, 160, 2109–2124. [Google Scholar] [CrossRef]
- Hussain, S.; Gomes, M.M.; Yano, K.; Nambara, E. Interactions between abscisic acid and other hormones. In Advances in Botanical Research; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 92, pp. 255–280. ISBN 9780081026205. [Google Scholar]
- Anderson, J.P.; Badruzsaufari, E.; Schenk, P.M.; Manners, J.M.; Desmond, O.J.; Ehlert, C.; Maclean, D.J.; Ebert, P.R.; Kazan, K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 2004, 16, 3460–3479. [Google Scholar] [CrossRef]
- Adie, B.A.T.; Pérez-Pérez, J.; Pérez-Pérez, M.M.; Godoy, M.; Sánchez-Serrano, J.-J.; Schmelz, E.A.; Solano, R. ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell 2007, 19, 1665–1681. [Google Scholar] [CrossRef] [PubMed]
- Mohr, P.G.; Cahill, D.M. Abscisic acid influences the susceptibility of Arabidopsis thaliana to Pseudomonas syringae pv. tomato and Peronospora parasitica. Funct. Plant Biol. 2003, 30, 461. [Google Scholar] [CrossRef] [PubMed]
- Mauch-Mani, B.; Mauch, F. The role of abscisic acid in plant–pathogen interactions. Curr. Opin. Plant Biol. 2005, 8, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Ishikawa, A.; Jikumaru, Y.; Seki, M.; Umezawa, T.; Asami, T.; Maruyama-Nakashita, A.; Kudo, T.; Shinozaki, K.; Yoshida, S.; et al. Antagonistic Interaction between Systemic Acquired Resistance and the Abscisic Acid-Mediated Abiotic Stress Response in Arabidopsis. Plant Cell 2008, 20, 1678–1692. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, J.; Zhang, Y.; Lu, J. Muscadinia rotundifolia ‘Noble’ defense response to Plasmopara viticola inoculation by inducing phytohormone-mediated stilbene accumulation. Protoplasma 2018, 255, 95–107. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Chrysargyris, A.; Aziz, A. Adaptive Response of a Native Mediterranean Grapevine Cultivar Upon Short-Term Exposure to Drought and Heat Stress in the Context of Climate Change. Agronomy 2020, 10, 249. [Google Scholar] [CrossRef]
- Copper, A.W.; Karaolis, C.; Koundouras, S.; Savvides, S.; Bastian, S.E.P.; Johnson, T.E.; Collins, C. Vine performance benchmarking of indigenous Cypriot grape varieties Xynisteri and Maratheftiko. OENO One 2020, 54, 935–954. [Google Scholar] [CrossRef]
- Harman, G.E.; Latorre, B.; Agosin, E.; San Martin, R.; Riegel, D.G.; Nielsen, P.A.; Tronsmo, A.; Pearson, R.C. Biological and Integrated Control of Botrytis Bunch Rot of Grape Using Trichoderma spp. Biol. Control 1996, 7, 259–266. [Google Scholar] [CrossRef]
- Doupis, G.; Chartzoulakis, K.; Beis, A.; Patakas, A. Allometric and biochemical responses of grapevines subjected to drought and enhanced ultraviolet-B radiation. Aust. J. Grape Wine Res. 2011, 17, 36–42. [Google Scholar] [CrossRef]
- Tognetti, V.B.; Mühlenbock, P.; Van Breusegem, F. Stress homeostasis—The redox and auxin perspective. Plant. Cell Environ. 2012, 35, 321–333. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Caffi, T.; Legler, S.E.; González-domínguez, E.; Rossi, V. Effect of temperature and wetness duration on infection by Plasmopara viticola and on post-inoculation efficacy of copper. Eur. J. Plant Pathol. 2016, 144, 737–750. [Google Scholar] [CrossRef]
- Angelotti, F.; Hamada, E.; Magalhães, E.E.; Ghini, R.; Garrido, L.d.R.; Jùnior, M.J.P. Climate change and the occurrence of downy mildew in Brazilian grapevines. Pesqui. Agropecuária Bras. 2017, 52, 426–434. [Google Scholar] [CrossRef][Green Version]
- Williams, M.G.; Magarey, P.A.; Sivasithamparam, K. Effect of temperature and light intensity on early infection behaviour of a Western Australian isolate of Plasmopara viticola, the downy mildew pathogen of grapevine. Australas. Plant Pathol. 2007, 36, 325–331. [Google Scholar] [CrossRef]
- Heyman, L.; Höfle, R.; Kicherer, A.; Trapp, O.; Ait Barka, E.; Töpfer, R.; Höfte, M. The durability of quantitative host resistance and variability in pathogen virulence in the interaction between European grapevine cultivars and Plasmopara viticola. Manuscript in preparation.
- Figueiredo, A.; Martins, J.; Sebastiana, M.; Guerreiro, A.; Silva, A.; Rita, A.; Monteiro, F.; Salomé, M.; Roepstorff, P.; Varela, A.; et al. Specific adjustments in grapevine leaf proteome discriminating resistant and susceptible grapevine genotypes to Plasmopara viticola. J. Proteomics 2017, 152, 48–57. [Google Scholar] [CrossRef]
- Nascimento, R.; Maia, M.; Ferreira, A.E.N.; Silva, A.B.; Freire, A.P.; Cordeiro, C.; Silva, M.S.; Figueiredo, A. Early stage metabolic events associated with the establishment of Vitis vinifera—Plasmopara viticola compatible interaction. Plant Physiol. Biochem. 2019, 137, 1–13. [Google Scholar] [CrossRef]
- Guerreiro, A.; Figueiredo, J.; Sousa Silva, M.; Figueiredo, A. Linking Jasmonic Acid to Grapevine Resistance against the Biotrophic Oomycete Plasmopara viticola. Front. Plant Sci. 2016, 7, 565. [Google Scholar] [CrossRef] [PubMed]
- Polesani, M.; Bortesi, L.; Ferrarini, A.; Zamboni, A.; Fasoli, M.; Zadra, C.; Lovato, A.; Pezzotti, M.; Delledonne, M.; Polverari, A. General and species-specific transcriptional responses to downy mildew infection in a susceptible (Vitis vinifera) and a resistant (V. riparia) grapevine species. BMC Genom. 2010, 11, 117. [Google Scholar] [CrossRef]
- Marchive, C.; Léon, C.; Kappel, C.; Coutos-Thévenot, P.; Corio-Costet, M.-F.; Delrot, S.; Lauvergeat, V. Over-Expression of VvWRKY1 in Grapevines Induces Expression of Jasmonic Acid Pathway-Related Genes and Confers Higher Tolerance to the Downy Mildew. PLoS ONE 2013, 8, e54185. [Google Scholar] [CrossRef]
- Gauthier, A.; Trouvelot, S.; Kelloniemi, J.; Frettinger, P.; Wendehenne, D.; Daire, X.; Joubert, J.; Ferrarini, A.; Delledonne, M.; Flors, V.; et al. The Sulfated Laminarin Triggers a Stress Transcriptome before Priming the SA- and ROS-Dependent Defenses during Grapevine’s Induced Resistance against Plasmopara viticola. PLoS ONE 2014, 9, e88145. [Google Scholar] [CrossRef]
- Figueiredo, A.; Monteiro, F.; Sebastiana, M. First clues on a jasmonic acid role in grapevine resistance against the biotrophic fungus Plasmopara viticola. Eur. J. Plant Pathol. 2015, 142, 645–652. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; Yin, L.; Zhang, Y.; Qu, J.; Lu, J. Comparative transcriptome analysis reveals defense-related genes and pathways against downy mildew in Vitis amurensis grapevine. Plant Physiol. Biochem. 2015, 95, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hamiduzzaman, M.M.; Jakab, G.; Barnavon, L.; Neuhaus, J.M.; Mauch-Mani, B. β -Aminobutyric Acid-Induced Resistance Against Downy Mildew in Grapevine Acts Through the Potentiation of Callose Formation and Jasmonic Acid Signaling. Mol. Plant Microbe Interact. 2005, 18, 819–829. [Google Scholar] [CrossRef]
- Yin, C.; Park, J.-J.; Gang, D.R.; Hulbert, S.H. Characterization of a Tryptophan 2-Monooxygenase Gene from Puccinia graminis f. sp. tritici Involved in Auxin Biosynthesis and Rust Pathogenicity. Mol. Plant Microbe Interact. 2014, 27, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Pospíšilová, J. Participation of phytohormones in the stomatal regulation of gas exchange during water stress. Biol. Plant. 2003, 46, 491–506. [Google Scholar] [CrossRef]
- Stoll, M.; Schultz, H.R.; Berkelmann-Loehnertz, B. Exploring the sensitivity of thermal imaging for Plasmopara viticola pathogen detection in grapevines under different water status. Funct. Plant Biol. 2008, 35, 281. [Google Scholar] [CrossRef] [PubMed]
- Selim, M. Elicitation of grapevine defense responses against Plasmopara viticola, the causal agent of downy mildew. Ph.D. Thesis, Justus Liebig Universität, Gießen, Germany, 2013. [Google Scholar]
- Jermini, M.; Blaise, P.; Gessler, C. Influence of Plasmopara viticola on gas exchange parameters on field-grown Vitis vinifera “Merlot”. Vitis 2010, 49, 87–93. [Google Scholar]
- Gamm, M.; Héloir, M.; Bligny, R.; Vaillant-gaveau, N.; Trouvelot, S.; Alcaraz, G.; Frettinger, P.; Clément, C.; Pugin, A.; Wendehenne, D.; et al. Changes in Carbohydrate Metabolism in Plasmopara viticola-Infected Grapevine Leaves. Mol. Plant Microbe Interact. 2011, 24, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Moriondo, M.; Orlandini, S.; Giuntoli, A.; Bindi, M. The Effect of Downy and Powdery Mildew on Grapevine (Vitis vinifera L.) Leaf Gas Exchange. J. Phytopathol. 2005, 153, 350–357. [Google Scholar] [CrossRef]
- Cséfalvay, L.; Di Gaspero, G.; Matouš, K.; Bellin, D.; Ruperti, B.; Olejníčková, J. Pre-symptomatic detection of Plasmopara viticola infection in grapevine leaves using chlorophyll fluorescence imaging. Eur. J. Plant Pathol. 2009, 125, 291–302. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kobayashi, Y.; Matsumoto, H. Lipid Peroxidation Is an Early Symptom Triggered by Aluminum, But not the Primary Cause of Elongation Inhibition in Pea Roots. Plant Physiol. 2001, 125, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Klessig, D.F.; Durner, J.; Noad, R.; Navarre, D.A.; Wendehenne, D.; Kumar, D.; Zhou, J.M.; Shah, J.; Zhang, S.; Kachroo, P.; et al. Nitric oxide and salicylic acid signaling in plant defense. Proc. Natl. Acad. Sci. USA 2000, 97, 8849–8855. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox Homeostasis and Antioxidant Signaling: A Metabolic Interface between Stress Perception and Physiological Responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Maltese, F.; Figueiredo, A.; Rex, M.; Margarida, A.; Zyprian, E.; Salomé, M.; Verpoorte, R.; Hae, Y.; Fortes, A.M.; et al. Alterations in grapevine leaf metabolism upon inoculation with Plasmopara viticola in different time-points. Plant Sci. 2012, 191–192, 100–107. [Google Scholar] [CrossRef]
- Matysik, J.; Alia; Bhalu, B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 82, 525–532. [Google Scholar]
- Audenaert, K.; De Meyer, G.B.; Höfte, M. Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid- dependent signaling mechanisms. Plant Physiol. 2002, 128, 491–501. [Google Scholar] [CrossRef]
- Asselbergh, B.; Achuo, A.E.; Höfte, M.; Van Gijsegem, F. Abscisic acid deficiency leads to rapid activation of tomato defence responses upon infection with Erwinia chrysanthemi. Mol. Plant Pathol. 2007, 9, 11–24. [Google Scholar] [CrossRef]
- Gupta, A.; Hisano, H.; Hojo, Y.; Matsuura, T.; Ikeda, Y.; Mori, I.C.; Senthil-Kumar, M. Global profiling of phytohormone dynamics during combined drought and pathogen stress in Arabidopsis thaliana reveals ABA and JA as major regulators. Sci. Rep. 2017, 7, 4017. [Google Scholar] [CrossRef]
- Allègre, M.; Héloir, M.C.; Trouvelot, S.; Daire, X.; Pugin, A.; Wendehenne, D.; Adrian, M. Are grapevine stomata involved in the elicitor-induced protection against downy mildew? MPMI 2009, 22, 977–986. [Google Scholar] [CrossRef]
- Liu, L.; Liu, C.-Y.; Wang, H.; Yu, S.-Y.; Guan, T.-S.; Huang, Y.-F.; Li, R.-C. The abscisic acid receptor gene VvPYL4 positively regulates grapevine resistance to Plasmopara viticola. Plant Cell Tissue Organ Cult. 2020, 142, 483–492. [Google Scholar] [CrossRef]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant Stomata Function in Innate Immunity against Bacterial Invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Ton, J.; Mauch-Mani, B. β -amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. Plant J. 2004, 38, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, J.; Zhang, P.; Hasi, G.; Huang, Y.; Lu, J.; Zhang, Y. Response of phytohormones and correlation of SAR signal pathway genes to the different resistance levels of grapevine against Plasmopara viticola infection. Plant Physiol. Biochem. 2016, 107, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Unger, S.; Büche, C.; Boso, S.; Kassemeyer, H. The Course of Colonization of Two Different Vitis Genotypes by Plasmopara viticola Indicates Compatible and Incompatible Host-Pathogen Interactions. Phytopathology 2007, 97, 780–786. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Toumi, I.; M’Sehli, W.; Bourgou, S.; Jallouli, N.; Bensalem-Fnayou, A.; Ghorbel, A.; Mliki, A. Response of ungrafted and grafted grapevine cultivars and rootstocks (Vitis sp.) to water stress. OENO One 2007, 41, 85. [Google Scholar] [CrossRef]
- Serra, I.; Strever, A.; Myburgh, P.A.; Deloire, A. Review: The interaction between rootstocks and cultivars (Vitis vinifera L.) to enhance drought tolerance in grapevine. Aust. J. Grape Wine Res. 2014, 20, 1–14. [Google Scholar] [CrossRef]
- Romero Azorín, P.; García García, J. The Productive, Economic, and Social Efficiency of Vineyards Using Combined Drought-Tolerant Rootstocks and Efficient Low Water Volume Deficit Irrigation Techniques under Mediterranean Semiarid Conditions. Sustainability 2020, 12, 1930. [Google Scholar] [CrossRef]
- Poni, S.; Bernizzoni, F.; Civardi, S.; Gatti, M.; Porro, D.; Camin, F. Performance and water-use efficiency (single-leaf vs. whole-canopy) of well-watered and half-stressed split-root Lambrusco grapevines grown in Po Valley (Italy). Agric. Ecosyst. Environ. 2009, 129, 97–106. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Litskas, V.; Stavrinides, M.; Heyman, L.; Demeestere, K.; Höfte, M.; Tzortzakis, N. Assessing the Impact of Drought Stress and Soil Cultivation in Chardonnay and Xynisteri Grape Cultivars. Agronomy 2020, 10, 670. [Google Scholar] [CrossRef]
- OIV. Descriptor List for Grape Varieties and Vitis Species, 2nd ed.; Office International de la Vigne et du Vin: Paris, France, 2009. [Google Scholar]
- Schwander, F.; Eibach, R.; Fechter, I.; Hausmann, L.; Zyprian, E.; Töpfer, R. Rpv10: A new locus from the Asian Vitis gene pool for pyramiding downy mildew resistance loci in grapevine. Theor. Appl. Genet. 2012, 124, 163–176. [Google Scholar] [CrossRef]
- Haeck, A.; Van Langenhove, H.; Harinck, L.; Kyndt, T.; Gheysen, G.; Höfte, M.; Demeestere, K. Trace analysis of multi-class phytohormones in Oryza sativa using different scan modes in high-resolution Orbitrap mass spectrometry: Method validation, concentration levels, and screening in multiple accessions. Anal. Bioanal. Chem. 2018, 410, 4527–4539. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Botsaris, G.; Tzortzakis, N. Antioxidant and antibacterial activities, mineral and essential oil composition of spearmint (Mentha spicata L.) affected by the potassium levels. Ind. Crops Prod. 2017, 103, 202–212. [Google Scholar] [CrossRef]
- Khedr, A.H.A.; Abbas, M.A.; Wahid, A.A.A.; Quick, W.P.; Abogadallah, G.M. Proline induces the expression of salt-stress-responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt-stress. J. Exp. Bot. 2003, 54, 2553–2562. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Michailidi, E.; Tzortzakis, N. Physiological and Biochemical Responses of Lavandula angustifolia to Salinity Under Mineral Foliar Application. Front. Plant Sci. 2018, 9, 1–23. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Involvement of plasma-membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta 2002, 215, 1022–1030. [Google Scholar] [CrossRef]
- Tarchoune, I.; Sgherri, C.; Izzo, R.; Lachaâl, M.; Navari-Izzo, F.; Ouerghi, Z. Changes in the antioxidative systems of Ocimum basilicum L. (cv. Fine) under different sodium salts. Acta Physiol. Plant. 2012, 34, 1873–1881. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]


| Effect of Drought Stress | Effect of Pathogen Stress | |||||||
|---|---|---|---|---|---|---|---|---|
| Xynisteri | Chardonnay | Xynisteri | Chardonnay | |||||
| Response Variables | 9 dot | 16 dot | 9 dot | 16 dot | 9 dot | 16 dot | 9 dot | 16 dot |
| Stomatal conductance | 0.029 * | 0.057 | 0.029 * | 0.050 * | 0.886 | 0.057 | 0.686 | 0.200 |
| Chlorophyll fluorescence | 0.100 | 0.663 | 0.400 | 0.029 * | 0.164 | 0.657 | 0.268 | 1.000 |
| Abscisic acid (ABA) | 0.008 * | 0.008 * | 0.008 * | 0.032 * | 0.310 | 0.690 | 0.841 | 0.690 |
| indole-3-acetic acid (IAA) | 0.310 | 0.151 | 0.032 * | 0.151 | 0.151 | 0.016 * | 0.841 | 0.151 |
| Jasmonic acid (JA) | 0.008 * | 0.008 * | 0.095 | 0.012 * | 0.222 | 0.008 * | 0.095 | 0.056 |
| Salicylic acid (SA) | 1.000 | 0.841 | 0.151 | 0.008 * | 0.032 * | 0.548 | 0.056 | 1.000 |
| Hydrogen peroxide (H2O2) | 1.000 | 0.151 | 0.151 | 1.000 | 0.690 | 0.421 | 0.548 | 0.151 |
| Catalase (CAT) | 0.151 | 0.421 | 0.008 * | 0.095 | 0.008 * | 0.008 * | 0.008 * | 0.008 * |
| Peroxidase (POD) | 0.056 | 0.690 | 0.310 | 0.310 | 0.151 | 0.008 * | 0.151 | 0.008 * |
| Superoxide dismutase (SOD) | 0.421 | 0.222 | 0.310 | 0.032 * | 0.548 | 0.016 * | 0.310 | 1.000 |
| Malondialdehyde (MDA) | 0.032 * | 1.000 | 1.000 | 0.310 | 0.690 | 0.032 * | 0.690 | 0.151 |
| Proline | 0.310 | 0.151 | 0.222 | 0.151 | 0.008 * | 0.008 * | 0.008 * | 0.008 * |
| Chlorophyll a (Chl a) | 0.095 | 0.841 | 1.000 | 0.421 | 1.000 | 0.222 | 0.841 | 0.032 * |
| Chlorophyll b (Chl b) | 0.151 | 0.548 | 1.000 | 0.421 | 0.841 | 0.310 | 1.000 | 0.095 |
| Supplementary Variables | R2 | p-Value | ||||
|---|---|---|---|---|---|---|
| Dim1 | Dim2 | Dim3 | Dim1 | Dim2 | Dim3 | |
| Cultivar | 0.703 | 3.12 × 10−22 | ||||
| Irrigation | 0.355 | 5.40 × 10−9 | ||||
| Duration | 0.188 | 0.063 | 5.74 × 10−5 | 2.51 × 10−2 | ||
| Pathogen | 0.089 | 0.490 | 7.12 × 10−3 | 5.18 × 10−13 | ||
| Active Variables | Correlation | p-Value | ||||
|---|---|---|---|---|---|---|
| Dim1 | Dim2 | Dim3 | Dim1 | Dim2 | Dim3 | |
| ABA | −0.292 | 0.624 | 8.61 × 10−3 | 6.21 × 10−10 | ||
| IAA | 0.361 | 0.602 | 1.01 × 10−3 | 3.51 × 10−9 | ||
| JA | −0.806 | 1.90 × 10−19 | ||||
| SA | −0.523 | −0.283 | 6.50 × 10−7 | 1.11 × 10−2 | ||
| H2O2 | 0.369 | 7.67 × 10−4 | ||||
| CAT | 0.505 | 0.586 | 1.75 × 10−6 | 1.14 × 10−8 | ||
| POD | 0.850 | 0.225 | 1.88 × 10−23 | 4.48 × 10−2 | ||
| SOD | 0.815 | 0.382 | 3.86 × 10−20 | 4.69 × 10−4 | ||
| MDA | 0.481 | 0.467 | 6.24 × 10−6 | 1.25 × 10−5 | ||
| Proline | 0.812 | 5.85 × 10−20 | ||||
| Chl a | −0.759 | 0.428 | 3.32 × 10−16 | 7.37 × 10−5 | ||
| Chl b | −0.793 | 0.413 | 1.84 × 10−18 | 1.41 × 10−4 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heyman, L.; Chrysargyris, A.; Demeestere, K.; Tzortzakis, N.; Höfte, M. Responses to Drought Stress Modulate the Susceptibility to Plasmopara viticola in Vitis vinifera Self-Rooted Cuttings. Plants 2021, 10, 273. https://doi.org/10.3390/plants10020273
Heyman L, Chrysargyris A, Demeestere K, Tzortzakis N, Höfte M. Responses to Drought Stress Modulate the Susceptibility to Plasmopara viticola in Vitis vinifera Self-Rooted Cuttings. Plants. 2021; 10(2):273. https://doi.org/10.3390/plants10020273
Chicago/Turabian StyleHeyman, Lisa, Antonios Chrysargyris, Kristof Demeestere, Nikolaos Tzortzakis, and Monica Höfte. 2021. "Responses to Drought Stress Modulate the Susceptibility to Plasmopara viticola in Vitis vinifera Self-Rooted Cuttings" Plants 10, no. 2: 273. https://doi.org/10.3390/plants10020273
APA StyleHeyman, L., Chrysargyris, A., Demeestere, K., Tzortzakis, N., & Höfte, M. (2021). Responses to Drought Stress Modulate the Susceptibility to Plasmopara viticola in Vitis vinifera Self-Rooted Cuttings. Plants, 10(2), 273. https://doi.org/10.3390/plants10020273








