Silicon Cycling in Soils Revisited
Abstract
:1. Introduction
2. Si Extraction Methods
3. Silicic Acid and Soluble Silica Species in Soils and Sediments
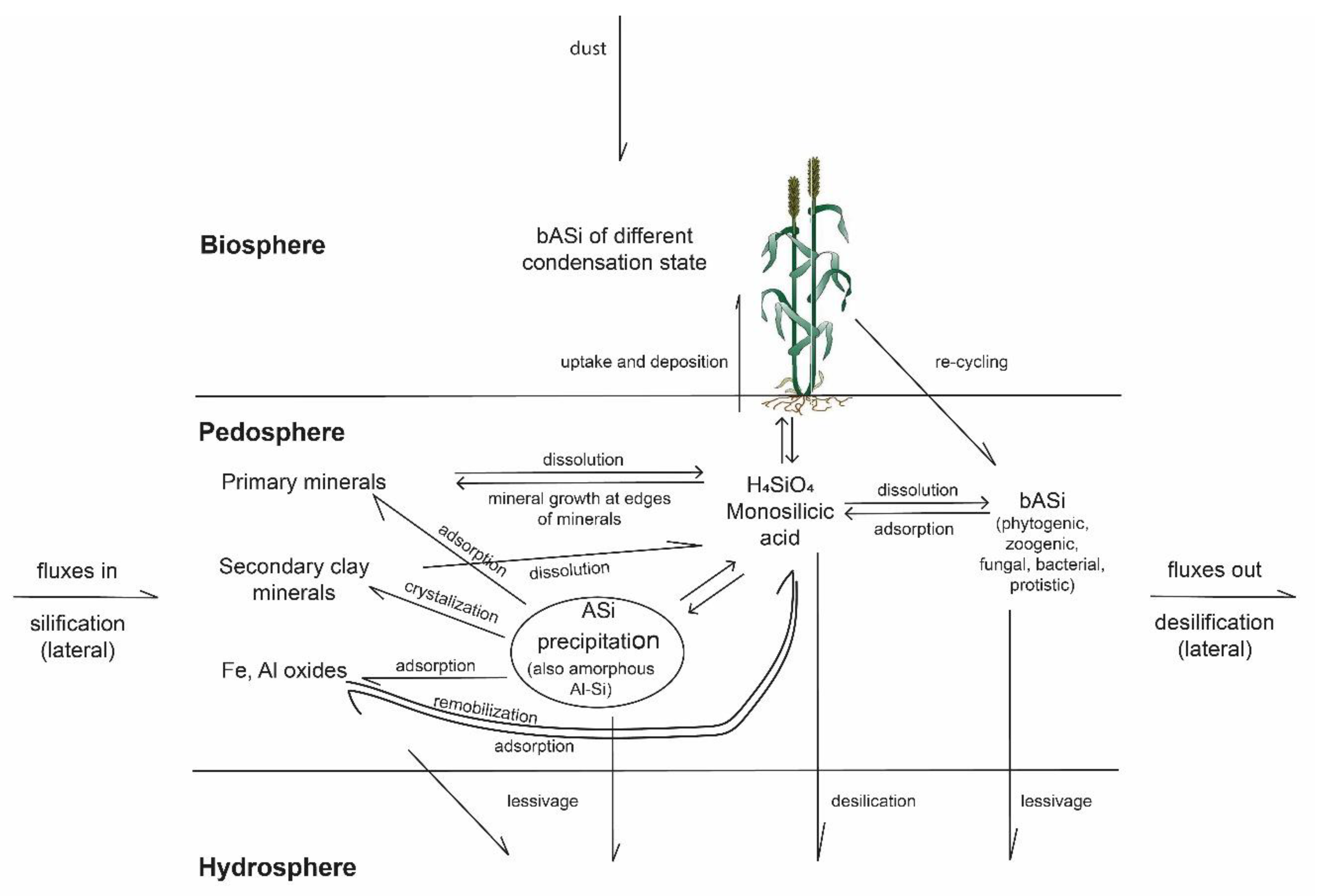
4. Biological Controls on Si Availability and Si Cycling in Terrestrial Ecosystems
4.1. Biogenic Silica Pools in Soils and Their Relevance for Si Cycling
4.2. Plants and Phytogenic Silica
4.3. Further Organisms and Corresponding bASi Pools
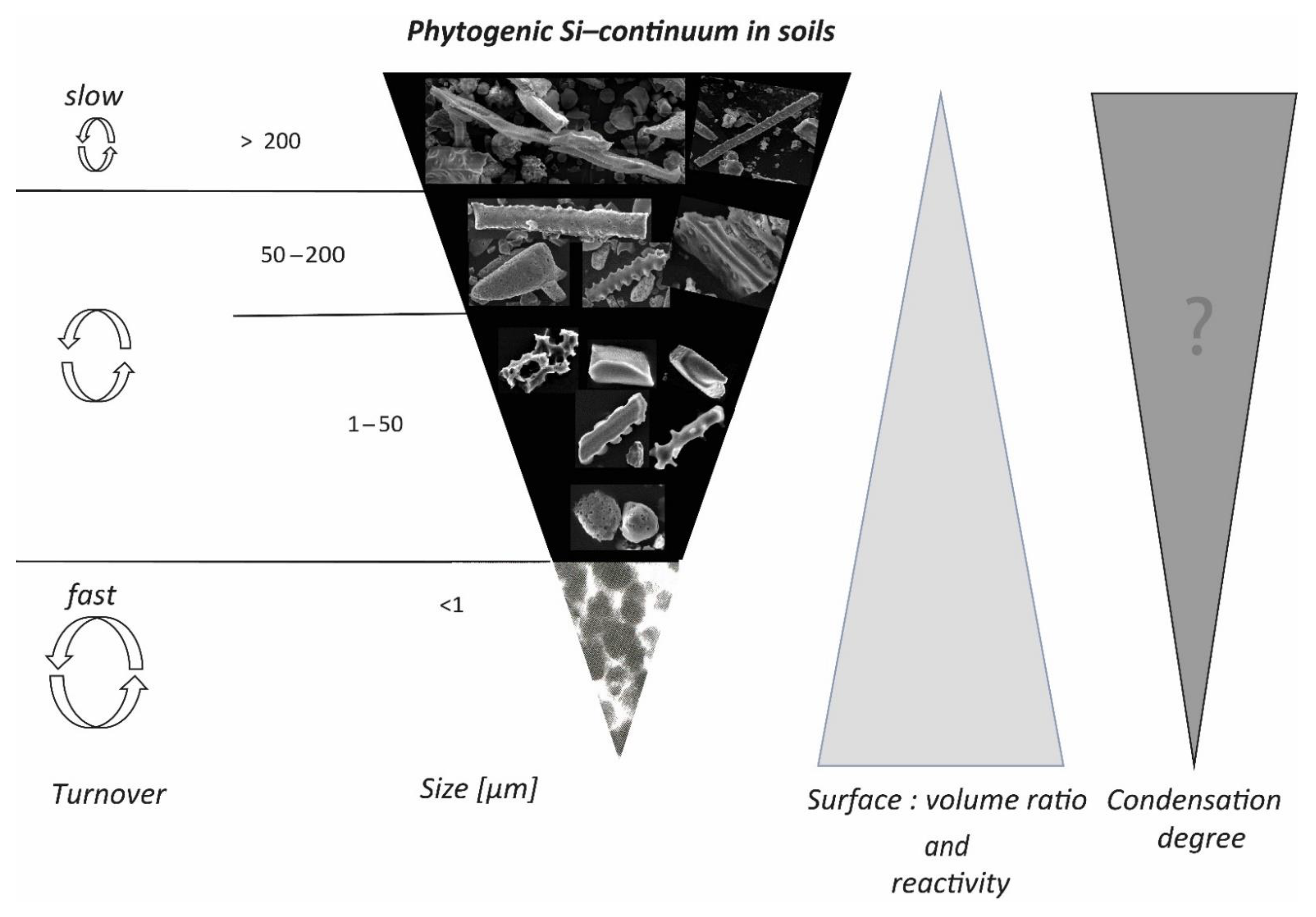
4.4. The Phytogenic Si Continuum in Soils
5. Constraints on Clay Neoformation
6. Human Impacts and Global Change Effects on Soil Si Cycling
6.1. Si Availability Depending on Soil pH in Forests, Pastures, and Arable Crop Fields
6.2. Amorphous Silica Contents in Forests, Pastures, and Arable Crop Fields
6.3. Si Fertilization
7. Importance of Si for Crop Production
7.1. The Need of Crops for Si
7.2. The Importance of Si for Mitigating Abiotic Stress
7.3. The Importance of Si for Mitigating Biotic Stress
8. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Sommer, M.; Jochheim, H.; Hoehn, A.; Breuer, J.; Zagorski, Z.; Busse, J.; Barkusky, D.; Meier, K.; Puppe, D.; Wanner, M.; et al. Si cycling in a forest biogeosystem—The importance of transient state biogenic Si pools. Biogeosciences 2013, 10, 4991–5007. [Google Scholar] [CrossRef] [Green Version]
- Gérard, F.; Mayer, K.; Hodson, M.; Ranger, J. Modelling the biogeochemical cycle of silicon in soils: Application to a temperate forest ecosystem. Geochim. Cosmochim. Acta 2008, 72, 741–758. [Google Scholar] [CrossRef]
- Cornelis, J.T.; Delvaux, B. Soil processes drive the biological silicon feedback loop. Funct. Ecol. 2016, 30, 1298–1310. [Google Scholar] [CrossRef]
- Chadwick, O.A.; Chorover, J. The chemistry of pedogenic thresholds. Geoderma 2001, 100, 321–353. [Google Scholar] [CrossRef]
- Köhler, P.; Hartmann, J.; Wolf-Gladrow, D.A. Geoengineering potential of artificially enhanced silicate weathering of olivine. Proc. Natl. Acad. Sci. USA 2010, 107, 20228–20233. [Google Scholar] [CrossRef] [Green Version]
- Beerling, D.J.; Kantzas, E.P.; Lomas, M.R.; Wade, P.; Eufrasio, R.M.; Renforth, P.; Sarkar, B.; Andrews, M.G.; James, R.H.; Pearce, C.R. Potential for large-scale CO 2 removal via enhanced rock weathering with croplands. Nature 2020, 583, 242–248. [Google Scholar] [CrossRef]
- Parr, J.; Sullivan, L.; Chen, B.; Ye, G.; Zheng, W. Carbon bio-sequestration within the phytoliths of economic bamboo species. Glob. Change Biol. 2010, 16, 2661–2667. [Google Scholar] [CrossRef]
- Song, Z.; Liu, H.; Strömberg, C.A.; Yang, X.; Zhang, X. Phytolith carbon sequestration in global terrestrial biomes. Sci. Total Environ. 2017, 603, 502–509. [Google Scholar] [CrossRef]
- Harrison, C.C. Evidence for intramineral macromolecules containing protein from plant silicas. Phytochemistry 1996, 41, 37–42. [Google Scholar] [CrossRef]
- Sauer, D.; Saccone, L.; Conley, D.J.; Herrmann, L.; Sommer, M. Review of methodologies for extracting plant-available and amorphous Si from soils and aquatic sediments. Biogeochemistry 2006, 80, 89–108. [Google Scholar] [CrossRef]
- Matichenkov, V.; Bocharnikova, E. The relationship between silicon and soil physical and chemical properties. In Studies in Plant Science; Datnoff, L.E., Snyder, G.H., Korndörfer, G.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 8, pp. 209–219. [Google Scholar]
- Cornelis, J.T.; Delvaux, B.; Cardinal, D.; Andre, L.; Ranger, J.; Opfergelt, S. Tracing mechanisms controlling the release of dissolved silicon in forest soil solutions using Si isotopes and Ge/Si ratios. Geochim. Cosmochim. Acta 2010, 74, 3913–3924. [Google Scholar] [CrossRef]
- Opfergelt, S.; de Bournonville, G.; Cardinal, D.; André, L.; Delstanche, S.; Delvaux, B. Impact of soil weathering degree on silicon isotopic fractionation during adsorption onto iron oxides in basaltic ash soils, Cameroon. Geochim. Cosmochim. Acta 2009, 73, 7226–7240. [Google Scholar] [CrossRef]
- Drees, R.L.; Wilding, L.P.; Smeck, N.E.; Senkayi, A.L. Silica in soils: Quartz and disordered silica polymorphs. Miner. Soil Environ. 1989, 1, 913–974. [Google Scholar]
- Monger, H.C.; Kelly, E.F. Silica minerals. Soil Mineral. Environ. Appl. 2002, 7, 611–636. [Google Scholar]
- Lucas, Y.; Chauvel, A. Soil formation in tropically weathered terrains. In Handbook of Exploration Geochemistry; Elsevier: Amsterdam, The Netherlands, 1992; Volume 4, pp. 57–77. [Google Scholar]
- Sommer, M.; Kaczorek, D.; Kuzyakov, Y.; Breuer, J. Silicon pools and fluxes in soils and landscapes—A review. J. Plant Nutr. Soil Sci. Z. Pflanzenernahr. Bodenkd. 2006, 169, 310–329. [Google Scholar] [CrossRef]
- Franzmeier, D.; Norton, L.; Steinhardt, G. Fragipan Formation in Loess of the Midwestern United States1. Fragipans Occurr. Classif. Genes. 1989, 24, 69–97. [Google Scholar]
- Veerhoff, M.; Brummer, G.W. Bildung schlechtkristalliner bis amorpher verwitterungsprodukte in stark bis extrem versauerten waldböden. Z. Pflanzenernähr. Bodenkd. 1993, 156, 11–17. [Google Scholar] [CrossRef]
- Chadwick, O.; Hendricks, D.; Nettleton, W. Silica in duric soils: I. A depositional model. Soil Sci. Soc. Am. J. 1987, 51, 975–982. [Google Scholar] [CrossRef]
- Chadwick, O.; Hendricks, D.; Nettleton, W. Silica in duric soils: II. Mineralogy. Soil Sci. Soc. Am. J. 1987, 51, 982–985. [Google Scholar] [CrossRef]
- Chartres, C.; Kirby, J.; Raupach, M. Poorly ordered silica and aluminosilicates as temporary cementing agents in hard-setting soils. Soil Sci. Soc. Am. J. 1990, 54, 1060–1067. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef]
- Meunier, J.D.; Barboni, D.; Anwar-ul-Haq, M.; Levard, C.; Chaurand, P.; Vidal, V.; Grauby, O.; Huc, R.; Laffont-Schwob, I.; Rabier, J. Effect of phytoliths for mitigating water stress in durum wheat. New Phytol. 2017, 215, 229–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-H.; Khan, A.L.; Kim, D.-H.; Lee, S.-Y.; Kim, K.-M.; Waqas, M.; Jung, H.-Y.; Shin, J.-H.; Kim, J.-G.; Lee, I.-J. Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant Biol. 2014, 14, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaller, J.; Brackhage, C.; Bäucker, E.; Dudel, E. UV-screening of grasses by plant silica layer? J. Biosci. 2013, 38, 413–416. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Muneer, S.; Ko, C.H.; Jeong, B.R. Silicon mitigates salinity stress by regulating the physiology, antioxidant enzyme activities, and protein expression in Capsicum annuum ‘Bugwang’. BioMed Res. Int. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Neu, S.; Schaller, J.; Dudel, E.G. Silicon availability modifies nutrient use efficiency and content, C:N:P stoichiometry, and productivity of winter wheat (Triticum aestivum L.). Sci. Rep. 2017, 7, 40829. [Google Scholar] [CrossRef]
- Massey, F.P.; Hartley, S.E. Physical defences wear you down: Progressive and irreversible impacts of silica on insect herbivores. J. Anim. Ecol. 2009, 78, 281–291. [Google Scholar] [CrossRef]
- Fauteux, F.; Remus-Borel, W.; Menzies, J.G.; Belanger, R.R. Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol. Lett. 2005, 249, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Christl, I.; Brechbühl, Y.; Graf, M.; Kretzschmar, R. Polymerization of silicate on hematite surfaces and its influence on arsenic sorption. Environ. Sci. Technol. 2012, 46, 13235–13243. [Google Scholar] [CrossRef]
- Steefel, C.I.; Van Cappellen, P. A new kinetic approach to modeling water-rock interaction: The role of nucleation, precursors, and Ostwald ripening. Geochim. Cosmochim. Acta 1990, 54, 2657–2677. [Google Scholar] [CrossRef]
- Morse, J.W.; Casey, W.H. Ostwald processes and mineral paragenesis in sediments. Am. J. Sci. 1988, 288, 537–560. [Google Scholar] [CrossRef]
- Fox, R.L.; Silva, J.; Younge, O.; Plucknett, D.; Sherman, G. Soil and plant silicon and silicate response by sugar cane. Soil Sci. Soc. Am. J. 1967, 31, 775–779. [Google Scholar] [CrossRef]
- Khalid, R.; Silva, J. Residual effects of calcium silicate in tropical soils: II. Biological extraction of residual soil silicon. Soil Sci. Soc. Am. J. 1978, 42, 94–97. [Google Scholar] [CrossRef]
- Schachtschabel, P.; Heinemann, C. Wasserlösliche Kieselsäure in Lößböden. Z. Pflanzenernähr. Bodenkd. 1967, 118, 22–35. [Google Scholar] [CrossRef]
- McKeague, J.; Cline, M. Silica in soil solutions: I. The form and concentration of dissolved silica in aqueous extracts of some soils. Can. J. Soil Sci. 1963, 43, 70–82. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Limmer, M.A.; Seyfferth, A.L. Quantitative assessment of plant-available silicon extraction methods in rice paddy soils under different management. Soil Sci. Soc. Am. J. 2020, 84, 618–626. [Google Scholar] [CrossRef]
- Haysom, M.; Chapman, L. Some aspects of the calcium silicate trials at Mackay. Proceedings 1975, 1975, 117–122. [Google Scholar]
- Korndörfer, G.H.; Coelho, N.; Snyder, G.H.; Mizutani, C. Avaliação de métodos de extração de silício em solos cultivados com arroz de sequeiro. Rev. Bras. Ciênc. Solo 1999, 23, 101–106. [Google Scholar] [CrossRef]
- Snyder, G. Development of a silicon soil test for Histosol-grown rice. Belle Glade EREC Res. Rep. EV-Fla. Univ. Agric. Res. Educ. Cent. (USA) 1991, 1, 29–39. [Google Scholar]
- Sauer, D.; Burghardt, W. The occurrence and distribution of various forms of silica and zeolites in soils developed from wastes of iron production. Catena 2006, 65, 247–257. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich-3 soil test extractant—A modification of Mehlich-2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Wang, J.J.; Dodla, S.K.; Henderson, R.E. Soil silicon extractability with seven selected extractants in relation to colorimetric and ICP determination. Soil Sci. 2004, 169, 861–870. [Google Scholar] [CrossRef]
- Ehrlich, H.L. How microbes influence mineral growth and dissolution. Chem. Geol. 1996, 132, 5–9. [Google Scholar] [CrossRef]
- Schwertmann, U. Differenzierung der Eisenoxide des Bodens durch Extraktion mit Ammoniumoxalat-Lösung. Z. Pflanzenernähr. Düng. Bodenkd. 1964, 105, 194–202. [Google Scholar] [CrossRef]
- Hashimoto, I.; Jackson, M. Rapid dissolution of allophane and kaolinite-halloysite after dehydration. In Clay Clay Minerals; Elsevier: Amsterdam, The Netherlands, 1960; pp. 102–113. [Google Scholar]
- Georgiadis, A.; Sauer, D.; Breuer, J.; Herrmann, L.; Rennert, T.; Stahr, K. Optimising the extraction of amorphous silica by NaOH from soils of temperate-humid climate. Soil Res. 2015, 53, 392–400. [Google Scholar] [CrossRef]
- Schaller, J.; Brackhage, C.; Paasch, S.; Brunner, E.; Bäucker, E.; Dudel, E.G. Silica uptake from nanoparticles and silica condensation state in different tissues of Phragmites australis. Sci. Total Environ. 2013, 442, 6–9. [Google Scholar] [CrossRef] [PubMed]
- DeMaster, D.J. The supply and accumulation of silica in the marine environment. Geochim. Cosmochim. Acta 1981, 45, 1715–1732. [Google Scholar] [CrossRef]
- DeMaster, D.J. Measuring biogenic silica in marine sediments and suspended matter. Mar. Part. Anal. Charact. 1991, 63, 363–367. [Google Scholar]
- Saccone, L.; Conley, D.J.; Koning, E.; Sauer, D.; Sommer, M.; Kaczorek, D.; Blecker, S.W.; Kelly, E.F. Assessing the extraction and quantification of amorphous silica in soils of forest and grassland ecosystems. Eur. J. Soil Sci. 2007, 58, 1446–1459. [Google Scholar] [CrossRef]
- Meunier, J.D.; Keller, C.; Guntzer, F.; Riotte, J.; Braun, J.J.; Anupama, K. Assessment of the 1% Na2CO3 technique to quantify the phytolith pool. Geoderma 2014, 216, 30–35. [Google Scholar] [CrossRef]
- Cornelis, J.-T.; Titeux, H.; Ranger, J.; Delvaux, B. Identification and distribution of the readily soluble silicon pool in a temperate forest soil below three distinct tree species. Plant Soil 2011, 342, 369–378. [Google Scholar] [CrossRef]
- Kodama, H.; Ross, G.J. Tiron dissolution method used to remove and characterize inorganic components in soils. Soil Sci. Soc. Am. J. 1991, 55, 1180–1187. [Google Scholar] [CrossRef] [Green Version]
- Georgiadis, A.; Sauer, D.; Herrmann, L.; Breuer, J.; Zarei, M.; Stahr, K. Development of a method for sequential Si extraction from soils. Geoderma 2013, 209, 251–261. [Google Scholar] [CrossRef]
- Madella, M.; Powers-Jones, A.H.; Jones, M.K. A simple method of extraction of opal phytoliths from sediments using a non-toxic heavy liquid. J. Archaeol. Sci. 1998, 25, 801–803. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Iler, R.K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry; Wiley: New York, NY, USA, 1979. [Google Scholar]
- Dietzel, M. Interaction of polysilicic and monosilicic acid with mineral surfaces. In Water-Rock Interaction; Stober, I., Bucher, K., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 207–235. [Google Scholar] [CrossRef]
- Alexander, G. The reaction of low molecular weight silicic acids with molybdic acid. J. Am. Chem. Soc. 1953, 75, 5655–5657. [Google Scholar] [CrossRef]
- Belton, D.J.; Deschaume, O.; Perry, C.C. An overview of the fundamentals of the chemistry of silica with relevance to biosilicification and technological advances. FEBS J. 2012, 279, 1710–1720. [Google Scholar] [CrossRef]
- Perry, C.C.; Keeling-Tucker, T. Aspects of the bioinorganic chemistry of silicon in conjunction with the biometals calcium, iron and aluminium. J. Inorg. Biochem. 1998, 69, 181–191. [Google Scholar] [CrossRef]
- Wonisch, H.; Gérard, F.; Dietzel, M.; Jaffrain, J.; Nestroy, O.; Boudot, J.-P. Occurrence of polymerized silicic acid and aluminum species in two forest soil solutions with different acidity. Geoderma 2008, 144, 435–445. [Google Scholar] [CrossRef]
- Audsley, A.; Aveston, J. The polymerization of silicic acid. J. Chem. Soc. 1962, 15, 2320–2329. [Google Scholar] [CrossRef]
- Dietzel, M. Dissolution of silicates and the stability of polysilicic acid. Geochim. Cosmochim. Acta 2000, 64, 3275–3281. [Google Scholar] [CrossRef]
- Dietzel, M.; Usdowski, E. Depolymerization of soluble silicate in dilute aqueous solutions. Colloid Polymer Sci. 1995, 273, 590–597. [Google Scholar] [CrossRef]
- Icopini, G.A.; Brantley, S.L.; Heaney, P.J. Kinetics of silica oligomerization and nanocolloid formation as a function of pH and ionic strength at 25 °C. Geochim. Cosmochim. Acta 2005, 69, 293–303. [Google Scholar] [CrossRef]
- Conrad, C.F.; Icopini, G.A.; Yasuhara, H.; Bandstra, J.Z.; Brantley, S.L.; Heaney, P.J. Modeling the kinetics of silica nanocolloid formation and precipitation in geologically relevant aqueous solutions. Geochim. Cosmochim. Acta 2007, 71, 531–542. [Google Scholar] [CrossRef]
- Stein, M.; Georgiadis, A.; Gudat, D.; Rennert, T. Formation and properties of inorganic Si-contaminant compounds. Environ. Pollut. 2020, 265, 115032. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, S. Silica in aqueous environments. J. Non-Cryst. Solids 1996, 196, 51–57. [Google Scholar] [CrossRef]
- Klotzbücher, T.; Treptow, C.; Kaiser, K.; Klotzbücher, A.; Mikutta, R. Sorption competition with natural organic matter as mechanism controlling silicon mobility in soil. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Zhang, C.; Li, L.; Lockington, D. Numerical study of evaporation-induced salt accumulation and precipitation in bare saline soils: Mechanism and feedback. Water Resour. Res. 2014, 50, 8084–8106. [Google Scholar] [CrossRef] [Green Version]
- Olson, L.L.; O’Melia, C.R. The interactions of Fe(III) with Si(OH)4. J. Inorg. Nucl. Chem. 1973, 35, 1977–1985. [Google Scholar] [CrossRef]
- Weber, W.J.; Stumm, W. Formation of a silicato-iron(III) complex in dilute aqueous solution. J. Inorg. Nucl. Chem. 1965, 27, 237–239. [Google Scholar] [CrossRef]
- Santschi, P.H.; Schindler, P.W. Complex formation in the ternary systems CaII–H4SiO4–H2O and MgII–H4SiO4–H2O. J. Chem. Soc. Dalton Trans. 1974, 2, 181–184. [Google Scholar] [CrossRef]
- Schindler, P.; Fürst, B.; Dick, R.; Wolf, P. Ligand properties of surface silanol groups. I. Surface complex formation with Fe3+, Cu2+, Cd2+, and Pb2+. J. Colloid Interface Sci. 1976, 55, 469–475. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Schecher, W.D. The chemistry of aluminum in the environment. Environ. Geochem. Health 1990, 12, 28–49. [Google Scholar] [CrossRef]
- Ehrlich, H.; Demadis, K.D.; Pokrovsky, O.S.; Koutsoukos, P.G. Modern Views on Desilicification: Biosilica and Abiotic Silica Dissolution in Natural and Artificial Environments. Chem. Rev. 2010, 110, 4656–4689. [Google Scholar] [CrossRef] [PubMed]
- Puppe, D. Review on protozoic silica and its role in silicon cycling. Geoderma 2020, 365, 114224. [Google Scholar] [CrossRef]
- Dürr, H.; Meybeck, M.; Hartmann, J.; Laruelle, G.G.; Roubeix, V. Global spatial distribution of natural riverine silica inputs to the coastal zone. Biogeosciences 2011, 8, 597–620. [Google Scholar] [CrossRef] [Green Version]
- Street-Perrott, F.A.; Barker, P.A. Biogenic silica: A neglected component of the coupled global continental biogeochemical cycles of carbon and silicon. Earth Surf. Process. Landf. 2008, 33, 1436–1457. [Google Scholar] [CrossRef]
- Struyf, E.; Conley, D.J. Silica: An essential nutrient in wetland biogeochemistry. Front. Ecol. Environ. 2009, 7, 88–94. [Google Scholar] [CrossRef]
- Struyf, E.; Conley, D.J. Emerging understanding of the ecosystem silica filter. Biogeochemistry 2012, 107, 9–18. [Google Scholar] [CrossRef]
- Tréguer, P.J.; De La Rocha, C.L. The World Ocean Silica Cycle. In Annual Review of Marine Science; Carlson, C.A., Giovannoni, S.J., Eds.; Annual Reviews: Palo Alto, CA, USA, 2013; Volume 5, pp. 477–501. [Google Scholar]
- Tréguer, P.; Pondaven, P. Global change—Silica control of carbon dioxide. Nature 2000, 406, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E. Silicon. Annu. Rev. Plant Physiol. Plant Molec. Biol. 1999, 50, 641–664. [Google Scholar] [CrossRef]
- Puppe, D.; Sommer, M. Experiments, uptake mechanisms, and functioning of silicon foliar fertilization—A review focusing on maize, rice, and wheat. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2018; Volume 152, pp. 1–49. [Google Scholar]
- Haynes, R.J. A contemporary overview of silicon availability in agricultural soils. J. Plant Nutr. Soil Sci. 2014, 177, 831–844. [Google Scholar] [CrossRef]
- Haynes, R.J. The nature of biogenic Si and its potential role in Si supply in agricultural soils. Agric. Ecosyst. Environ. 2017, 245, 100–111. [Google Scholar] [CrossRef]
- Haynes, R.J. Significance and role of Si in crop production. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2017; Volume 146, pp. 83–166. [Google Scholar]
- Struyf, E.; Smis, A.; Van Damme, S.; Garnier, J.; Govers, G.; Van Wesemael, B.; Conley, D.J.; Batelaan, O.; Frot, E.; Clymans, W.; et al. Historical land use change has lowered terrestrial silica mobilization. Nat. Commun. 2010, 1, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandevenne, F.; Barão, L.; Ronchi, B.; Govers, G.; Meire, P.; Kelly, E.; Struyf, E. Silicon pools in human impacted soils of temperate zones. Glob. Biogeochem. Cycle 2015, 29, 1439–1450. [Google Scholar] [CrossRef]
- Vandevenne, F.I.; Delvaux, C.; Hughes, H.J.; André, L.; Ronchi, B.; Clymans, W.; Barao, L.; Cornelis, J.-T.; Govers, G.; Meire, P. Landscape cultivation alters δ 30 Si signature in terrestrial ecosystems. Sci. Rep. 2015, 5, 7732. [Google Scholar] [CrossRef] [Green Version]
- Amundson, R.; Berhe, A.A.; Hopmans, J.W.; Olson, C.; Sztein, A.E.; Sparks, D.L. Soil and human security in the 21st century. Science 2015, 348, 1261071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitousek, P.M.; Naylor, R.; Crews, T.; David, M.; Drinkwater, L.; Holland, E.; Johnes, P.; Katzenberger, J.; Martinelli, L.; Matson, P. Nutrient imbalances in agricultural development. Science 2009, 324, 1519–1520. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.; Von Braun, J. Climate change impacts on global food security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Meunier, J.; Guntzer, F.; Kirman, S.; Keller, C. Terrestrial plant-Si and environmental changes. Mineral. Mag. 2008, 72, 263–267. [Google Scholar] [CrossRef]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A review of silicon in soils and plants and its role in US agriculture: History and future perspectives. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef] [Green Version]
- Carey, J.C.; Fulweiler, R.W. Human appropriation of biogenic silicon–the increasing role of agriculture. Funct. Ecol. 2016, 30, 1331–1339. [Google Scholar] [CrossRef] [Green Version]
- Fraysse, F.; Pokrovsky, O.S.; Schott, J.; Meunier, J.-D. Surface properties, solubility and dissolution kinetics of bamboo phytoliths. Geochim. Cosmochim. Acta 2006, 70, 1939–1951. [Google Scholar] [CrossRef]
- Fraysse, F.; Pokrovsky, O.S.; Schott, J.; Meunier, J.D. Surface chemistry and reactivity of plant phytoliths in aqueous solutions. Chem. Geol. 2009, 258, 197–206. [Google Scholar] [CrossRef]
- Puppe, D.; Leue, M. Physicochemical surface properties of different biogenic silicon structures: Results from spectroscopic and microscopic analyses of protistic and phytogenic silica. Geoderma 2018, 330, 212–220. [Google Scholar] [CrossRef]
- Buján, E. Elemental composition of phytoliths in modern plants (Ericaceae). Quat. Int. 2013, 287, 114–120. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Wang, H.; Wang, C. The effects of chemical composition and distribution on the preservation of phytolith morphology. Appl. Phys. A 2014, 114, 503–507. [Google Scholar] [CrossRef]
- Kameník, J.; Mizera, J.; Řanda, Z. Chemical composition of plant silica phytoliths. Environ. Chem. Lett. 2013, 11, 189–195. [Google Scholar] [CrossRef]
- Hodson, M.J. The development of phytoliths in plants and its influence on their chemistry and isotopic composition. Implications for palaeoecology and archaeology. J. Archaeol. Sci. 2016, 68, 62–69. [Google Scholar] [CrossRef]
- Sangster, A.; Hodson, M.; Tubb, H. Silicon deposition in higher plants. In Studies in Plant Science; Elsevier: Amsterdam, The Netherlands, 2001; Volume 8, pp. 85–113. [Google Scholar]
- Frick, D.A.; Remus, R.; Sommer, M.; Augustin, J.; Kaczorek, D.; von Blanckenburg, F. Silicon uptake and isotope fractionation dynamics by crop species. Biogeosciences 2020, 17, 6475–6490. [Google Scholar] [CrossRef]
- Hodson, M.J.; Sangster, A.G. X-ray microanalysis of the seminal root of Sorghum bicolor (L.) Moench. with particular reference to silicon. Ann. Bot. 1989, 64, 659–667. [Google Scholar] [CrossRef]
- Piperno, D.R. Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists; Altamira Press: Lanham, MD, USA, 2006. [Google Scholar]
- Kaczorek, D.; Puppe, D.; Busse, J.; Sommer, M. Effects of phytolith distribution and characteristics on extractable silicon fractions in soils under different vegetation—An exploratory study on loess. Geoderma 2019, 356, 113917. [Google Scholar] [CrossRef]
- Puppe, D.; Höhn, A.; Kaczorek, D.; Wanner, M.; Wehrhan, M.; Sommer, M. How big is the influence of biogenic silicon pools on short-term changes in water-soluble silicon in soils? Implications from a study of a 10-year-old soil-plant system. Biogeosciences 2017, 14, 5239–5252. [Google Scholar] [CrossRef] [Green Version]
- Schaller, J.; Hines, J.; Brackhage, C.; Bäucker, E.; Gessner, M.O. Silica decouples fungal growth and litter decomposition without changing responses to climate warming and N enrichment. Ecology 2014, 95, 3181–3189. [Google Scholar] [CrossRef]
- Hodson, M.J. The relative importance of cell wall and lumen phytoliths in carbon sequestration in soil: A hypothesis. Front. Earth Sci. 2019, 7, 167. [Google Scholar] [CrossRef]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic variation in the silicon composition of plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.F.; Tamai, K.; Ichii, M.; Wu, G.F. A rice mutant defective in Si uptake. Plant Physiol. 2002, 130, 2111–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.F.; Yamaji, N. A cooperated system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Exley, C.; Guerriero, G.; Lopez, X. How is silicic acid transported in plants? Silicon 2020, 12, 2641–2645. [Google Scholar] [CrossRef] [Green Version]
- Exley, C. A possible mechanism of biological silicification in plants. Front. Plant Sci. 2015, 6, 853. [Google Scholar] [CrossRef] [Green Version]
- Schaller, J.; Roscher, C.; Hillebrand, H.; Weigelt, A.; Oelmann, Y.; Wilcke, W.; Ebeling, A.; Weisser, W.W. Plant diversity and functional groups affect Si and Ca pools in aboveground biomass of grassland systems. Oecologia 2016, 182, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Schaller, J.; Hodson, M.J.; Struyf, E. Is relative Si/Ca availability crucial to the performance of grassland ecosystems? Ecosphere 2017, 8, e01726. [Google Scholar] [CrossRef] [Green Version]
- Haynes, R.J. What effect does liming have on silicon availability in agricultural soils? Geoderma 2019, 337, 375–383. [Google Scholar] [CrossRef]
- Reithmaier, G.M.S.; Knorr, K.H.; Arnhold, S.; Planer-Friedrich, B.; Schaller, J. Enhanced silicon availability leads to increased methane production, nutrient and toxicant mobility in peatlands. Sci. Rep. 2017, 7, 8728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaller, J.; Fauchere, S.; Joss, H.; Obst, M.; Goeckede, M.; Planer-Friedrich, B.; Peiffer, S.; Gilfedder, B.; Elberling, B. Silicon increases the phosphorus availability of Arctic soils. Sci. Rep. 2019, 9, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datnoff, L.E.; Snyder, G.H.; Korndörfer, G.H. Silicon in Agriculture; Elsevier: Amsterdam, The Netherlands, 2001; Volume 8. [Google Scholar]
- Hinsinger, P.; Barros, O.N.F.; Benedetti, M.F.; Noack, Y.; Callot, G. Plant-induced weathering of a basaltic rock: Experimental evidence. Geochim. Cosmochim. Acta 2001, 65, 137–152. [Google Scholar] [CrossRef]
- Gattullo, C.E.; Allegretta, I.; Medici, L.; Fijan, R.; Pii, Y.; Cesco, S.; Mimmo, T.; Terzano, R. Silicon dynamics in the rhizosphere: Connections with iron mobilization. J. Plant Nutr. Soil Sci. 2016, 179, 409–417. [Google Scholar] [CrossRef]
- Blecker, S.W.; McCulley, R.L.; Chadwick, O.A.; Kelly, E.F. Biologic cycling of silica across a grassland bioclimosequence. Glob. Biogeochem. Cycle 2006, 20. [Google Scholar] [CrossRef]
- Alexandre, A.; Bouvet, M.; Abbadie, L. The role of savannas in the terrestrial Si cycle: A case-study from Lamto, Ivory Coast. Global Planet. Change 2011, 78, 162–169. [Google Scholar] [CrossRef]
- Bartoli, F. The biogeochemical cycle of silicon in two temperate forest ecosystems. Ecol. Bull. 1983, 469–476. [Google Scholar]
- Cornelis, J.T.; Ranger, J.; Iserentant, A.; Delvaux, B. Tree species impact the terrestrial cycle of silicon through various uptakes. Biogeochemistry 2010, 97, 231–245. [Google Scholar] [CrossRef]
- Keller, C.; Guntzer, F.; Barboni, D.; Labreuche, J.; Meunier, J.-D. Impact of agriculture on the Si biogeochemical cycle: Input from phytolith studies. C. R. Geosci. 2012, 344, 739–746. [Google Scholar] [CrossRef]
- Savant, N.; Snyder, G.; Datnoff, L. Silicon management and sustainable rice production. Adv. Agron. 1996, 58, 151–199. [Google Scholar]
- Savant, N.K.; Korndörfer, G.H.; Datnoff, L.E.; Snyder, G.H. Silicon nutrition and sugarcane production: A review. J. Plant Nutr. 1999, 22, 1853–1903. [Google Scholar] [CrossRef]
- Burki, F.; Roger, A.J.; Brown, M.W.; Simpson, A.G. The new tree of eukaryotes. Trends Ecol. Evol. 2020, 35, 43–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adl, S.M.; Simpson, A.G.; Lane, C.E.; Lukeš, J.; Bass, D.; Bowser, S.S.; Brown, M.W.; Burki, F.; Dunthorn, M.; Hampl, V. The revised classification of eukaryotes. J. Eukaryot. Microbiol. 2012, 59, 429–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meisterfeld, R. Testate amoebae with filopodia. Illus. Guide Protozoa 2002, 2, 1054–1084. [Google Scholar]
- Rauenbusch, V.K. Biologie und feinstruktur (REM-Untersuchungen) terrestrischer testaceen in Waldböden (Rhizopoda, Protozoa). Arch. Protistenkd. 1987, 134, 191–294. [Google Scholar] [CrossRef]
- Ogden, C.G. The biology and ultrastructure of an agglutinate testate amoeba Difflugia geosphaira sp. nov.(Protozoa, Rhizopoda). Arch. Protistenkd. 1991, 140, 141–150. [Google Scholar] [CrossRef]
- Anderson, O.R. Fine structure of the marine amoeba Vexillifera telmathalassa collected from a coastal site near Barbados with a description of salinity tolerance, feeding behavior and prey. J. Eukaryot. Microbiol. 1994, 41, 124–128. [Google Scholar] [CrossRef]
- Anderson, O.R.; Rogerson, A. Annual abundances and growth potential of gymnamoebae in the Hudson Estuary with comparative data from the Firth of Clyde. Eur. J. Protistol. 1995, 31, 223–233. [Google Scholar] [CrossRef]
- Clarke, J. The occurrence and significance of biogenic opal in the regolith. Earth Sci. Rev. 2003, 60, 175–194. [Google Scholar] [CrossRef]
- Cary, L.; Alexandre, A.; Meunier, J.-D.; Boeglin, J.-L.; Braun, J.-J. Contribution of phytoliths to the suspended load of biogenic silica in the Nyong basin rivers (Cameroon). Biogeochemistry 2005, 74, 101–114. [Google Scholar] [CrossRef]
- Conley, D.J.; Sommer, M.; Meunier, J.; Kaczorek, D.; Saccone, L. Silicon in the terrestrial biogeosphere. In The Silicon Cycle: Human Perturbations and Impacts on Aquatic Systems; Island Press: Washington, DC, USA, 2006; Volume 66, pp. 13–28. [Google Scholar]
- Aoki, Y.; Hoshino, M.; Matsubara, T. Silica and testate amoebae in a soil under pine–oak forest. Geoderma 2007, 142, 29–35. [Google Scholar] [CrossRef]
- Wilkinson, D.M. Testate amoebae and nutrient cycling: Peering into the black box of soil ecology. Trends Ecol. Evol. 2008, 23, 596–599. [Google Scholar] [CrossRef]
- Wilkinson, D.M.; Mitchell, E.A. Testate amoebae and nutrient cycling with particular reference to soils. Geomicrobiol. J. 2010, 27, 520–533. [Google Scholar] [CrossRef]
- Puppe, D.; Kaczorek, D.; Wanner, M.; Sommer, M. Dynamics and drivers of the protozoic Si pool along a 10-year chronosequence of initial ecosystem states. Ecol. Eng. 2014, 70, 477–482. [Google Scholar] [CrossRef]
- Creevy, A.L.; Fisher, J.; Puppe, D.; Wilkinson, D.M. Protist diversity on a nature reserve in NW England—With particular reference to their role in soil biogenic silicon pools. Pedobiologia 2016, 59, 51–59. [Google Scholar] [CrossRef]
- Puppe, D.; Ehrmann, O.; Kaczorek, D.; Wanner, M.; Sommer, M. The protozoic Si pool in temperate forest ecosystems—Quantification, abiotic controls and interactions with earthworms. Geoderma 2015, 243, 196–204. [Google Scholar] [CrossRef]
- Puppe, D.; Höhn, A.; Kaczorek, D.; Wanner, M.; Sommer, M. As time goes by—Spatiotemporal changes of biogenic Si pools in initial soils of an artificial catchment in NE Germany. Appl. Soil Ecol. 2016, 105, 9–16. [Google Scholar] [CrossRef]
- Geisen, S.; Mitchell, E.A.; Wilkinson, D.M.; Adl, S.; Bonkowski, M.; Brown, M.W.; Fiore-Donno, A.M.; Heger, T.J.; Jassey, V.E.; Krashevska, V. Soil protistology rebooted: 30 fundamental questions to start with. Soil Biol. Biochem. 2017, 111, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Conley, D.J.; Schelske, C.L. Potential role of sponge spicules in influencing the silicon biogeochemistry of Florida lakes. Can. J. Fish. Aquat. Sci. 1993, 50, 296–302. [Google Scholar] [CrossRef]
- Brehm, U.; Gorbushina, A.; Mottershead, D. The role of microorganisms and biofilms in the breakdown and dissolution of quartz and glass. In Geobiology: Objectives, Concepts, Perspectives; Elsevier: Amsterdam, The Netherlands, 2005; pp. 117–129. [Google Scholar]
- Kaczorek, D.; Vrydaghs, L.; Devos, Y.; Petó, Á.; Effland, W.R. Biogenic Siliceous Features. In Interpretation of Micromorphological Features of Soils and Regoliths; Elsevier: Amsterdam, The Netherlands, 2018; pp. 157–176. [Google Scholar]
- Golyeva, A. Biomorphic analysis as a part of soil morphological investigations. Catena 2001, 43, 217–230. [Google Scholar] [CrossRef]
- Runge, F. Opal-Phytolithe in den Tropen Afrikas und ihre Verwendung bei der Rekonstruktion Palaeooekologischer Umweltverhaltnisse; BoD–Books on Demand: Norderstedt, Germany, 2000. [Google Scholar]
- Watteau, F.; Villemin, G. Ultrastructural study of the biogeochemical cycle of silicon in the soil and litter of a temperate forest. Eur. J. Soil Sci. 2001, 52, 385–396. [Google Scholar] [CrossRef]
- Piperno, D.R. Phytolith Analysis: An Archaeological and Geological Perspective; Academic Press: San Diego, CA, USA, 1988; p. 280. [Google Scholar]
- Wilding, L.P.; Drees, L.R. Biogenic opal in Ohio soils. Soil Sci. Soc. Am. J. 1971, 35, 1004–1010. [Google Scholar] [CrossRef]
- Sommella, A.; Deacon, C.; Norton, G.; Pigna, M.; Violante, A.; Meharg, A. Total arsenic, inorganic arsenic, and other elements concentrations in Italian rice grain varies with origin and type. Environ. Pollut. 2013, 181, 38–43. [Google Scholar] [CrossRef]
- Borrelli, N.; Alvarez, M.F.; Osterrieth, M.L.; Marcovecchio, J.E. Silica content in soil solution and its relation with phytolith weathering and silica biogeochemical cycle in Typical Argiudolls of the Pampean Plain, Argentina—A preliminary study. J. Soils Sediments 2010, 10, 983–994. [Google Scholar] [CrossRef]
- Meunier, J.D.; Colin, F.; Alarcon, C. Biogenic silica storage in soils. Geology 1999, 27, 835–838. [Google Scholar] [CrossRef] [Green Version]
- Cabanes, D.; Shahack-Gross, R. Understanding fossil phytolith preservation: The role of partial dissolution in paleoecology and archaeology. PLoS ONE 2015, 10, e0125532. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Unzué-Belmonte, D.; Cornelis, J.-T.; Vander Linden, C.; Struyf, E.; Ronsse, F.; Delvaux, B. Effects of phytolithic rice-straw biochar, soil buffering capacity and pH on silicon bioavailability. Plant Soil 2019, 438, 187–203. [Google Scholar] [CrossRef]
- Nguyen, M.N.; Meharg, A.A.; Carey, M.; Dultz, S.; Marone, F.; Cichy, S.B.; Tran, C.T.; Le, G.H.; Mai, N.T.; Nguyen, T.T. Fern, Dicranopteris linearis, derived phytoliths in soil: Morphotypes, solubility and content in relation to soil properties. Eur. J. Soil Sci. 2019, 70, 507–517. [Google Scholar] [CrossRef]
- Osterrieth, M.; Borrelli, N.; Alvarez, M.F.; Honaine, M.F. Silica biogeochemical cycle in temperate ecosystems of the Pampean Plain, Argentina. J. S. Am. Earth Sci. 2015, 63, 172–179. [Google Scholar] [CrossRef]
- Bartoli, F.; Wilding, L. Dissolution of biogenic opal as a function of its physical and chemical properties. Soil Sci. Soc. Am. J. 1980, 44, 873–878. [Google Scholar] [CrossRef]
- Alexandre, A.; Meunier, J.-D.; Colin, F.; Koud, J.-M. Plant impact on the biogeochemical cycle of silicon and related weathering processes. Geochim. Cosmochim. Acta 1997, 61, 677–682. [Google Scholar] [CrossRef]
- Wilding, L.; Drees, L. Contributions of forest opal and associated crystalline phases to fine silt and clay fractions of soils. Clay Clay Min. 1974, 22, 295–306. [Google Scholar] [CrossRef]
- Fishkis, O.; Ingwersen, J.; Streck, T. Phytolith transport in sandy sediment: Experiments and modeling. Geoderma 2009, 151, 168–178. [Google Scholar] [CrossRef]
- Fishkis, O.; Ingwersen, J.; Lamers, M.; Denysenko, D.; Streck, T. Phytolith transport in soil: A field study using fluorescent labelling. Geoderma 2010, 157, 27–36. [Google Scholar] [CrossRef]
- Dudas, M.; Warren, C. Submicroscopic model of fly ash particles. Geoderma 1987, 40, 101–114. [Google Scholar] [CrossRef]
- Struyf, E.; Smis, A.; Van Damme, S.; Meire, P.; Conley, D.J. The Global Biogeochemical Silicon Cycle. Silicon 2009, 1, 207–213. [Google Scholar] [CrossRef]
- Marxen, A.; Klotzbücher, T.; Jahn, R.; Kaiser, K.; Nguyen, V.; Schmidt, A.; Schädler, M.; Vetterlein, D. Interaction between silicon cycling and straw decomposition in a silicon deficient rice production system. Plant Soil 2015, 398, 153–163. [Google Scholar] [CrossRef]
- Schaller, J.; Struyf, E. Silicon controls microbial decay and nutrient release of grass litter during aquatic decomposition. Hydrobiologia 2013, 709, 201–212. [Google Scholar] [CrossRef]
- de Tombeur, F.; Turner, B.; Laliberté, E.; Lambers, H.; Mahy, G.; Faucon, M.-P.; Zemunik, G.; Cornelis, J.-T. Plants sustain the terrestrial silicon cycle during ecosystem retrogression. Science 2020, 369, 1245–1248. [Google Scholar] [CrossRef]
- Struyf, E.; Van Damme, S.; Gribsholt, B.; Bal, K.; Beauchard, O.; Middelburg, J.J.; Meire, P. Phragmites australis and silica cycling in tidal wetlands. Aquat. Bot. 2007, 87, 134–140. [Google Scholar] [CrossRef]
- Harder, H. Kaolinit-synthese bei niedrigen Temperaturen. NW 1970, 57, 193. [Google Scholar] [CrossRef]
- Jasmund, K.; Lagaly, G. Tonminerale und Tone: Struktur, Eigenschaften, Anwendungen und Einsatz in Industrie und Umwelt; Springer-Verlag: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Essington, M.E. Soil and Water Chemistry: An Integrative Approach; CRC press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 126. [Google Scholar]
- Rai, D.; Kittrick, J.A. Mineral equilibria and the soil system. Min. Soil Environ. 1989, 1, 161–198. [Google Scholar]
- Lowe, D.J. Controls on the rates of weathering and clay mineral genesis in airfall tephras: A review and New Zealand case study. Rates Chem. Weather. Rocks Min. 1986, 265–330. [Google Scholar]
- Soil-Survey-Staff. Soil Taxonomy—A Basic System of Soil Classification for Making and Interpreting Soil Surveys: By Soil Survey Staff, 1999; USDA–NRCS, Agriculture Handbook number 436, Hardbound; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2014; pp. 1–203. [Google Scholar]
- Takahashi, T.; Shoji, S. Distribution and classification of volcanic ash soils. Global Environmental Research-English-Edition 2002, 6, 83–98. [Google Scholar]
- Shoji, S.; Nanzyo, M.; Dahlgren, R. Volcanic Ash Soils: Genesis, Properties and Utilization; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Wada, K. Allophane and imogolite. Min. Soil Environ. 1989, 1, 1051–1087. [Google Scholar]
- McDaniel, P.A.; Lowe, D.J.; Arnalds, O.; Ping, C.-L. Andisols. In Handbook of Soil Sciences: Properties and Processes; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Ping, C. Volcanic Soils–Encyclopedia of Volcanoes; Academic Press: San Francisco, CA, USA, 2000. [Google Scholar]
- Maeda, T.; Takenaka, H.; Warkentin, B. Physical properties of allophane soils. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 1977; Volume 29, pp. 229–264. [Google Scholar]
- Shoji, S.; Fujiwara, Y.; Yamada, I.; Saigusa, M. Chemistry and clay mineralogy of Ando soils, Brown forest soils, and Podzolic soils formed from recent Towada ashes, northeastern Japan. Soil Sci. 1982, 133, 69–86. [Google Scholar] [CrossRef]
- Hochella Jr, M.F. Nanogeoscience: From origins to cutting-edge applications. Elements 2008, 4, 373–379. [Google Scholar] [CrossRef]
- Michel, F.M.; Ehm, L.; Antao, S.M.; Lee, P.L.; Chupas, P.J.; Liu, G.; Strongin, D.R.; Schoonen, M.A.; Phillips, B.L.; Parise, J.B. The structure of ferrihydrite, a nanocrystalline material. Science 2007, 316, 1726–1729. [Google Scholar] [CrossRef] [Green Version]
- Shoji, S.; Nanzyo, M.; Dahlgren, R.A.; Quantin, P. Evaluation and proposed revisions of criteria for Andosols in the world reference base for soil resources. Soil Sci. 1996, 161, 604–615. [Google Scholar] [CrossRef] [Green Version]
- Exley, C.; Guerriero, G.; Lopez, X. Silicic acid: The omniscient molecule. Sci. Total Environ. 2019, 665, 432–437. [Google Scholar] [CrossRef]
- Pačes, T. Reversible control of aqueous aluminum and silica during the irreversible evolution of natural waters. Geochim. Cosmochim. Acta 1978, 42, 1487–1493. [Google Scholar] [CrossRef]
- Beardmore, J.; Lopez, X.; Mujika, J.I.; Exley, C. What is the mechanism of formation of hydroxyaluminosilicates? Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoji, S.; Fujiwara, Y. Active aluminum and iron in the humus horizons of andosols from northeastern Japan: Their forms, properties, and significance in clay weathering. Soil Sci. 1984, 137, 216–226. [Google Scholar] [CrossRef]
- McLean, E. Chemistry of soil aluminum. Commun. Soil Sci. Plant Anal. 1976, 7, 619–636. [Google Scholar] [CrossRef]
- Gustafsson, J.P.; Berggren, D.; Simonsson, M.; Zysset, M.; Mulder, J. Aluminium solubility mechanisms in moderately acid Bs horizons of podzolized soils. Eur. J. Soil Sci. 2001, 52, 655–665. [Google Scholar] [CrossRef]
- Wang, S.; Du, P.; Yuan, P.; Liu, Y.; Song, H.; Zhou, J.; Deng, L.; Liu, D. Structural alterations of synthetic allophane under acidic conditions: Implications for understanding the acidification of allophanic Andosols. Geoderma 2020, 376, 114561. [Google Scholar] [CrossRef]
- Parfitt, R. Allophane and imogolite: Role in soil biogeochemical processes. Clay Min. 2009, 44, 135–155. [Google Scholar] [CrossRef]
- Park, W.-P.; Koo, B.-J. Silicon and Aluminum Mobility in Soils of Jeju Island, Korea. Appl. Environ. Soil Sci. 2020, 2020. [Google Scholar] [CrossRef]
- Wada, K.; Wilson, M.; Kakuto, Y.; Wada, S.-I. Synthesis and characterization of a hollow spherical form of monolayer aluminosilicate. Clay Clay Min. 1988, 36, 11–18. [Google Scholar] [CrossRef]
- Mizota, C.; Wada, K. Implications of clay mineralogy to the weathering and chemistry of Ap horizons of Ando soils in Japan. Geoderma 1980, 23, 49–63. [Google Scholar] [CrossRef]
- Merl, T.; Koren, K. Visualizing NH3 emission and the local O2 and pH microenvironment of soil upon manure application using optical sensors. Environ. Int. 2020, 144, 106080. [Google Scholar] [CrossRef]
- Schaller, J.; Frei, S.; Rohn, L.; Gilfedder, B.S. Amorphous Silica Controls Water Storage Capacity and Phosphorus Mobility in Soils. Front. Environ. Sci. 2020, 8. [Google Scholar] [CrossRef]
- Schaller, J.; Cramer, A.; Carminati, A.; Zarebanadkouki, M. Biogenic amorphous silica as main driver for plant available water in soils. Sci. Rep. 2020, 10, 2424. [Google Scholar] [CrossRef] [Green Version]
- Parfitt, R.; Kimble, J. Conditions for formation of allophane in soils. Soil Sci. Soc. Am. J. 1989, 53, 971–977. [Google Scholar] [CrossRef]
- Schachtschabel, P.; Blume, H.; Brümmer, G.; Hartge, K.; Schwertmann, U. Scheffer/Schachtschabel-Lehrbuch der Bodenkunde (Scheffer/Schachtschabel-Textbook of Soil Science); Enke: Stuttgart, Germany, 1992. (in German) [Google Scholar]
- Michalopoulos, P.; Aller, R.C. Rapid clay mineral formation in Amazon delta sediments: Reverse weathering and oceanic elemental cycles. Science 1995, 270, 614–617. [Google Scholar] [CrossRef]
- Zabowski, D.; Ugolini, F. Seasonality in the mineral stability of a subalpine Spodosol. Soil Sci. 1992, 154, 497–507. [Google Scholar] [CrossRef]
- Zabowski, D.; Ugolini, F. Lysimeter and centrifuge soil solutions: Seasonal differences between methods. Soil Sci. Soc. Am. J. 1990, 54, 1130–1135. [Google Scholar] [CrossRef]
- Ugolini, F.; Dahlgren, R.; LaManna, J.; Nuhn, W.; Zachara, J. Mineralogy and weathering processes in recent and Holocene tephra deposits of the Pacific Northwest, USA. Geoderma 1991, 51, 277–299. [Google Scholar] [CrossRef]
- Rahman, S.; Aller, R.; Cochran, J. The missing silica sink: Revisiting the marine sedimentary Si cycle using cosmogenic 32Si. Glob. Biogeochem. Cycle 2017, 31, 1559–1578. [Google Scholar] [CrossRef]
- Vogt, T.; Larqué, P. Transformations and neoformations of clay in the cryogenic environment: Examples from Transbaikalia (Siberia) and Patagonia (Argentina). Eur. J. Soil Sci. 1998, 49, 367–376. [Google Scholar] [CrossRef]
- Monger, H.; Daugherty, L. Neoformation of palygorskite in a southern New Mexico Aridisol. Soil Sci. Soc. Am. J. 1991, 55, 1646–1650. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2018, 181, 104–136. [Google Scholar] [CrossRef] [Green Version]
- Kemper, W.; Rosenau, R. Soil cohesion as affected by time and water content. Soil Sci. Soc. Am. J. 1984, 48, 1001–1006. [Google Scholar] [CrossRef] [Green Version]
- Kemper, W.; Rosenau, R. Aggregate stability and size distribution. Methods Soil Anal. Part 1 Phys. Mineral. Methods 1986, 5, 425–442. [Google Scholar]
- Brewer, R.; Bettenay, E.; Churchward, H.M. Some Aspects of the Origin and Development of the Red and Brown Hardpan Soils of Bulloo Downs, Western Australia; CSIRO: Clayton, VIC, Australia, 1972. [Google Scholar]
- Flach, K.; Nettleton, W.; Nelson, R. Micromorphology of silica-cemented soil horizons in western North America. In Soil Microscopy, Proceedings of the International Working Meeting on Soil Micromorphology, Kingston, ON, Canada, 27–31 August 1973; Limestone Press: Kingston, ON, Canada, 1974. [Google Scholar]
- Norton, L. Micromorphology of silica cementation in soils. In Developments in Soil Science; Elsevier: Amsterdam, The Netherlands, 1993; Volume 22, pp. 811–824. [Google Scholar]
- Humphries, M.S.; Kindness, A.; Ellery, W.N.; Hughes, J.C. Sediment geochemistry, mineral precipitation and clay neoformation on the Mkuze River floodplain, South Africa. Geoderma 2010, 157, 15–26. [Google Scholar] [CrossRef]
- Frings, P.J.; De La Rocha, C.; Struyf, E.; van Pelt, D.; Schoelynck, J.; Hudson, M.M.; Gondwe, M.J.; Wolski, P.; Mosimane, K.; Gray, W.; et al. Tracing silicon cycling in the Okavango Delta, a sub-tropical flood-pulse wetland using silicon isotopes. Geochim. Cosmochim. Acta 2014, 142, 132–148. [Google Scholar] [CrossRef] [Green Version]
- Ishizawa, H.; Niiyama, K.; Iida, Y.; Shari, N.H.; Ripin, A.; Kitajima, K. Spatial variations of soil silicon availability and biogenic silicon flux in a lowland tropical forest in Malaysia. Ecol. Res. 2019, 34, 548–559. [Google Scholar] [CrossRef]
- Clymans, W.; Struyf, E.; Govers, G.; Vandevenne, F.; Conley, D. Anthropogenic impact on amorphous silica pools in temperate soils. Biogeosciences 2011, 8, 2281–2293. [Google Scholar] [CrossRef] [Green Version]
- Miles, N.; Manson, A.D.; Rhodes, R.; van Antwerpen, R.; Weigel, A. Extractable silicon in soils of the South African Sugar industry and relationships with crop uptake. Commun. Soil Sci. Plant Anal. 2014, 45, 2949–2958. [Google Scholar] [CrossRef]
- Schaller, J.; Turner, B.L.; Weissflog, A.; Pino, D.; Bielnicka, A.W.; Engelbrecht, B.M. Silicon in tropical forests: Large variation across soils and leaves suggests ecological significance. Biogeochemistry 2018, 140, 161–174. [Google Scholar] [CrossRef]
- Quigley, K.M.; Donati, G.L.; Anderson, T.M. Variation in the soil ‘silicon landscape’explains plant silica accumulation across environmental gradients in Serengeti. Plant Soil 2017, 410, 217–229. [Google Scholar] [CrossRef]
- Meunier, J.-D.; Sandhya, K.; Prakash, N.B.; Borschneck, D.; Dussouillez, P. pH as a proxy for estimating plant-available Si? A case study in rice fields in Karnataka (South India). Plant Soil 2018, 432, 143–155. [Google Scholar] [CrossRef]
- Caubet, M.; Cornu, S.; Saby, N.P.A.; Meunier, J.D. Agriculture increases the bioavailability of silicon, a beneficial element for crop, in temperate soils. Sci. Rep. 2020, 10, 19999. [Google Scholar] [CrossRef] [PubMed]
- Saccone, L.; Conley, D.J.; Likens, G.E.; Bailey, S.W.; Buso, D.C.; Johnson, C.E. Factors that control the range and variability of amorphous silica in soils in the Hubbard Brook Experimental Forest. Soil Sci. Soc. Am. J. 2008, 72, 1637–1644. [Google Scholar] [CrossRef]
- Vandevenne, F.; Struyf, E.; Clymans, W.; Meire, P. Agricultural silica harvest: Have humans created a new loop in the global silica cycle? Front. Ecol. Environ. 2012, 10, 243–248. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Poulton, P.R.; McGrath, S.P.; Meunier, J.-D. Long-term removal of wheat straw decreases soil amorphous silica at Broadbalk, Rothamsted. Plant Soil 2012, 352, 173–184. [Google Scholar] [CrossRef]
- Yang, S.; Hao, Q.; Liu, H.; Zhang, X.; Yu, C.; Yang, X.; Xia, S.; Yang, W.; Li, J.; Song, Z. Impact of grassland degradation on the distribution and bioavailability of soil silicon: Implications for the Si cycle in grasslands. Sci. Total Environ. 2019, 657, 811–818. [Google Scholar] [CrossRef]
- Desplanques, V.; Cary, L.; Mouret, J.C.; Trolard, F.; Bourrié, G.; Grauby, O.; Meunier, J.D. Silicon transfers in a rice field in Camargue (France). J. Geochem. Explor. 2006, 88, 190–193. [Google Scholar] [CrossRef]
- Yang, X.; Song, Z.; Yu, C.; Ding, F. Quantification of different silicon fractions in broadleaf and conifer forests of northern China and consequent implications for biogeochemical Si cycling. Geoderma 2020, 361, 114036. [Google Scholar] [CrossRef]
- Unzué-Belmonte, D.; Struyf, E.; Clymans, W.; Tischer, A.; Potthast, K.; Bremer, M.; Meire, P.; Schaller, J. Fire enhances solubility of biogenic silica. Sci. Total Environ. 2016, 572, 1289–1296. [Google Scholar] [CrossRef]
- Schaller, J.; Puppe, D. Heat improves silicon availability in mineral soils. Geoderma 2021, 386, 114909. [Google Scholar]
- Shan, Y.; Johnson-Beebout, S.; Buresh, R. Crop residue management for lowland rice-based cropping systems in Asia. Adv. Agron. 2008, 98, 117–199. [Google Scholar]
- Goswami, S.B.; Mondal, R.; Mandi, S.K. Crop residue management options in rice–rice system: A review. Arch. Agron. Soil Sci. 2020, 66, 1218–1234. [Google Scholar] [CrossRef]
- Schaller, J.; Wang, J.; Islam, M.R.; Planer-Friedrich, B. Black carbon yields highest nutrient and lowest arsenic release when using rice residuals in paddy soils. Sci. Rep. 2018, 8, 17004. [Google Scholar] [CrossRef] [Green Version]
- Guntzer, F.; Keller, C.; Meunier, J.-D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Datnoff, L.E.; Rodrigues, F.A. History of silicon and plant disease. In Silicon and Plant Diseases; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–5. [Google Scholar]
- Korndörfer, G.; Snyder, G.; Ulloa, M.; Powell, G.; Datnoff, L. Calibration of soil and plant silicon analysis for rice production. J. Plant Nutr. 2001, 24, 1071–1084. [Google Scholar] [CrossRef]
- Klotzbücher, T.; Marxen, A.; Vetterlein, D.; Schneiker, J.; Türke, M.; van Sinh, N.; Manh, N.H.; van Chien, H.; Marquez, L.; Villareal, S. Plant-available silicon in paddy soils as a key factor for sustainable rice production in Southeast Asia. Basic Appl. Ecol. 2014, 16, 665–673. [Google Scholar] [CrossRef]
- Velbel, M.A. Temperature dependence of silicate weathering in nature: How strong a negative feedback on long-term accumulation of atmospheric CO2 and global greenhouse warming? Geology 1993, 21, 1059–1062. [Google Scholar] [CrossRef]
- Li, G.; Hartmann, J.; Derry, L.A.; West, A.J.; You, C.-F.; Long, X.; Zhan, T.; Li, L.; Li, G.; Qiu, W. Temperature dependence of basalt weathering. Earth Planet. Sci. Lett. 2016, 443, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Lehner, B.; Döll, P.; Alcamo, J.; Henrichs, T.; Kaspar, F. Estimating the impact of global change on flood and drought risks in Europe: A continental, integrated analysis. Clim. Change 2006, 75, 273–299. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.T. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Bockheim, J. Properties and classification of cold desert soils from Antarctica. Soil Sci. Soc. Am. J. 1997, 61, 224–231. [Google Scholar] [CrossRef]
- Mitani, N.; Ma, J.F. Uptake system of silicon in different plant species. J. Exp. Bot. 2005, 56, 1255–1261. [Google Scholar] [CrossRef] [Green Version]
- Katz, O.; Puppe, D.; Kaczorek, D.; Prakash, N.B.; Schaller, J. Silicon in the soil-plant continuum: Intricate feedbacks within ecosystems. Plants. in preparation.
- Savant, N.K.; Datnoff, L.E.; Snyder, G.H. Depletion of plant-available silicon in soils: A possible cause of declining rice yields. Commun. Soil Sci. Plant Anal. 1997, 28, 1245–1252. [Google Scholar] [CrossRef]
- Deren, C.; Datnoff, L.; Snyder, G.; Martin, F. Silicon concentration, disease response, and yield components of rice genotypes grown on flooded organic histosols. Crop Sci. 1994, 34, 733–737. [Google Scholar] [CrossRef]
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef]
- Berthelsen, S.; Noble, A.; Kingston, G.; Hurney, A.; Rudd, A.; Garside, A. Improving yield and ccs in sugarcane through the application of silicon based amendments. Final Report on SRDC Project CLW009; CSIRO: Clayton, VIC, Australia, 2003. [Google Scholar]
- Eneji, A.E.; Inanaga, S.; Muranaka, S.; Li, J.; Hattori, T.; An, P.; Tsuji, W. Growth and nutrient use in four grasses under drought stress as mediated by silicon fertilizers. J. Plant Nutr. 2008, 31, 355–365. [Google Scholar] [CrossRef]
- Ma, J.F.; Takahashi, E. Effect of silicon on the growth and phosphorus uptake of rice. Plant Soil 1990, 126, 115–119. [Google Scholar] [CrossRef]
- Ma, J.F.; Takahashi, E. Effect of silicate on phosphate availability for rice in a P-deficient soil. Plant Soil 1991, 133, 151–155. [Google Scholar]
- Hömberg, A.; Obst, M.; Knorr, K.-H.; Kalbitz, K.; Schaller, J. Increased silicon concentration in fen peat leads to a release of iron and phosphate and changes in the composition of dissolved organic matter. Geoderma 2020, 374, 114422. [Google Scholar] [CrossRef]
- Yang, X.; Post, W.M. Phosphorus transformations as a function of pedogenesis: A synthesis of soil phosphorus data using Hedley fractionation method. Biogeosciences 2011, 8, 2907–2916. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Post, W.M.; Thornton, P.E.; Jain, A. The distribution of soil phosphorus for global biogeochemical modeling. Biogeosciences 2013, 10, 2525–2537. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; Chen, K. The regulatory role of silicon on water relations, photosynthetic gas exchange, and carboxylation activities of wheat leaves in field drought conditions. Acta Physiol. Plant. 2012, 34, 1589–1594. [Google Scholar] [CrossRef]
- Pei, Z.; Ming, D.; Liu, D.; Wan, G.; Geng, X.; Gong, H.; Zhou, W. Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. J. Plant Growth Regul. 2010, 29, 106–115. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.; Ali, S.; Ibrahim, M.; Farid, M.; Adrees, M.; Bharwana, S.A.; Zia-ur-Rehman, M.; Qayyum, M.F.; Abbas, F. Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: A review. Environ. Sci. Pollut. Res. 2015, 22, 15416–15431. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, Y.; Kuşvuran, A.; Alharby, H.F.; Kuşvuran, S.; Rady, M.M. The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotox. Environ. Safe. 2018, 154, 187–196. [Google Scholar] [CrossRef]
- del Carmen Gutiérrez-Castorena, M.; Stoops, G.; Solorio, C.A.O.; Avila, G.L. Amorphous silica materials in soils and sediments of the Ex-Lago de Texcoco, Mexico: An explanation for its subsidence. Catena 2005, 60, 205–226. [Google Scholar] [CrossRef]
- Iler, R.K. Surface and Colloid Science; Matijevic, E., Ed.; Wiley: New York, NY, USA, 1973; Volume 6. [Google Scholar]
- Goto, M.; Ehara, H.; Karita, S.; Takabe, K.; Ogawa, N.; Yamada, Y.; Ogawa, S.; Yahaya, M.S.; Morita, O. Protective effect of silicon on phenolic biosynthesis and ultraviolet spectral stress in rice crop. Plant Sci. 2003, 164, 349–356. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Meharg, C.; Meharg, A.A. Silicon, the silver bullet for mitigating biotic and abiotic stress, and improving grain quality, in rice? Environ. Exp. Bot. 2015, 120, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.J.; Ma, J.F.; Meharg, A.A.; McGrath, S.P. Arsenic uptake and metabolism in plants. New Phytol. 2009, 181, 777–794. [Google Scholar] [CrossRef] [PubMed]
- You-Qiang, F.; Hong, S.; Dao-Ming, W.; Kun-Zheng, C. Silicon-mediated amelioration of Fe2+ toxicity in rice (Oryza sativa L.) roots. Pedosphere 2012, 22, 795–802. [Google Scholar]
- Li, P.; Song, A.L.; Li, Z.J.; Fan, F.L.; Liang, Y.C. Silicon ameliorates manganese toxicity by regulating manganese transport and antioxidant reactions in rice (Oryza sativa L.). Plant Soil 2012, 354, 407–419. [Google Scholar] [CrossRef]
- Massey, F.P.; Smith, M.J.; Lambin, X.; Hartley, S.E. Are silica defences in grasses driving vole population cycles? Biol. Lett. 2008, 4, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Alhousari, F.; Greger, M. Silicon and Mechanisms of Plant Resistance to Insect Pests. Plants 2018, 7, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massey, F.P.; Massey, K.; Ennos, A.R.; Hartley, S.E. Impacts of silica-based defences in grasses on the feeding preferences of sheep. Basic Appl. Ecol. 2009, 10, 622–630. [Google Scholar] [CrossRef]
- Dallagnol, L.J.; Rodrigues, F.A.; Mielli, M.V.; Ma, J.F. Rice grain resistance to brown spot and yield are increased by silicon. Trop. Plant Pathol. 2014, 39, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Datnoff, L.E.; Snyder, G.H.; Deren, C.W. Influence of Silicon Fertilizer Grades on Blast and Brown Spot Development and on Rice Yields. Plant Dis. 1992, 76, 1011–1013. [Google Scholar] [CrossRef]
- Ning, D.; Song, A.; Fan, F.; Li, Z.; Liang, Y. Effects of slag-based silicon fertilizer on rice growth and brown-spot resistance. PLoS ONE 2014, 9, e102681. [Google Scholar] [CrossRef] [Green Version]
- Laing, M.; Gatarayiha, M.; Adandonon, A. Silicon use for pest control in agriculture: A review. In Proceedings of the Congress of the South African Sugar Technologists’ Association, Durban, South Africa, 18–20 July 2006; pp. 278–286. [Google Scholar]






| Extractant | Procedure (Soil: Extractant Ratio/Extraction Time/Temperature | Si Fraction Supposed to Be Extracted | References |
|---|---|---|---|
| H2O | 10 g: 50 mL/21 days/room temperature | Water-soluble Si | [37] |
| H2O | 10 g: 100 mL/4 h/room temperature | Water-soluble Si | [35,36] |
| 0.01 M CaCl2 | 1 g: 20 mL/16 h/room temperature | Readily available + above listed fractions | [40] |
| 0.01 M CaCl2 | 10 g: 100 mL/1 h/room temperature | Readily available + above listed fractions | [41] |
| 0.5 M NH4-acetate; adjusted to pH 4.8 | 1 g: 10 mL/1 h/room temperature | Soluble and some exchangeable Si + above listed fractions | [35] |
| 0.5 M acetic acid | 1 g: 10 mL/1 h at room temperature//--resting | Soluble and some exchangeable Si + above listed fractions | [42] |
| NH4 citrate | 10 g: 25 mL/80 h/room temperature | Soluble, exchangeable and specifically adsorbed Si + above listed fractions | [43] |
| 0.2 M NH4 oxalate; adjusted to pH 3.0 | 2 g: 100 mL/1 h room temperature//dark room | Si bound in amorphous and poorly crystalline pedogenic oxides + above listed fractions | [47] |
| Mehlich-III solution at pH 2 | 2 g: 42 mL/5 min/room temperature | Si bound in amorphous and poorly crystalline pedogenic oxides + above listed fractions potential share of crystalline Si is smaller | [44,45] |
| 0.5 M NaOH | soil: extractant ratio of less than 100 mg: 100 mL/2.5 min/boiling | Si from allophanes and amorphous silica + above listed fractions | [48] |
| 0.2 M NaOH | 1 g: 400 mL/5 h, ~120–168 h/room temperature | Amorphous silica and low amounts of crystalline Si + above listed fractions | [49] |
| 0.1 M Na2CO3 | 30 mg: 40 mL/5 h/85 °C/aliquots after 2, 4 and 5 h/ | Biogenic silica in sediments and water + above listed fractions | [51,52] |
| 0.1 M Tiron, pH 10.5 | 25 mg: 30 mL/1 h/80 °C | Si bound in amorphous silica in soils + above listed fractions | [56] |
| Sequential extraction | [57] | ||
| Step 1: 0.01 M CaCl2 | 1 g: 5 mL/TIME/rinsed with pure water room temperature/ | Readily plant-available Si | |
| Step 2: 0.01 M acetic acid | soil solution ratio = 1:10/24 h/room temperature/ | Adsorbed Si fraction | |
| Step 3: H2O2 (17.5% in water) | soil solution ratio = 1:20/24 h/plus 10 mL 35% H2O2/85 °C until reaction is complete | Si bound to organic matter | |
| Step 4: 0.2 M NH4 oxalate and 0.2 M oxalic acid | 1:50/8 h/overnight treatment with UV light/room temperature/ | Si occluded in pedogenic (hydr)oxides | |
| Step 5a: sodium polytungstate 0.2 M NaOH solution | 50% residue of step 4 pre-preparation with sodium polytungstate, afterwards soil solution ratio = 1:400/168 h/room temperature/ | Biogenic ASi | |
| Step 5b: 0.2 M NaOH solution | Other 50% residue of step 4 soil solution ratio = 1:400/168 h/room temperature | Total ASi, also calculate minerogenic ASi (total ASi—biogenic ASi) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaller, J.; Puppe, D.; Kaczorek, D.; Ellerbrock, R.; Sommer, M. Silicon Cycling in Soils Revisited. Plants 2021, 10, 295. https://doi.org/10.3390/plants10020295
Schaller J, Puppe D, Kaczorek D, Ellerbrock R, Sommer M. Silicon Cycling in Soils Revisited. Plants. 2021; 10(2):295. https://doi.org/10.3390/plants10020295
Chicago/Turabian StyleSchaller, Jörg, Daniel Puppe, Danuta Kaczorek, Ruth Ellerbrock, and Michael Sommer. 2021. "Silicon Cycling in Soils Revisited" Plants 10, no. 2: 295. https://doi.org/10.3390/plants10020295






