Effects of Different Planting Densities on Photosynthesis in Maize Determined via Prompt Fluorescence, Delayed Fluorescence and P700 Signals
Abstract
:1. Introduction
2. Results
2.1. Effect of Planting Density on Yield
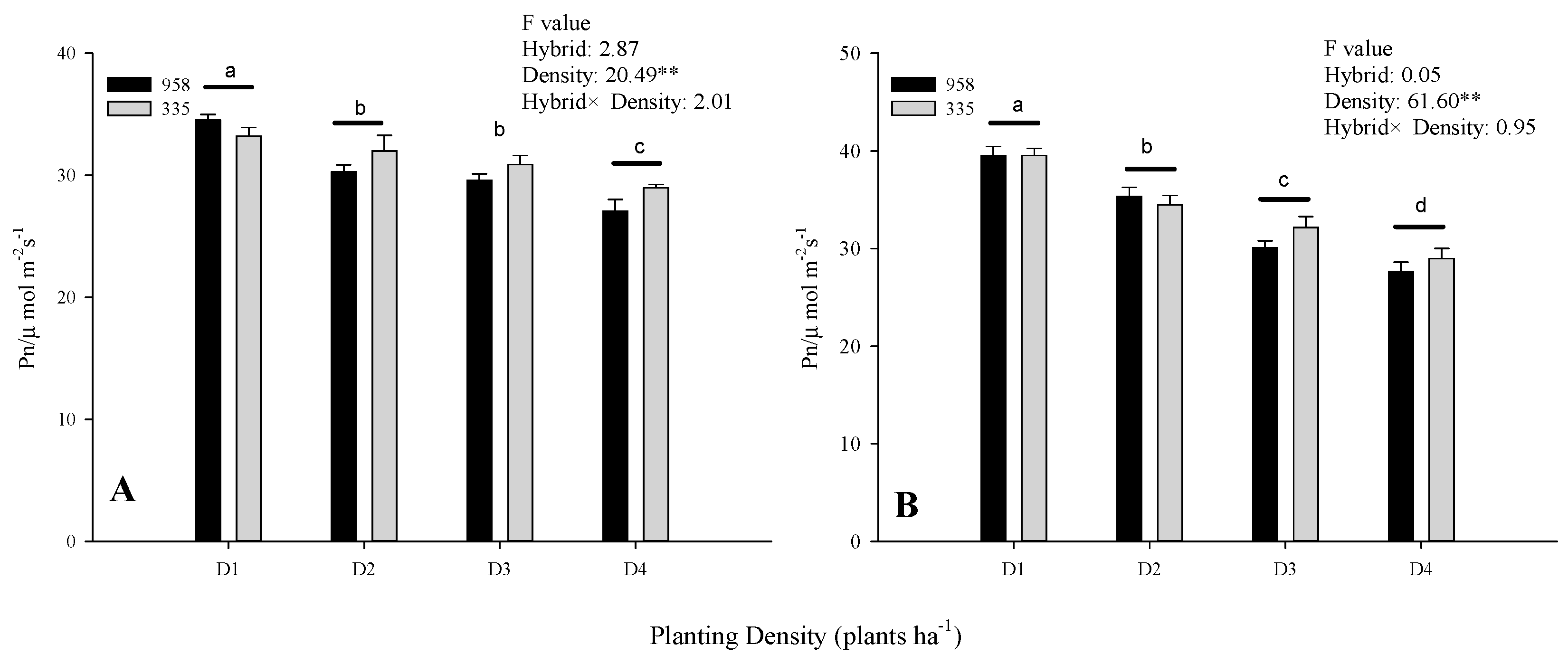
2.2. Effect of Planting Density on the Net Photosynthetic Rate
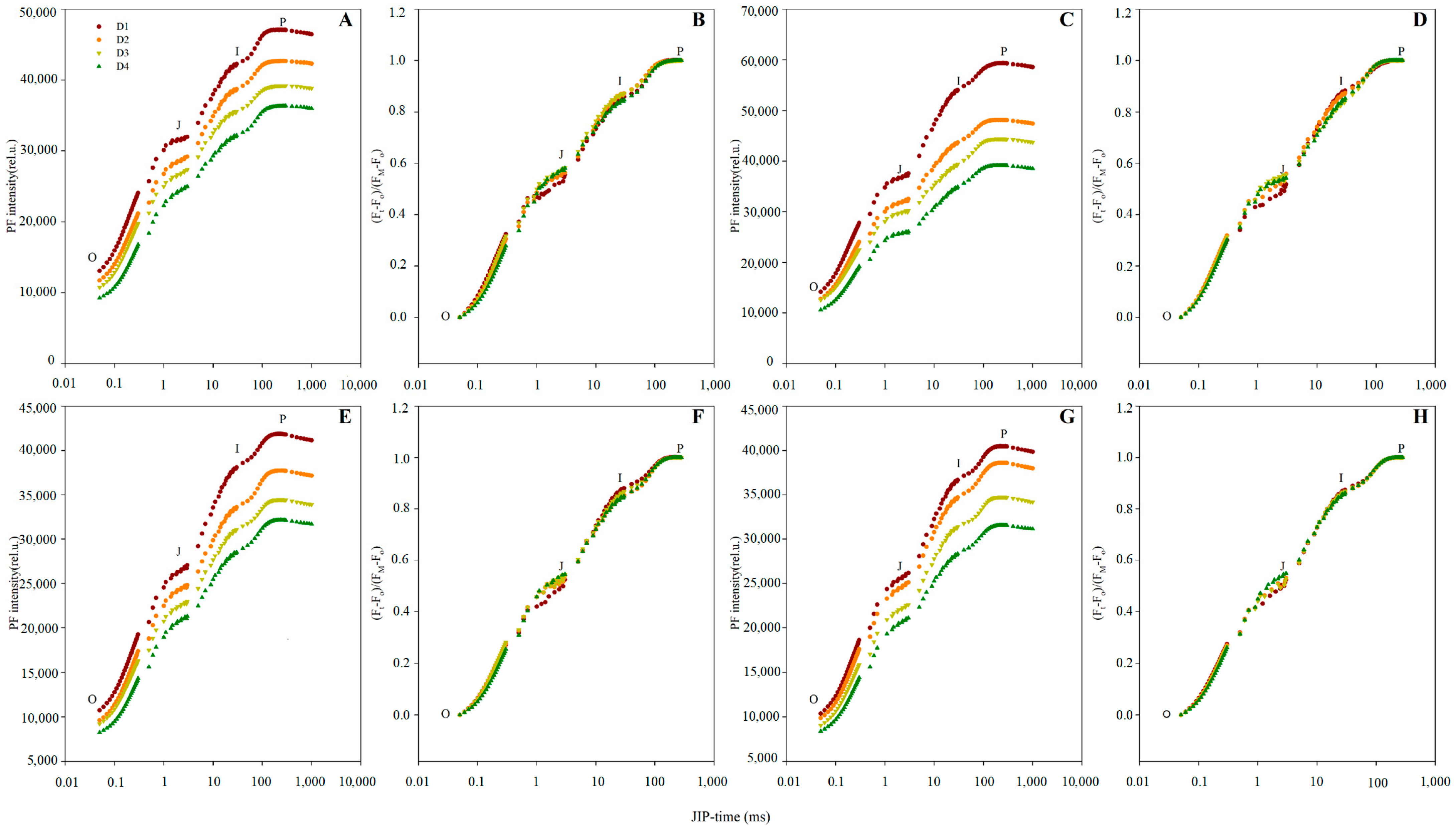
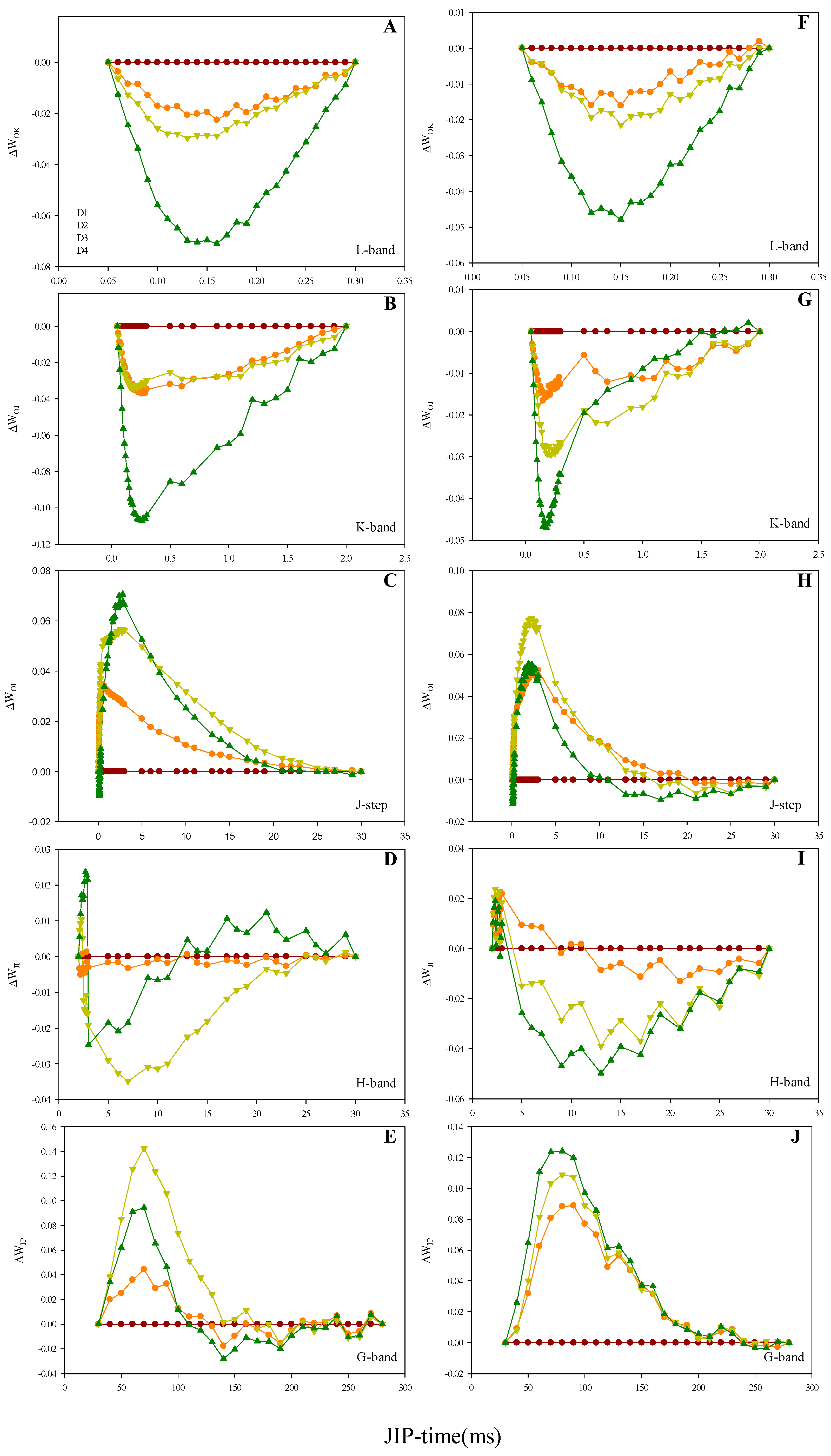
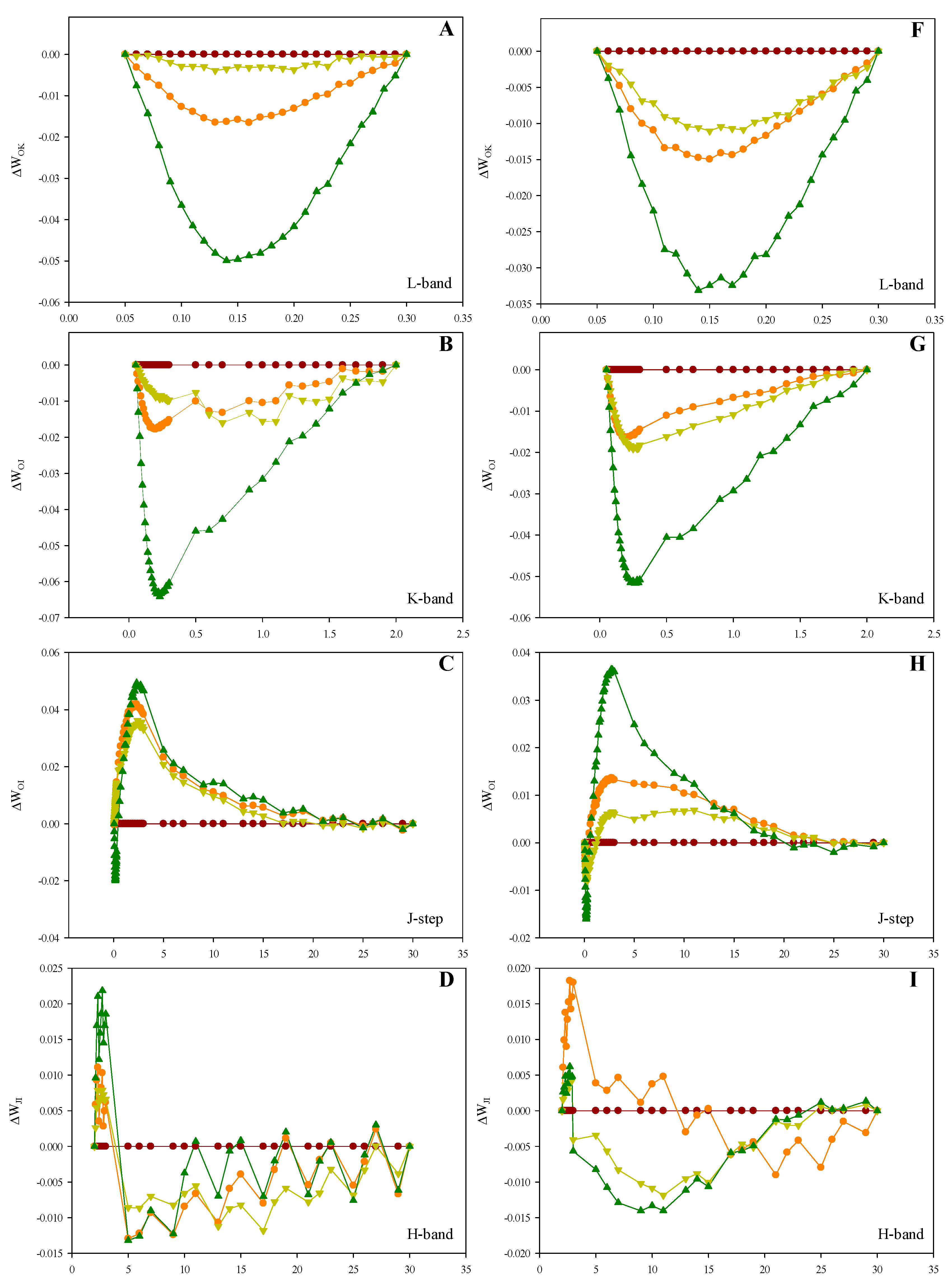
2.3. Prompt Fluorescence OJIP Transient Analysis
2.4. MR/MRO Transient Analysis
2.5. DF Induction and Decay Transient Analysis
2.6. Correlation Analysis between Photosynthetic Parameters
3. Discussion
4. Materials and Methods
4.1. Plant Material Growth and Treatment
4.2. Determination of Yield
4.3. Determination of Net Photosynthetic Rate
4.4. Prompt Chlorophyll a Fluorescence, Delayed Chlorophyll a Fluorescence and Modulated 820 nm Reflection Measurements
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PF | instantaneous chlorophyll fluorescence |
| MR | modulated 820 nm light reflection |
| DF | chlorophyll a delayed fluorescence |
| PSI | photosystem I |
| PSII | photosystem II |
| P680 | PSII reaction center |
| P700 | PSI reaction center |
| N | number of QA reduction events |
| RC/CSO | density of PSII RC per unit area |
| TRO/ABS | ratio of captured light energy to absorbed light energy |
| ETO/TRO | efficiency of electron transport at QA- |
| ABS/RC | light energy absorbed by the unit reaction center |
| PIABS | performance index (potential) for energy conservation from photons absorbed by PSII to the reduction of intersystem electron acceptors |
| REO/ETO | efficiency of an electron beyond QA− reduces PSI acceptors |
| Pn | net photosynthetic rate |
| QA | photosystem II primary quinone acceptor |
| QB | photosystem II secondary quinone acceptor |
| PQ | plastoquinone |
| VPSI | maximum falling slope of the MR/MRO curve |
| VPSII -PSI | maximum rising slope of the MR/MRO curve |
| OEC | Oxygen-evolving complex |
| L1, L2 and L3 | amplitudes of the DF decay kinetic components |
References
- Tollenaar, M.; Lee, E.A. Yield potential, yield stability and stress tolerance in maize. Field Crops Res. 2002, 75, 161–169. [Google Scholar] [CrossRef]
- Tollenaar, M.; Deen, W.; Echarte, L.; Liu, W. Effect of Crowding Stress on Dry Matter Accumulation and Harvest Index in Maize. Agron. J. 2006, 98, 930–937. [Google Scholar] [CrossRef]
- Lu, R.S. Soil Agricultural Chemical Analysis Method; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Hassink, J. Density fractions of soil macro organic matter and microbial biomass as predictors of C and N mineralization. Soilbiol. Biochem. 1995, 27, 1099–1108. [Google Scholar] [CrossRef]
- Logan, B.A. Chlorophyll a Fluorescence: A Signature of Photosynthesis. J. Torrey Bot. Soc. 2005, 132, 650. [Google Scholar] [CrossRef]
- Govindjee, P.G. Chlorophyll a Fluorescence: A Bit of Basics and History, Chlorophyll a Fluorescence; Springer: Dordrecht, The Netherlands, 2004; pp. 1–42. [Google Scholar]
- Gao, J.; Li, P.M.; Ma, F.W.; Goltsev, V. Photosynthetic performance during leaf expansion in Malus micromalus probed by chlorophyll a fluorescence and modulated 820nm reflection. J. Photochem. Photobiol. B 2014, 137, 144–150. [Google Scholar] [CrossRef]
- Strasser, R.J. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll A Fluorescence a Signature of Photosynthesis; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 321–362. [Google Scholar]
- Oukarroum, A.; Madidi, S.E.; Schansker, G.; Strasser, R.J. Probing the responses of barley cultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress and re-watering. Environ. Exp. Bot. 2007, 60, 438–446. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, W.; Wang, X.X.; Yang, L.H.; Han, S.; Chen, S.G.; Strasser, R.J.; Valerde, B.E.; Qiang, S. Comparative phytotoxicity of usnic acid, salicylic acid, cinnamic acid and benzoic acid on photosynthetic apparatus of Chlamydomonas reinhardtii. Plant Physiol. Biochem. 2018, 128, 1–12. [Google Scholar] [CrossRef]
- Tóth, S.Z.; Schansker, G.; Garab, G.; Strasser, R.J. Photosynthetic electron transport activity in heat-treated barley leaves: The role of internal alternative electron donors to photosystem II. Biochim. Biophys. Acta 2007, 1767, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Momchil, P.; Lyubka, K.; Andon, V.; Jaco, V.; Vasilij, G. Effects of Different Metals on Photosynthesis: Cadmium and Zinc Affect Chlorophyll Fluorescence in Durum Wheat. Int. J. Mol. Sci. 2018, 19, 787. [Google Scholar]
- Goltsev, V.; Zaharieva, I.; Lambrev, P.; Yordanov, I.; Strasser, R. Simultaneous 20analysis of prompt and delayed chlorophyll a fluorescence in leaves during the induction period of dark to light adaptation. J. Theor. Biol. 2003, 225, 171–183. [Google Scholar] [CrossRef]
- Goltsev, V.; Zaharieva, I.; Chernev, P.; Strasser, R.J. Delayed fluorescence in photosynthesis. Photosynth. Res. 2009, 101, 217. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.Y.; Li, C.D.; Zheng, J.F.; Bi, C.R.; Tang, G.L. Effects of Planting Density on Physiological Characteristics and Yield of Maize Xianyu335 and Zhengdan958. North China Agric. J. 2010, 25, 166–169. [Google Scholar]
- Dąbrowski, P.; Pawluśkiewicz, B.; Baczewska, A.H.; Oglęcki, P.; Kalaji, H. Chlorophyll a fluorescence of perennial ryegrass (Lolium perenne L.) varieties under long term exposure to shade. Zemdirbyste 2015, 102, 305–312. [Google Scholar]
- Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S. Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol. Biochem. 2010, 48, 16–20. [Google Scholar] [CrossRef]
- Zhang, R.H.; Zhang, X.H.; Camberato, J.J.; Xue, J.Q. Photosynthetic performance of maize hybrids to drought stress. Russ. J. Plant Physiol. 2015, 62, 788–796. [Google Scholar] [CrossRef]
- Kan, X.; Ren, J.J.; Chen, T.T.; Cui, M.; Li, C.L.; Zhou, R.H.; Zhang, Y.; Liu, H.H.; Deng, D.X.; Yin, Z.T. Effects of salinity on photosynthesis in maize probed by prompt fluorescence, delayed fluorescence and P700 signals. Environ. Exp. Bot. 2017, 140, 56–64. [Google Scholar] [CrossRef]
- Pavlovi, I.; Mlinari, S.; Tarkowská, D.; Oklestková, J.; Novák, O. Early Brassica Crops Responses to Salinity Stress: A Comparative Analysis between Chinese Cabbage, White Cabbage, and Kale. Front. Plant Sci. 2019, 10, 450. [Google Scholar] [CrossRef] [Green Version]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta (BBA)-Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef] [Green Version]
- Schansker, G.; Tóth, S.Z.; Strasser, R.J. Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim. Biophys. Acta 2005, 1706, 250–261. [Google Scholar] [CrossRef] [Green Version]
- Goltsev, V.; Zaharieva, I.; Chernev, P. Drought-induced modifications of photosynthetic electron transport in intact leaves: Analysis and use of neural networks as a tool for a rapid non-invasive estimation. Biochim. Biophys. Acta 2012, 1817, 1490–1498. [Google Scholar] [CrossRef] [Green Version]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. Probing Photosynth. Mech. Regul. Adapt. 2000, 25, 445–483. [Google Scholar]
- Begovic, L.; Galic, V.; Abicic, I.; Loncaric, Z.; Mlinaric, S. Implications of intra-seasonal climate variations on chlorophyll a fluorescence and biomass in winter barley breeding program. Photosynthetica 2020, 58, 995–1008. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Baczewska-Dąbrowska, A.H.; Kalaji, H.M.; Goltsev, V.; Paunov, M.; Rapacz, M.; Wójcik-Jagła, M.; Pawluśkiewicz, B.; Bąba, W.; Brestic, M. Exploration of Chlorophyll a Fluorescence and Plant Gas Exchange Parameters as Indicators of Drought Tolerance in Perennial Ryegrass. Sensors 2019, 19, 2736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, A.; Zivcak, M.; Tripathi, D.K.; Yadav, S.; Kalaji, H.M.; Brestic, M. Phytotoxic effect of silver nanoparticles in Triticum aestivum: Improper regulation of photosystem I activity as the reason for oxidative damage in the chloroplast. Photosynthetica 2019, 57, 209–216. [Google Scholar] [CrossRef]
- Guissé, B.; Srivastava, A.; Strasser, R.J. Effects of high temperature and water stress on the polyphasic chlorophyll a fluorescence transient of potato leaves. In Photosynthesis: From Light to Biosphere; Mathis, P., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 913–916. [Google Scholar]
- Dąbrowski, P.; Baczewska, A.H.; Pawluśkiewicz, B. Prompt chlorophyll a fluorescence as a rapid tool for diagnostic changes in PSII structure inhibited by salt stress in Perennial ryegrass. J. Photochem. Photobiol. B Biol. 2016, 157, 22–31. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Račková, L.; Paganová, V.; Swoczyna, T.; Rusinowski, S.; Sitko, K. Can chlorophyll a fluorescence parameters be used as bio-indicators to distinguish between drought and salinity stress in Tilia cordata Mill? Environ. Exp. Bot. 2018, 152, 149–157. [Google Scholar] [CrossRef]
- Zhou, R.H.; Kan, X.; Chen, J.J.; Hua, H.L.; Yin, Z.T. Drought-induced changes in photosynthetic electron transport in maize probed by prompt fluorescence, delayed fluorescence, P700 and cyclic electron flow signals. Environ. Exp. Bot. 2018, 158, 51–62. [Google Scholar] [CrossRef]
- Chen, S.G.; Yang, J.; Zhang, M.S.; Strasser, R.J.; Qiang, S. Classification and characteristics of heat tolerance in Ageratina adenophora populations using fast chlorophyll a fluorescence rise O-J-I-P. Environ. Exp. Bot. 2016, 122, 126–140. [Google Scholar] [CrossRef]
- Zhang, W.T.; Li, P.M. Application of simultaneous measurement technology of instantaneous and delayed chlorophyll fluorescence and 820nm light reflection dynamics in the research of photosynthesis. J. Biophys. 2015, 31, 221–229. [Google Scholar]
- Schansker, G.; Srivastava, A.; Govindjee Strasser, R.J. Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct. Plant Biol. 2003, 30, 785–796. [Google Scholar] [CrossRef] [Green Version]
- Oukarroum, A.; Goltsev, V.; Strasser, R.J. Temperature Effects on Pea Plants Probed by Simultaneous Measurements of the Kinetics of Prompt Fluorescence, Delayed Fluorescence and Modulated 820 nm Reflection. PLoS ONE 2013, 8, e59433. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, E.; Fusaro, L.; Gottardini, E.; Pollastrini, M.; Strasser, R.J.; Goltsev, V.; Bussotti, F. Plant stress analysis: Application of prompt, delayed chlorophyll fluorescence and 820 nm modulated reflectance: Insights from independent experiments. Plant Physiol. Biochem. 2014, 85, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Goltsev, V.; Chernev, P.; Zaharieva, I.; Lambrev, P.; Strasser, R.J. Kinetics of delayed chlorophyll a fluorescence registered in milliseconds time range. Photosynth. Res. 2005, 84, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Oukarroum, A.; El Gharous, M.; Goltsev, V.; Strasser, R.J. Desiccation-induced changes of photosynthetic transport in Parmelina tiliacea (Hoffm.) Ach. analysed by simultaneous measurements of the kinetics of prompt fluorescence, delayed fluorescence and modulated 820 nm reflection. J. Lumin. 2018, 198, 302–308. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.L.; Strasser, R.J.; Goltsev, V. Experimental in vivo measurements of light emission in plants: A perspective dedicated to David Walker. Photosynth. Res. 2012, 114, 69–96. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, P.; Kalaji, M.H.; Baczewska, A.H.; Pawluśkiewicz, B.; Mastalerczuk, G. Delayed chlorophyll a fluorescence, MR 820, and gas exchange changes in perennial ryegrass under salt stress. J. Lumin. 2017, 183, 322–333. [Google Scholar] [CrossRef]
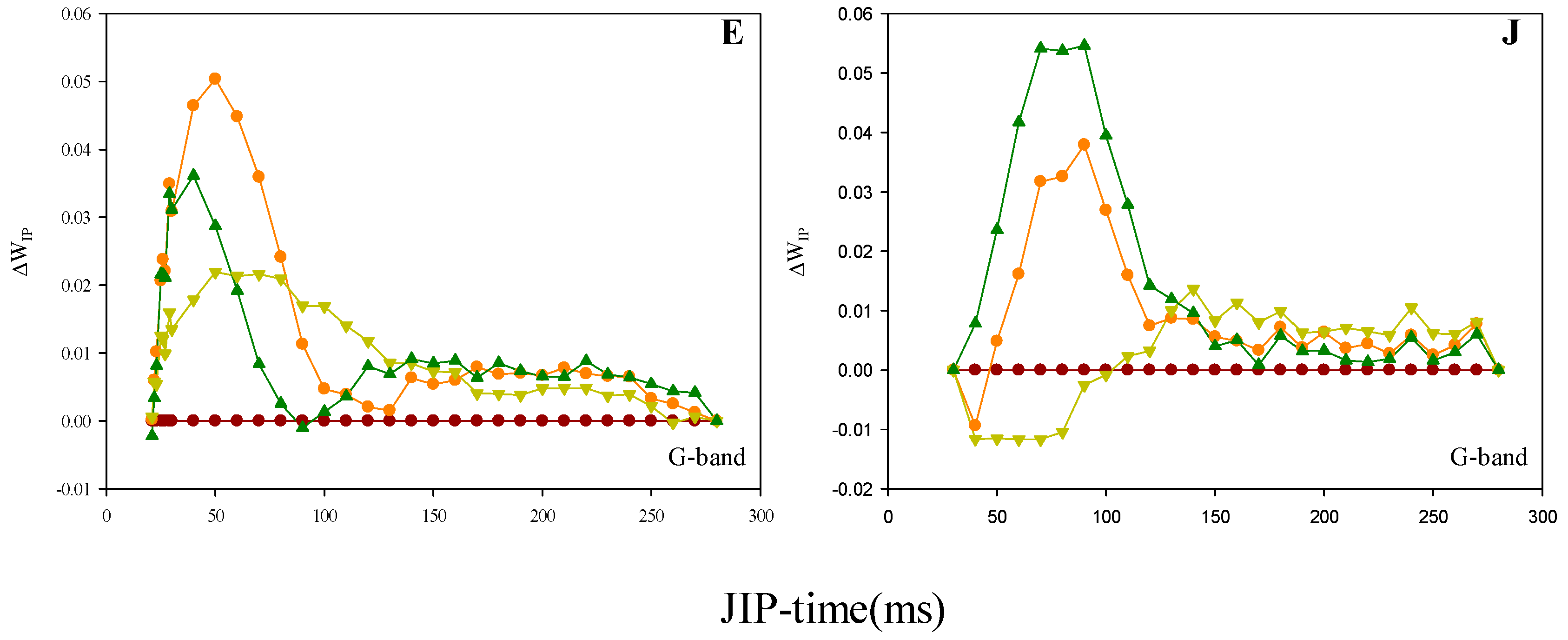
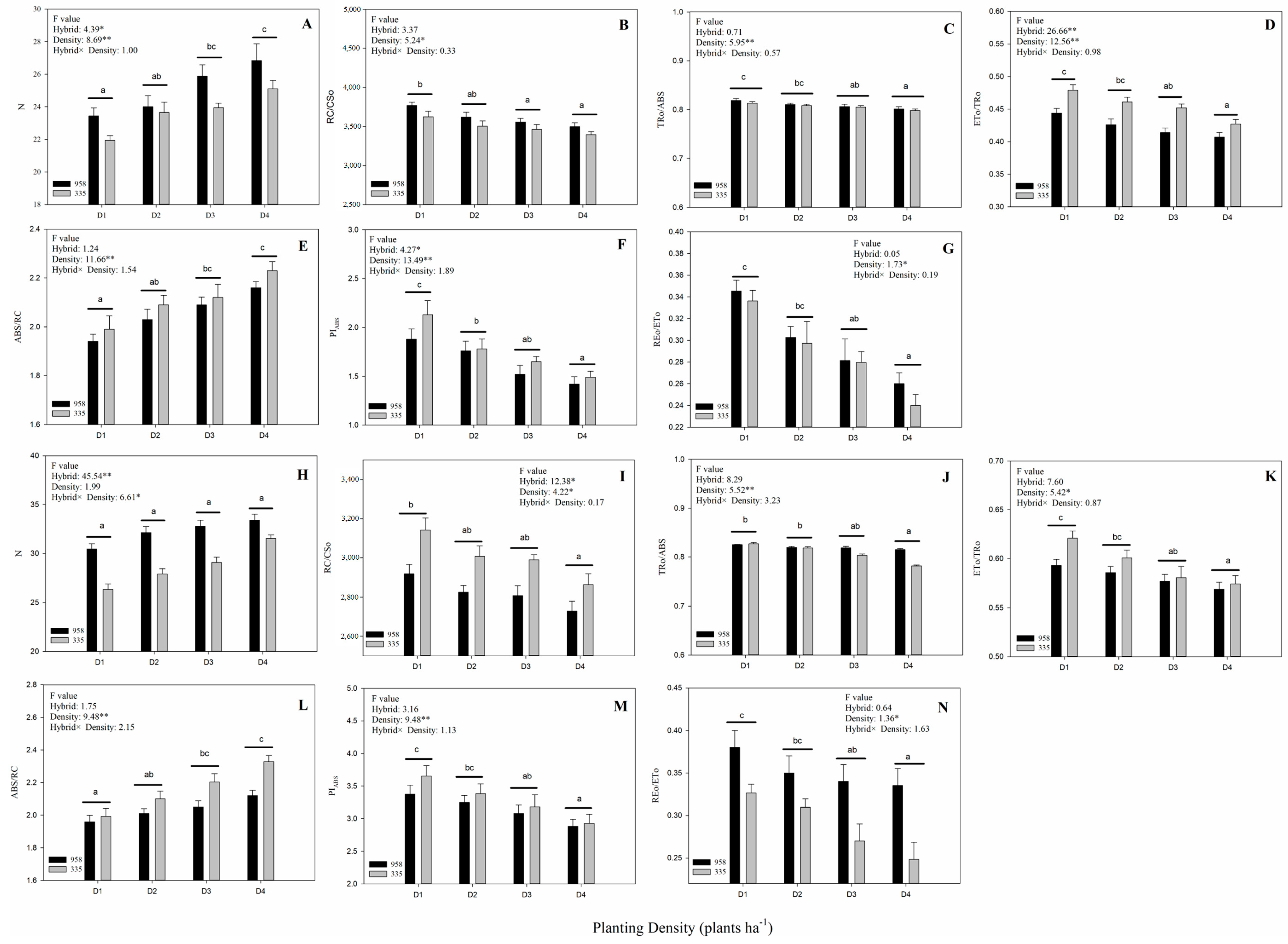


| Hybrid | Planting Density (Plants ha−1) | Yield (kg ha−1) | Hybrid | Planting Density (Plants ha−1) | Yield (kg ha−1) | ||
|---|---|---|---|---|---|---|---|
| 2019 | Zhengdan958 | D1 | 8205a | 2020 | Zhengdan958 | D1 | 8645a |
| D2 | 10,294a | D2 | 12,009b | ||||
| D3 | 13,601b | D3 | 15,348c | ||||
| D4 | 13,147b | D4 | 13,487bc | ||||
| Xianyu335 | D1 | 8456a | Xianyu335 | D1 | 8519a | ||
| D2 | 11,565b | D2 | 11,934b | ||||
| D3 | 12,923b | D3 | 13,477b | ||||
| D4 | 12,300b | D4 | 12,645b | ||||
| F value | Hybrid | 3 × 10−6 | F value | Hybrid | 19.26 ** | ||
| Density | 27.29 ** | Density | 52.21 ** | ||||
| Hybrid × Density | 1.59 | Hybrid × Density | 4.12 ** |
| VPSI | VPSII-PSI | VPSII | ||
|---|---|---|---|---|
| 2019 | Zhengdan958 | |||
| D1 | 1.0045 ± 0.00018a | 1.0055 ± 0.00022a | 2.0100 ± 0.00038a | |
| D2 | 1.0039 ± 0.00018ab | 1.0047 ± 0.00025ab | 2.0086 ± 0.00042ab | |
| D3 | 1.0037 ± 0.00016b | 1.0046 ± 0.00022b | 2.0083 ± 0.00037b | |
| D4 | 1.0033 ± 0.00013b | 1.0042 ± 0.00021b | 2.0076 ± 0.00033b | |
| Xianyu335 | ||||
| D1 | 1.0032 ± 0.00014a | 1.0037 ± 0.00015a | 2.0069 ± 0.00029a | |
| D2 | 1.0029 ± 0.00009ab | 1.0035 ± 0.00021a | 2.0064 ± 0.00041ab | |
| D3 | 1.0028 ± 0.00014ab | 1.0033 ± 0.00014a | 2.0061 ± 0.00027ab | |
| D4 | 1.0023 ± 0.00018b | 1.0026 ± 0.00020b | 2.0049 ± 0.00037b | |
| 2020 | Zhengdan958 | |||
| D1 | 1.0069 ± 0.00022a | 1.0075 ± 0.00025a | 2.0144 ± 0.00047a | |
| D2 | 1.0061 ± 0.00026ab | 1.0065 ± 0.00028ab | 2.0126 ± 0.00053ab | |
| D3 | 1.0058 ± 0.00045ab | 1.0059 ± 0.00041b | 2.0118 ± 0.00086b | |
| D4 | 1.0053 ± 0.00025b | 1.0055 ± 0.00031b | 2.0109 ± 0.00055b | |
| Xianyu335 | ||||
| D1 | 1.0074 ± 0.00042a | 1.0080 ± 0.00049a | 2.0154 ± 0.00091a | |
| D2 | 1.0070 ± 0.00018ab | 1.0078 ± 0.00020a | 2.0148 ± 0.00037ab | |
| D3 | 1.0061 ± 0.00027bc | 1.0069 ± 0.00022a | 2.0130 ± 0.00049bc | |
| D4 | 1.0060 ± 0.00021c | 1.0060 ± 0.00027b | 2.0127 ± 0.00048c |
| L1 | L2 | L3 | τ1 | τ2 | ||
|---|---|---|---|---|---|---|
| 2019 | Zhengdan958 | |||||
| D1 | 331.93 ± 9.88a | 63.67 ± 3.01a | 22.17 ± 2.03a | 0.02 ± 0.00a | 0.28 ± 0.02a | |
| D2 | 308.33 ± 7.99ab | 54.16 ± 3.06ab | 19.00 ± 1.96a | 0.02 ± 0.00a | 0.31 ± 0.03a | |
| D3 | 282.87 ± 9.95bc | 52.06 ± 3.10ab | 18.74 ± 1.90a | 0.02 ± 0.00a | 0.29 ± 0.03a | |
| D4 | 243.60 ± 10.01c | 46.17 ± 2.81b | 17.27 ± 1.88a | 0.02 ± 0.00a | 0.27 ± 0.04a | |
| Xianyu335 | ||||||
| D1 | 272.96 ± 9.83a | 56.25 ± 3.08a | 20.09 ± 2.03a | 0.02 ± 0.00a | 0.30 ± 0.00a | |
| D2 | 263.53 ± 9.90a | 50.58 ± 2.85ab | 18.38 ± 1.97a | 0.02 ± 0.00a | 0.29 ± 0.01a | |
| D3 | 242.83 ± 8.74ab | 49.36 ± 3.01ab | 17.67 ± 1.91a | 0.02 ± 0.00a | 0.30 ± 0.01a | |
| D4 | 222.63 ± 9.90b | 44.36 ± 3.05b | 16.84 ± 2.91a | 0.02 ± 0.00a | 0.29 ± 0.02a | |
| 2020 | Zhengdan958 | |||||
| D1 | 296.34 ± 10.79a | 56.26 ± 2.90a | 23.81 ± 2.81a | 0.02 ± 0.00a | 0.30 ± 0.03a | |
| D2 | 224.09 ± 13.84b | 48.82 ± 2.88ab | 20.12 ± 2.73a | 0.02 ± 0.00a | 0.32 ± 0.01a | |
| D3 | 209.20 ± 9.90bc | 46.35 ± 3.05ab | 19.66 ± 3.71a | 0.02 ± 0.00a | 0.32 ± 0.01a | |
| D4 | 184.86 ± 9.77c | 43.75 ± 2.86b | 18.46 ± 2.89a | 0.02 ± 0.00a | 0.34 ± 0.02a | |
| Xianyu335 | ||||||
| D1 | 247.05 ± 9.66a | 49.93 ± 2.96a | 23.81 ± 1.97a | 0.02 ± 0.00a | 0.28 ± 0.03a | |
| D2 | 224.38 ± 7.95ab | 47.55 ± 2.83a | 20.70 ± 1.88a | 0.02 ± 0.00a | 0.30 ± 0.02a | |
| D3 | 216.77 ± 9.98ab | 43.36 ± 3.97a | 19.60 ± 1.91a | 0.02 ± 0.00a | 0.30 ± 0.02a | |
| D4 | 206.02 ± 9.84b | 41.54 ± 3.02b | 18.46 ± 2.98a | 0.02 ± 0.00a | 0.32 ± 0.03a |
| N | RC/ CSO | TRO/ ABS | ETO/ TRO | ABS/RC | PI ABS | REO/ ETO | VPSI | VPSII- PSI | L1 | L2 | Pn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | −0.46 | −0.70 | −0.92 ** | 0.74* | -0.94 ** | −0.76 * | −0.10 | −0.01 | −0.37 | −0.61 | −0.87 ** | |
| RC/ CSO | −0.99 ** | 0.94 ** | 0.22 | −0.86 ** | 0.65 | 0.90 ** | 0.91 ** | 0.87 ** | 0.94 ** | 0.97 ** | 0.70 | |
| TRO/ ABS | −0.24 | 0.22 | 0.52 | −0.93 ** | 0.82* | 0.99 ** | 0.76* | 0.70 | 0.88 ** | 0.98 ** | 0.88 ** | |
| ETO/ TRO | -0.88 ** | 0.88 ** | 0.55 | -0.58 | 0.84 ** | 0.61 | 0.16 | 0.24 | 0.09 | 0.39 | 0.78* | |
| ABS/ RC | 0.16 | −0.17 | −0.96 ** | −0.53 | −0.90 ** | −0.96** | -0.65 | −0.58 | −0.77 * | −0.88 ** | -0.78* | |
| PIABS | −0.84 ** | 0.85 ** | 0.64 | 0.97 ** | -0.64 | 0.89 ** | 0.31 | 0.22 | 0.52 | 0.74* | 0.86 ** | |
| REO/ ETO | 0.18 | 0.17 | 0.87 ** | 0.22 | −0.92 ** | 0.34 | 0.68 | 0.61 | 0.81* | 0.95 ** | 0.89 ** | |
| VPSI | −0.88 ** | 0.88 ** | 0.43 | 0.95 ** | −0.44 | 0.94 ** | 0.17 | 0.99 ** | 0.94 ** | 0.82* | 0.41 | |
| VPSII- PSI | −0.91 ** | 0.90 ** | 0.47 | 0.91 ** | −0.44 | 0.94 ** | 0.16 | 0.97 ** | 0.90 ** | 0.76* | 0.32 | |
| L1 | −0.44 | 0.48 | 0.49 | 0.59 | −0.61 | 0.70 | 0.52 | 0.74 * | 0.71 * | 0.91 ** | 0.63 | |
| L2 | −0.27 | 0.30 | 0.76* | 0.57 | −0.86 ** | 0.69 | 0.81* | 0.64 | 0.62 | 0.91 ** | 0.84 ** | |
| Pn | −0.66 | 0.69 | 0.64 | 0.85 ** | −0.71* | 0.93 ** | 0.50 | 0.88 ** | 0.87 ** | 0.89 ** | 0.86 ** |
| Parameters of PF | |
| N = (SM/SS) = SMMO(1/VJ) | the number of QA reduction events |
| RC/CSO = φPo (VJ/MO) (ABS/CSO) | the density of PSII RC per unit area |
| φPo = TRO/ABS = [1 − (FO/FM)] | the ratio of captured light energy to absorbed light energy |
| ψEo = ETO/TRO = (1 − VJ) | the efficiency of electron transport at QA- |
| PIABS = (RC/ABS)·[φPo/(1 − φPo)]·[ψo/(1 − ψo)] | performance index (potential) for energy conservation from photons absorbed by PSII to the reduction of intersystem electron acceptors |
| δRo = REO/ETO = (1 − VI)/(1 − VJ) | the efficiency of an electron beyond QA- reduced PSI acceptors |
| Parameters of DF | |
| L1, L2 and L3 | the amplitude of the emission component |
| τ1 and τ2 | the lifetime of the DF component |
| I1 | the first maxima of the DF induction curve |
| I2 | the second maxima of the DF induction curve |
| I2/I1 | the second maxima divided by the first maxima of the DF induction curve |
| Parameters of MR | |
| VPSI | The maximum PSI oxidation rate |
| VPSII-PSI | maximum PSI reduction rate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Jia, B.; Chen, J.; Feng, Y.; Li, Y.; Chen, M.; Liu, H.; Yin, Z. Effects of Different Planting Densities on Photosynthesis in Maize Determined via Prompt Fluorescence, Delayed Fluorescence and P700 Signals. Plants 2021, 10, 276. https://doi.org/10.3390/plants10020276
Chen W, Jia B, Chen J, Feng Y, Li Y, Chen M, Liu H, Yin Z. Effects of Different Planting Densities on Photosynthesis in Maize Determined via Prompt Fluorescence, Delayed Fluorescence and P700 Signals. Plants. 2021; 10(2):276. https://doi.org/10.3390/plants10020276
Chicago/Turabian StyleChen, Wanying, Bo Jia, Junyu Chen, Yujiao Feng, Yue Li, Miantai Chen, Huanhuan Liu, and Zhitong Yin. 2021. "Effects of Different Planting Densities on Photosynthesis in Maize Determined via Prompt Fluorescence, Delayed Fluorescence and P700 Signals" Plants 10, no. 2: 276. https://doi.org/10.3390/plants10020276






