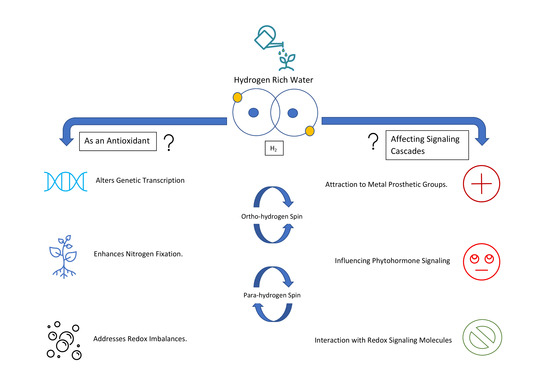
Downstream Signalling from Molecular Hydrogen
Abstract
:1. Introduction
2. Downstream Effects
2.1. Effects on Reactive Oxygen Species and Antioxidant Capacity
2.2. Impact on Reactive Nitrogen Species Metabolism
2.3. Stress, Heme Oxygenase and H2
2.4. Paramagnetic Properties and Possible Cellular Effects
3. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ge, L.; Yang, M.; Yang, N.-N.; Yin, X.-X.; Song, W.-G. Molecular hydrogen: A preventive and therapeutic medical gas for various diseases. Oncotarget 2017, 8, 102653–102673. [Google Scholar] [CrossRef] [Green Version]
- Huang, L. Molecular hydrogen: A therapeutic antioxidant and beyond. Med. Gas Res. 2016, 6, 219–222. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Gong, T.; Bian, B.; Liao, W. Roles of hydrogen gas in plants: A review. Funct. Plant Biol. 2018, 45, 783–792. [Google Scholar] [CrossRef]
- Zeng, J.; Ye, Z.; Sun, X. Progress in the study of biological effects of hydrogen on higher plants and its promising application in agriculture. Med. Gas Res. 2014, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Li, P.; Wang, Y.; Gu, R. Hydrogen-rich water delays postharvest ripening and senescence of kiwifruit. Food Chem. 2014, 156, 100–109. [Google Scholar] [CrossRef]
- Wilson, H.R.; Veal, D.; Whiteman, M.; Hancock, J.T. Hydrogen gas and its role in cell signalling. CAB Rev. 2017, 12, 1–3. [Google Scholar] [CrossRef]
- Russell, G.; Zulfiqar, F.; Hancock, J.T. Hydrogenases and the Role of Molecular Hydrogen in Plants. Plants 2020, 9, 1136. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Su, N.; Huang, X.; Ling, X.; Yu, M.; Cui, J.; Shabala, S. Hydrogen-rich water promotes elongation of hypocotyls and roots in plants through mediating the level of endogenous gibberellin and auxin. Funct. Plant Biol. 2020, 47, 771. [Google Scholar] [CrossRef] [PubMed]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Cory, J.S.; Myers, J.H. Adaptation in an insect host-plant pathogen interaction. Ecol. Lett. 2004, 7, 632–639. [Google Scholar] [CrossRef]
- Keyster, M.; Niekerk, L.-A.; Basson, G.; Carelse, M.; Bakare, O.; Ludidi, N.; Klein, A.; Mekuto, L.; Gokul, A. Decoding Heavy Metal Stress Signalling in Plants: Towards Improved Food Security and Safety. Plants 2020, 9, 1781. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Molecular Regulation of Plant Responses to Environmental Temperatures. Mol. Plant 2020, 13, 544–564. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Vanhaelewyn, L.; Prinsen, E.; Van Der Straeten, D.; Vandenbussche, F. Hormone-controlled UV-B responses in plants. J. Exp. Bot. 2016, 67, 4469–4482. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2016, 90, 856–867. [Google Scholar] [CrossRef]
- Sharma, A.; Soares, C.; Sousa, B.; Martins, M.; Kumar, V.; Shahzad, B.; Sidhu, G.P.; Bali, A.S.; Asgher, M.; Bhardwaj, R.; et al. Nitric oxide-mediated regulation of oxidative stress in plants under metal stress: A review on molecular and biochemical aspects. Physiol. Plant. 2019, 168, 318–344. [Google Scholar] [CrossRef] [PubMed]
- Černý, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohatý, B. Hydrogen Peroxide: Its Role in Plant Biology and Crosstalk with Signalling Networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umbreen, S.; Lubega, J.; Cui, B.; Pan, Q.; Jiang, J.; Loake, G.J. Specificity in nitric oxide signalling. J. Exp. Bot. 2018, 69, 3439–3448. [Google Scholar] [CrossRef]
- Speckmann, B.; Steinbrenner, H.; Grune, T.; Klotz, L.-O. Peroxynitrite: From interception to signaling. Arch. Biochem. Biophys. 2016, 595, 153–160. [Google Scholar] [CrossRef]
- Ventimiglia, L.; Mutus, B. The Physiological Implications of S-Nitrosoglutathione Reductase (GSNOR) Activity Mediating NO Signalling in Plant Root Structures. Antioxidants 2020, 9, 1206. [Google Scholar] [CrossRef]
- Lindermayr, C. Crosstalk between reactive oxygen species and nitric oxide in plants: Key role of S-nitrosoglutathione reductase. Free. Radic. Biol. Med. 2018, 122, 110–115. [Google Scholar] [CrossRef]
- Hancock, J.; Whiteman, M. Hydrogen sulfide and cell signaling: Team player or referee? Plant Physiol. Biochem. 2014, 78, 37–42. [Google Scholar] [CrossRef]
- Shivaraj, S.M.; Vats, S.; Bhat, J.A.; Dhakte, P.; Goyal, V.; Khatri, P.; Kumawat, S.; Singh, A.; Prasad, M.; Sonah, H.; et al. Nitric oxide and hydrogen sulfide crosstalk during heavy metal stress in plants. Physiol. Plant. 2019, 168, 437–455. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jiang, W.; Han, W.; Li, J.; Liu, Y. Effects of Hydrogen-Rich Water on Fitness Parameters of Rice Plants. Agron. J. 2017, 109, 2033–2039. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Su, N.; Shabala, L.; Huang, L.; Yu, M.; Shabala, S. Understanding the mechanistic basis of ameliorating effects of hydrogen rich water on salinity tolerance in barley. Environ. Exp. Bot. 2020, 177, 104136. [Google Scholar] [CrossRef]
- Su, J.; Yang, X.; Shao, Y.; Chen, Z.; Shen, W. Molecular hydrogen–induced salinity tolerance requires melatonin signalling in Arabidopsis thaliana. Plant Cell Environ. 2021, 44, 476–490. [Google Scholar] [CrossRef]
- Cui, W.; Yao, P.; Pan, J.; Dai, C.; Cao, H.; Chen, Z.; Zhang, S.; Xu, S.; Shen, W. Transcriptome analysis reveals insight into molecular hydrogen-induced cadmium tolerance in alfalfa: The prominent role of sulfur and (homo)glutathione metabolism. BMC Plant Biol. 2020, 20, 19–58. [Google Scholar] [CrossRef] [PubMed]
- Renwick, G.M.; Giumarro, C.; Siegel, S.M. Hydrogen Metabolism in Higher Plants. Plant Physiol. 1964, 39, 303–306. [Google Scholar] [CrossRef]
- Vargas, S.R.; Dos Santos, P.V.; Giraldi, L.A.; Zaiat, M.; Calijuri, M.D.C. Anaerobic phototrophic processes of hydrogen production by different strains of microalgae Chlamydomonas sp. FEMS Microbiol. Lett. 2018, 365, fny073. [Google Scholar] [CrossRef] [Green Version]
- Hemschemeier, A.; Fouchard, S.; Cournac, L.; Peltier, G.; Happe, T. Hydrogen production by Chlamydomonas reinhardtii: An elaborate interplay of electron sources and sinks. Planta 2007, 227, 397–407. [Google Scholar] [CrossRef]
- Sanadze, G.A. Absorption of molecular hydrogen by green leaves in light. Fiziol. Rast. 1961, 8, 555–559. [Google Scholar]
- Zeng, J.; Zhang, M.; Sun, X. Molecular Hydrogen Is Involved in Phytohormone Signaling and Stress Responses in Plants. PLoS ONE 2013, 8, e71038. [Google Scholar] [CrossRef]
- Cao, Z.; Duan, X.; Yao, P.; Cui, W.; Cheng, D.; Zhang, J.; Jin, Q.; Chen, J.; Dai, T.; Shen, W. Hydrogen Gas Is Involved in Auxin-Induced Lateral Root Formation by Modulating Nitric Oxide Synthesis. Int. J. Mol. Sci. 2017, 18, 2084. [Google Scholar] [CrossRef]
- Jin, T.; Liu, Y.; Wei, J.; Wu, M.; Lei, G.; Chen, H.; Lan, Y. Modeling and analysis of the flammable vapor cloud formed by liquid hydrogen spills. Int. J. Hydrogen Energy 2017, 42, 26762–26770. [Google Scholar] [CrossRef]
- Molecular Hydrogen Institute. Concentration and Solubility of H2. Available online: http://www.molecularhydrogeninstitute.com/concentration-and-solubility-of-h2 (accessed on 13 January 2021).
- Wilhelm, E.; Battino, R.; Wilcock, R.J. Low-pressure solubility of gases in liquid water. Chem. Rev. 1977, 77, 219–262. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, W.; Qi, F.; Cui, W.; Xie, Y.; Shen, W. Hydrogen-rich water regulates cucumber adventitious root development in a heme oxygenase-1/carbon monoxide-dependent manner. J. Plant Physiol. 2014, 171, 1–8. [Google Scholar] [CrossRef]
- Fang, S.; Li, X.; Wei, X.; Zhang, Y.; Ma, Z.; Wei, Y.; Wang, W. Beneficial effects of hydrogen gas inhalation on a murine model of allergic rhinitis. Exp. Ther. Med. 2018, 16, 5178–5184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Z.; Wu, L.; Kettlewell, B.; Caldwell, C.D.; Layzell, D.B. Hydrogen fertilization of soils–is this a benefit of legumes in rotation? Plant Cell Environ. 2003, 26, 1875–1879. [Google Scholar] [CrossRef]
- Hancock, J.T. Methods for the addition of redox compounds. In Redox-Mediated Signal Transduction; Hancock, J.T., Conway, M.E., Eds.; Humana: New York, NY, USA, 2019; pp. 13–25. [Google Scholar]
- Deller, M.C. Cell surface receptors. Curr. Opin. Struct. Biol. 2000, 10, 213–219. [Google Scholar] [CrossRef]
- Gasc, J.M.; Baulieu, E.E. Steroid hormone receptors: Intracellular distribution. Biol. Cell 1986, 56, 1–6. [Google Scholar] [CrossRef]
- Mazaira, G.I.; Zgajnar, N.R.; Lotufo, C.M.; Daneri-Becerra, C.; Sivils, J.C.; Soto, O.B.; Cox, M.B.; Galigniana, M.D. Nuclear Receptors: A Historical Perspective. Adv. Struct. Saf. Stud. 2019, 1966, 1–5. [Google Scholar] [CrossRef]
- Sandner, P.; Zimmer, D.P.; Milne, G.T.; Follmann, M.; Hobbs, A.; Stasch, J.-P. Soluble Guanylate Cyclase Stimulators and Activators. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–39. [Google Scholar] [CrossRef] [Green Version]
- Astier, J.; Mounier, A.; Santolini, J.; Jeandroz, S.; Wendehenne, D. The evolution of nitric oxide signalling diverges between animal and green lineages. J. Exp. Bot. 2019, 70, 4355–4364. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2018, 221, 1197–1214. [Google Scholar] [CrossRef]
- Feng, J.; Chen, L.; Zuo, J. Protein S -Nitrosylation in plants: Current progresses and challenges. J. Integr. Plant Biol. 2019, 61, 1206–1223. [Google Scholar] [CrossRef]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fryzova, R.; Pohanka, M.; Martinkova, P.; Cihlarova, H.; Brtnicky, M.; Hladky, J.; Kynicky, J. Oxidative Stress and Heavy Metals in Plants. Rev. Environ. Contam. Toxicol. 2017, 245, 129–156. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Bao, M.; Wang, L.; Khan, I.; Ullah, E.; Tung, S.A.; Samad, R.A.; Shahzad, B. Cadmium toxicity in Maize (Zea mays L.): Consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ. Sci. Pollut. Res. 2015, 22, 17022–17030. [Google Scholar] [CrossRef]
- Górska-Czekaj, M.; Borucki, W. A correlative study of hydrogen peroxide accumulation after mercury or copper treatment observed in root nodules of Medicago truncatula under light, confocal and electron microscopy. Micron 2013, 52–53, 24–32. [Google Scholar] [CrossRef]
- Camejo, D.; Guzmán-Cedeño, Á.; Moreno, A. Reactive oxygen species, essential molecules, during plant–pathogen interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar] [CrossRef]
- Santos, A.D.A.; Da Silveira, J.A.G.; Bonifacio, A.; Rodrigues, A.C.; Figueiredo, M.D.V.B. Antioxidant response of cowpea co-inoculated with plant growth-promoting bacteria under salt stress. Braz. J. Microbiol. 2018, 49, 513–521. [Google Scholar] [CrossRef] [PubMed]
- AbdelGawad, H.; Zinta, G.; Hamed, B.A.; Selim, S.; Beemster, G.; Hozzein, W.N.; Wadaan, M.A.; Asard, H.; Abuelsoud, W.; Badreldin, A.H. Maize roots and shoots show distinct profiles of oxidative stress and antioxidant defense under heavy metal toxicity. Environ. Pollut. 2020, 258, 113705. [Google Scholar] [CrossRef] [PubMed]
- Airaki, M.; Leterrier, M.; Mateos, R.M.; Valderrama, R.; Chaki, M.; Barroso, J.B.; Del Río, L.A.; Palma, J.M.; Corpas, F.J. Metabolism of reactive oxygen species and reactive nitrogen species in pepper (Capsicum annuum L.) plants under low temperature stress. Plant Cell Environ. 2012, 35, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhu, S.; Jiang, Y.; Wang, N.; Wang, R.; Shen, W.; Yang, J. Hydrogen-rich water alleviates salt stress in rice during seed germination. Plant Soil 2013, 370, 47–57. [Google Scholar] [CrossRef]
- Xie, Y.; Mao, Y.; Lai, D.; Zhang, W.; Shen, W. H2 enhances Arabidopsis salt tolerance by manipulating ZAT10/12-mediated antioxidant defence and controlling sodium exclusion. PLoS ONE 2012, 7, e49800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Cui, W.; Zhu, K.; Xie, Y.; Zhang, C.; Shen, W. Hydrogen-rich water alleviates aluminium-induced inhibition of root elongation in alfalfa via decreasing nitric oxide production. J. Hazard. Mater. 2014, 267, 40–47. [Google Scholar] [CrossRef]
- Xu, D.; Cao, H.; Fang, W.; Pan, J.; Chen, J.; Zhang, J.; Shen, W. Linking hydrogen-enhanced rice aluminum tolerance with the reestablishment of GA/ABA balance and miRNA-modulated gene expression: A case study on germination. Ecotoxicol. Environ. Saf. 2017, 145, 303–312. [Google Scholar] [CrossRef]
- Cui, W.; Gao, C.; Fang, P.; Lin, G.; Shen, W. Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J. Hazard. Mater. 2013, 260, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Fang, P.; Zhu, K.; Mao, Y.; Gao, C.; Xie, Y.; Wang, J.; Shen, W. Hydrogen-rich water confers plant tolerance to mercury toxicity in alfalfa seedlings. Ecotoxicol. Environ. Saf. 2014, 105, 103–111. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Hu, L.; Liao, W.; Dawuda, M.M.; Li, C. Carbon monoxide is involved in hydrogen gas-induced adventitious root development in cucumber under simulated drought stress. Front. Plant Sci. 2017, 8, 128. [Google Scholar] [CrossRef]
- Jin, Q.; Zhu, K.; Cui, W.; Li, L.; Shen, W. Hydrogen-Modulated Stomatal Sensitivity to Abscisic Acid and Drought Tolerance Via the Regulation of Apoplastic pH in Medicago sativa. J. Plant Growth Regul. 2016, 35, 565–573. [Google Scholar] [CrossRef]
- Jin, Q.; Zhu, K.; Cui, W.; Xie, Y.; Han, B.; Shen, W. Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of heme oxygenase-1 signalling system. Plant Cell Environ. 2012, 36, 956–969. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, S.; Zhang, J.-M. Hydrogen as a Selective Antioxidant: A Review of Clinical and Experimental Studies. J. Int. Med. Res. 2010, 38, 1893–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Veal, E.A.; Day, A.M.; Morgan, B.A. Hydrogen Peroxide Sensing and Signaling. Mol. Cell 2007, 26, 1–14. [Google Scholar] [CrossRef]
- Pastina, B.; LaVerne, J.A. Effect of Molecular Hydrogen on Hydrogen Peroxide in Water Radiolysis. J. Phys. Chem. A 2001, 105, 9316–9322. [Google Scholar] [CrossRef]
- Richards, S.L.; Wilkins, K.A.; Swarbreck, S.M.; Anderson, A.A.; Habib, N.; Smith, A.G.; McAinsh, M.; Davies, J.M. The hydroxyl radical in plants: From seed to seed. J. Exp. Bot. 2015, 66, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Pottosin, I.; Zepeda-Jazo, I.; Bose, J.; Shabala, S. An Anion Conductance, the Essential Component of the Hydroxyl-Radical-Induced Ion Current in Plant Roots. Int. J. Mol. Sci. 2018, 19, 897. [Google Scholar] [CrossRef] [Green Version]
- Demidchik, V.; Cuin, T.A.; Svistunenko, D.; Smith, S.J.; Miller, A.J.; Shabala, S.; Sokolik, A.; Yurin, V. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: Single-channel properties, genetic basis and involvement in stress-induced cell death. J. Cell Sci. 2010, 123, 1468–1479. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.J.; Lin, K.H.; Hsu, M.J.; Chou, D.S.; Hsiao, G.; Sheu, J.R. Suppression of NF-κB signaling by andrographolide with a novel mechanism in human platelets: Regulatory roles of the p38 MAPK-hydroxyl radical-ERK2 cascade. Biochem. Pharmacol. 2012, 84, 914–924. [Google Scholar] [CrossRef]
- Qu, Y.; Yan, M.; Zhang, Q. Functional regulation of plant NADPH oxidase and its role in signaling. Plant Signal. Behav. 2017, 12, e1356970. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Yadav, S.; Hasanuzzaman, M.; Fujita, M.; Mishra, P.; Sabat, S.C.; Tuteja, N. Superoxide dismutase—Mentor of abiotic stress tolerance in crop plants. Environ. Sci. Pollut. Res. 2015, 22, 10375–10394. [Google Scholar] [CrossRef]
- Fong, K.-L.; McCay, P.B.; Poyer, J.; Misra, H.P.; Keele, B.B. Evidence for superoxide-dependent reduction of Fe3+ and its role in enzyme-generated hydroxyl radical formation. Chem. Interact. 1976, 15, 77–89. [Google Scholar] [CrossRef]
- Halliwell, B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron chelates: Is it a mechanism for hydroxyl radical production in biochemical systems? FEBS Lett. 1978, 92, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Prasad, A.; Sedlářová, M.; Pospíšil, P. Data on detection of singlet oxygen, hydroxyl radical and organic radical in Arabidopsis thaliana. Data Brief 2018, 21, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ding, S.; Wu, J.; Shi, G.; Zhu, A. In situ detection of hydroxyl radicals in mitochondrial oxidative stress with a nanopipette electrode. Chem. Commun. 2020, 56, 13225–13228. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, A.; Hendrix, S.; Dos Reis, R.A.; De Smet, S.; Deckers, J.; Gielen, H.; Jozefczak, M.; Loix, C.; Vercampt, H.; Vangronsveld, J.; et al. Hydrogen Peroxide, Signaling in Disguise during Metal Phytotoxicity. Front. Plant Sci. 2016, 7, 470. [Google Scholar] [CrossRef] [Green Version]
- Babbs, C.F.; Pham, J.A.; Coolbaugh, R.C. Lethal Hydroxyl Radical Production in Paraquat-Treated Plants. Plant Physiol. 1989, 90, 1267–1270. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Jensen, R.G.; Bohnert, H.J. Mannitol Protects against Oxidation by Hydroxyl Radicals. Plant Physiol. 1997, 115, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.J.; Cai, B.B.; Sun, S.N.; Bi, H.G.; Ai, X.Z. Effect of hydrogen-rich water soaked cucumber seeds on cold tolerance and its physiological mechanism in cucumber seedlings. Sci. Agric. Sin. 2017, 50, 881–889. [Google Scholar]
- Yadav, D.K.; Pospíšil, P. Role of chloride ion in hydroxyl radical production in photosystem II under heat stress: Electron paramagnetic resonance spin-trapping study. J. Bioenerg. Biomembr. 2012, 44, 365–372. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, X.; Lei, D.; Hu, S.; Shen, Z.; Shen, W.; Xu, X. Hydrogen-rich water pretreatment alters photosynthetic gas exchange, chlorophyll fluorescence, and antioxidant activities in heat-stressed cucumber leaves. Plant Growth Regul. 2017, 83, 69–82. [Google Scholar] [CrossRef]
- Lipinski, B. Hydroxyl Radical and Its Scavengers in Health and Disease. Oxidative Med. Cell. Longev. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matros, A.; Peshev, D.; Peukert, M.; Mock, H.-P.; Ende, W.V.D. Sugars as hydroxyl radical scavengers: Proof-of-concept by studying the fate of sucralose in Arabidopsis. Plant J. 2015, 82, 822–839. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; Galisteo, J. Hydroxyl radical reactions and the radical scavenging activity of β-carboline alkaloids. Food Chem. 2015, 172, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Imai, J.; Ito, T.; Takagaki, H.; Ui, M.; Hatta, S. The novel antioxidant TA293 reveals the role of cytoplasmic hydroxyl radicals in oxidative stress-induced senescence and inflammation. Biochem. Biophys. Res. Commun. 2017, 482, 1183–1189. [Google Scholar] [CrossRef]
- Penders, J.; Kissner, R.; Koppenol, W.H. ONOOH does not react with H2: Potential beneficial effects of H2 as an antioxidant by selective reaction with hydroxyl radicals and peroxynitrite. Free Radic. Biol. Med. 2014, 75, 191–194. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, P.; Ross, A.B. Critical-review of rate constants for reactions of hydrated electrons, hydrogen-atoms and hydroxyl radicals (OH/O−) in aqueous-solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Matheson, M.S.; Rabani, J. Pulse radiolysis of aqueous hydrogen solutions. I. Rate constants for reaction of eaq− with itself and other transients. II. The interconvertibility of eaq− and H. J. Phys. Chem. 1965, 69, 1324–1335. [Google Scholar] [CrossRef]
- Wood, K.C.; Gladwin, M.T. The hydrogen highway to reperfusion therapy. Nat. Med. 2007, 13, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Ding, X.-W.; Jiang, R.; Ouyang, P.-L.; Gui, J.; Feng, L.; Yang, L.; Song, L.-H. Effects of hydrogen-rich water on the nutrient composition and antioxidative characteristics of sprouted black barley. Food Chem. 2019, 299, 125095. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, Q.; Wang, Y.; Shen, Z.; Shen, W.; Xueqiang, Z. Hydrogen-rich water induces aluminum tolerance in maize seedlings by enhancing antioxidant capacities and nutrient homeostasis. Ecotoxicol. Environ. Saf. 2017, 144, 369–379. [Google Scholar] [CrossRef]
- Wu, Q.; Su, N.; Cai, J.; Shen, Z.; Cui, J. Hydrogen-rich water enhances cadmium tolerance in Chinese cabbage by reducing cadmium uptake and increasing antioxidant capacities. J. Plant Physiol. 2015, 175, 174–182. [Google Scholar] [CrossRef]
- Kolbert, Z.; Barroso, J.; Brouquisse, R.; Corpas, F.; Gupta, K.; Lindermayr, C.; Loake, G.; Palma, J.; Petřivalský, M.; Wendehenne, D.; et al. A forty year journey: The generation and roles of NO in plants. Nitric Oxide 2019, 93, 53–70. [Google Scholar] [CrossRef] [Green Version]
- Arasimowicz, M.; Floryszak-Wieczorek, J. Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant Sci. 2007, 172, 876–887. [Google Scholar] [CrossRef]
- Schubert, K.R.; Evans, H.J. Hydrogen evolution: A major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc. Natl. Acad. Sci. USA 1976, 73, 1207–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, C.J.; Wheeler, J.A.; Sedlacek, J.; Cortés, A.J.; Rixen, C. Small-scale drivers: The importance of nutrient availability and snowmelt timing on performance of the alpine shrub Salix herbacea. Oecologia 2015, 180, 1015–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedlacek, J.F.; Bossdorf, O.; Cortés, A.J.; Wheeler, J.A.; Van Kleunen, M. What role do plant–soil interactions play in the habitat suitability and potential range expansion of the alpine dwarf shrub Salix herbacea? Basic Appl. Ecol. 2014, 15, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, J.A.; Schnider, F.; Sedlacek, J.; Cortés, A.J.; Wipf, S.; Hoch, G.; Rixen, C. With a little help from my friends: Community facilitation increases performance in the dwarf shrub Salix herbacea. Basic Appl. Ecol. 2015, 16, 202–209. [Google Scholar] [CrossRef]
- Li, C.; Huang, D.; Wang, C.; Wang, N.; Yao, Y.; Li, W.; Liao, W. NO is involved in H2-induced adventitious rooting in cucumber by regulating the expression and interaction of plasma membrane H+-ATPase and 14-3-3. Planta 2020, 252, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liao, W.; Niu, L.; Wang, M.; Ma, Z. Nitric oxide is involved in hydrogen gas-induced cell cycle activation during adventitious root formation in cucumber. BMC Plant Biol. 2016, 16, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Mao, Y.; Zhang, W.; Lai, D.; Wang, Q.; Shen, W. Reactive Oxygen Species-Dependent Nitric Oxide Production Contributes to Hydrogen-Promoted Stomatal Closure in Arabidopsis. Plant Physiol. 2014, 165, 759–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogg, N.; Darley-Usmar, V.M.; Wilson, M.T.; Moncada, S. Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem. J. 1992, 281, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Vandelle, E.; Delledonne, M. Peroxynitrite formation and function in plants. Plant Sci. 2011, 181, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Staszek, P.; Gniazdowska, A. Peroxynitrite induced signaling pathways in plant response to non-proteinogenic amino acids. Planta 2020, 252, 1–11. [Google Scholar] [CrossRef]
- Kolbert, Z.; Feigl, G.; Bordé, Á.; Molnár, Á.; Erdei, L. Protein tyrosine nitration in plants: Present knowledge, computational prediction and future perspectives. Plant Physiol. Biochem. 2017, 113, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, B.; Radi, R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids 2003, 25, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, T.; Kamimura, N.; Yokota, T.; Takai, S.; Ohta, S. Molecular hydrogen protects chondrocytes from oxidative stress and indirectly alters gene expressions through reducing peroxynitrite derived from nitric oxide. Med. Gas Res. 2011, 1, 18. [Google Scholar] [CrossRef] [Green Version]
- Shen, N.Y.; Bi, J.B.; Zhang, J.Y.; Zhang, S.M.; Gu, J.X.; Qu, K.; Liu, C. Hydrogen-rich water protects against inflammatory bowel disease in mice by inhibiting endoplasmic reticulum stress and promoting heme oxygenase-1 expression. World J. Gastroenterol. 2017, 23, 1375. [Google Scholar] [CrossRef]
- Cortés, A.J.; Chavarro, C.M.; Madriñán, S.; This, D.; Blair, M.W. Molecular ecology and selection in the drought-related Asr gene polymorphisms in wild and cultivated common bean (Phaseolus vulgaris L.). BMC Genet. 2012, 13, 58. [Google Scholar] [CrossRef] [Green Version]
- Cortés, A.J.; This, D.; Chavarro, C.; Madriñán, S.; Blair, M.W. Nucleotide diversity patterns at the drought-related DREB2 encoding genes in wild and cultivated common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2012, 125, 1069–1085. [Google Scholar] [CrossRef]
- Blair, M.W.; Cortés, A.J.; This, D. Identification of an ERECTA gene and its drought adaptation associations with wild and cultivated common bean. Plant Sci. 2016, 242, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Cortés, A.J.; Monserrate, F.A.; Ramírez-Villegas, J.; Madriñán, S.; Blair, M.W. Drought Tolerance in Wild Plant Populations: The Case of Common Beans (Phaseolus vulgaris L.). PLoS ONE 2013, 8, e62898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés, A.J.; Blair, M.W. Genotyping by Sequencing and Genome–Environment Associations in Wild Common Bean Predict Widespread Divergent Adaptation to Drought. Front. Plant Sci. 2018, 9, 128. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, J.A.; Cortés, A.J.; Sedlacek, J.; Karrenberg, S.; Van Kleunen, M.; Wipf, S.; Hoch, G.; Bossdorf, O.; Rixen, C. The snow and the willows: Earlier spring snowmelt reduces performance in the low-lying alpine shrub Salix herbacea. J. Ecol. 2016, 104, 1041–1050. [Google Scholar] [CrossRef]
- López-Hernández, F.; Cortés, A.J. Last-Generation Genome–Environment Associations Reveal the Genetic Basis of Heat Tolerance in Common Bean (Phaseolus vulgaris L.). Front. Genet. 2019, 10, 954. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, J.A.; Hoch, G.; Cortés, A.J.; Sedlacek, J.; Wipf, S.; Rixen, C. Increased spring freezing vulnerability for alpine shrubs under early snowmelt. Oecologia 2014, 175, 219–229. [Google Scholar] [CrossRef] [Green Version]
- He, H.; He, L. Heme oxygenase 1 and abiotic stresses in plants. Acta Physiol. Plant. 2013, 36, 581–588. [Google Scholar] [CrossRef]
- Wilks, A. Heme Oxygenase: Evolution, Structure, and Mechanism. Antioxid. Redox Signal. 2002, 4, 603–614. [Google Scholar] [CrossRef]
- Wegiel, B.; Nemeth, Z.; Correa-Costa, M.; Bulmer, A.C.; Otterbein, L.E. Heme Oxygenase-1: A Metabolic Nike. Antioxid. Redox Signal. 2014, 20, 1709–1722. [Google Scholar] [CrossRef] [Green Version]
- Rychlewski, J. Magnetic effects for the hydrogen molecule in excited states: b3Σ+u of H2. Mol. Phys. 1986, 59, 327–336. [Google Scholar] [CrossRef]
- Rychlewski, J. Magnetic effects for the hydrogen molecule in excited states: B1 Σu+ of H2. Phys. Rev. A Gen. Phys. 1985, 31, 2091–2095. [Google Scholar] [CrossRef]
- Steiner, U.E.; Ulrich, T. Magnetic field effects in chemical kinetics and related phenomena. Chem. Rev. 1989, 89, 51–147. [Google Scholar] [CrossRef] [Green Version]
- Buntkowsky, G.; Walaszek, B.; Adamczyk, A.; Xu, Y.; Limbach, H.-H.; Chaudret, B. Mechanism of nuclear spin initiated para-H2 to ortho-H2 conversion. Phys. Chem. Chem. Phys. 2006, 8, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.T.; Hancock, T.H. Hydrogen gas, ROS metabolism, and cell signaling: Are hydrogen spin states important? React. Oxyg. Species 2018, 6, 389–395. [Google Scholar] [CrossRef]
- Marais, A.; Adams, B.; Ringsmuth, A.K.; Ferretti, M.; Gruber, J.M.; Hendrikx, R.; Schuld, M.; Smith, S.L.; Sinayskiy, I.; Krüger, T.P.J.; et al. The future of quantum biology. J. R. Soc. Interface 2018, 15, 20180640. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Bertagna, F.; D’Souza, E.M.; Heyes, D.J.; Johannissen, L.O.; Nery, E.T.; Pantelias, A.; Jimenez, A.S.-P.; Slocombe, L.; Spencer, M.G.; et al. Quantum Biology: An Update and Perspective. Quantum Rep. 2021, 3, 80–126. [Google Scholar] [CrossRef]
- Huang, C.-S.; Kawamura, T.; Toyoda, Y.; Nakao, A. Recent advances in hydrogen research as a therapeutic medical gas. Free. Radic. Res. 2010, 44, 971–982. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Liu, Y.-H.; Wang, S.; Du, H.-M.; Shen, W.-B. Hydrogen agronomy: Research progress and prospects. J. Zhejiang Univ. Sci. B 2020, 21, 841–855. [Google Scholar] [CrossRef]
- Chuai, Y.; Gao, F.; Li, B.; Zhao, L.; Qian, L.; Cao, F.; Wang, L.; Sun, X.; Cui, J.; Cai, J. Hydrogen-rich saline attenuates radiation-induced male germ cell loss in mice through reducing hydroxyl radicals. Biochem. J. 2012, 442, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Gutteridge, J.M.; Aruoma, O.I. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1987, 165, 215–219. [Google Scholar] [CrossRef]
- Xu, G.; Chance, M.R. Hydroxyl Radical-Mediated Modification of Proteins as Probes for Structural Proteomics. Chem. Rev. 2007, 107, 3514–3543. [Google Scholar] [CrossRef]
- El-Bahr, S.M. Biochemistry of Free Radicals and Oxidative Stress. Sci. Int. 2013, 1, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Tejero, I.; González-Lafont, À.; Lluch, J.M.; Eriksson, L.A. Theoretical Modeling of Hydroxyl-Radical-Induced Lipid Peroxidation Reactions. J. Phys. Chem. B 2007, 111, 5684–5693. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, B.C.; King, D.M.; Thomas, C. The oxidation of some polysaccharides by the hydroxyl radical: An e.s.r. investigation. Carbohydr. Res. 1984, 125, 217–235. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Jaruga, P. Mechanisms of free radical-induced damage to DNA. Free Radic. Res. 2012, 46, 382–419. [Google Scholar] [CrossRef] [PubMed]
- Gansäuer, A.; Otte, M.; Piestert, F.; Fan, C.-A. Sustainable radical reduction through catalyzed hydrogen atom transfer reactions (CHAT-reactions). Tetrahedron 2009, 65, 4984–4991. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hancock, J.T.; Russell, G. Downstream Signalling from Molecular Hydrogen. Plants 2021, 10, 367. https://doi.org/10.3390/plants10020367
Hancock JT, Russell G. Downstream Signalling from Molecular Hydrogen. Plants. 2021; 10(2):367. https://doi.org/10.3390/plants10020367
Chicago/Turabian StyleHancock, John T., and Grace Russell. 2021. "Downstream Signalling from Molecular Hydrogen" Plants 10, no. 2: 367. https://doi.org/10.3390/plants10020367
APA StyleHancock, J. T., & Russell, G. (2021). Downstream Signalling from Molecular Hydrogen. Plants, 10(2), 367. https://doi.org/10.3390/plants10020367