Biofortification of Silage Maize with Zinc, Iron and Selenium as Affected by Nitrogen Fertilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Greenhouse Experiment
2.2. Plant Sampling and Chemical Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cakmak, I.; Kutman, U. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil. Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Bouis, H.E.; Hotz, C.; McClafferty, B.; Meenakshi, J.; Pfeiffer, W.H. Biofortification: A new tool to reduce micronutrient malnutrition. Food Nutr. Bull. 2011, 32, S31–S40. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.A.; Gregory, P.J. Soil, food security and human health: A review. Eur. J. Soil. Sci. 2015, 66, 257–276. [Google Scholar] [CrossRef]
- Paul, S.; Dey, A. Nutrition in health and immune function of ruminants. Indian J. Anim. Sci. 2015, 85, 103–112. [Google Scholar]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil. 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Welch, R.M.; Graham, R.D.; Cakmak, I. Linking agricultural production practices to improving human nutrition and health. In Proceedings of the ICN2 Second International Conference on Nutrition Preparatory Technical Meeting, Rome, Italy, 13–15 November 2013. [Google Scholar]
- Hill, G.M.; Shannon, M.C. Copper and Zinc Nutritional Issues for Agricultural Animal Production. Biol. Trace Elemres 2019, 188, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Schiavon, M.; Nardi, S.; dalla Vecchia, F.; Ertani, A. Selenium biofortification in the 21st century: Status and challenges for healthy human nutrition. Plant Soil. 2020, 453, 245–270. [Google Scholar] [CrossRef]
- Dalgaard, T.S.; Briens, M.; Engberg, R.M.; Lauridsen, C. The influence of selenium and selenoproteins on immune responses of poultry and pigs. Anim. Feed Sci. Technol. 2018, 238, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Lee, J.; Wu, C.; Guo, X.; Lee, B.J.; Chun, J.S.; Kim, J.H. The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp. Mol. Med. 2020, 52, 1198–1208. [Google Scholar] [CrossRef]
- Welch, R.M.; Graham, R.D. A new paradigm for world agriculture: Meeting human needs and productive, sustainable, nutritious. Field Crops Res. 1999, 60, 1–10. [Google Scholar] [CrossRef]
- Pingali, P.; Mittra, B.; Rahman, A. The bumpy road from food to nutrition security—Slow evolution of India’s food policy. Glob. Food Sec. 2017, 15, 77–84. [Google Scholar] [CrossRef]
- Rengel, Z.; Römheld, V.; Marschner, H. Uptake of zinc and iron by wheat genotypes differing in tolerance to zinc deficiency. J. Plant Physiol. 1998, 152, 433–438. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Manojlović, M.; Singh, B.R. Trace elements in soils and food chains of the Balkan region. Acta Agric. Scand. B Soil. Plant Sci. 2012, 62, 673–695. [Google Scholar] [CrossRef]
- Kincaid, R.L. Assessment of trace mineral status of ruminants: A review. J. Anim. Sci. 2000, 77, 1–10. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Zinc through the three domains of life. J. Proteome Res. 2006, 5, 31–73. [Google Scholar] [CrossRef]
- Kluska, K.; Adamczyk, J.; Krężel, A. Metal binding properties, stability and reactivity of zinc fingers Coordination. Chem. Rev. 2018, 367, 18–64. [Google Scholar]
- McDonald, P.; Edwards, R.; Greenhalgh, J.; Morgan, C.; Sinclair, L.A.; Wilkinson, R.G. Animal Nutrition, 7th ed.; Prentice-Hall: London, UK, 2007; Volume 11, pp. 264–271. [Google Scholar]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Ademi, A.; Govasmark, E.; Bernhoft, A.; Bytyqi, H.; Djikic, M.; Manojlović, M.; Loncaric, Z.; Drinic, M.; Filipovic, A.; Singh, B.R. Status of selenium in sheep and dairy cow blood in Western Balkan countries. Acta Agric. Scand. A Anim. Sci. 2015, 65, 9–16. [Google Scholar] [CrossRef]
- Shiferaw, B.; Prasanna, B.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Sec. 2011, 3, 307–327. [Google Scholar] [CrossRef] [Green Version]
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 13121, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Micronutrients and Crop Production: An Introduction. In Micronutrient Deficiencies in Global Crop Production; Springer: Dordrecht, The Netherlands, 2008; pp. 1–39. [Google Scholar]
- Eurostat. Available online: https://ec.europa.eu/eurostat (accessed on 20 January 2021).
- La Frano, M.R.; de Moura, F.F.; Boy, E.; Lönnerdal, B.; Burri, B.J. Bioavailability of iron, zinc, and provitamin A carotenoids in biofortified staple crops. Nutr. Rev. 2014, 72, 289–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grujcic, D.; Drinic, M.; Zivanovic, I.; Cakmak, I.; Singh, B.R. Micronutrient availability in soils of Northwest Bosnia and Herzegovina in relation to silage maize production. Acta Agric. Scand. B Soil. Plant Sci. 2018, 68, 301–310. [Google Scholar] [CrossRef]
- Subedi, K.D.; Ma, B.L. Assessment of some major yield-limiting factors on maize production in a humid temperate environment. Field Crops Res. 2009, 110, 21–26. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, X.; Li, H.; Li, H.; Cheng, L.; Zhang, F.; Rengel, Z.; Shen, J. Localized application of NH4+-N plus P enhances zinc and iron accumulation in maize via modifying root traits and rhizosphere processes. Field Crops Res. 2014, 164, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Mortvedt, J.J. Crop response to level of water-soluble zinc in granular zinc fertilizers. Fertil. Res. 1992, 33, 249–255. [Google Scholar] [CrossRef]
- Gangloff, W.J.; Westfall, D.G.; Peterson, G.A.; Mortvedt, J.J. Relative availability coefficients of organic and inorganic fertilizers. J. Plant Nutr. 2002, 25, 259–273. [Google Scholar] [CrossRef]
- Aciksoz, S.B.; Yazici, A.; Ozturk, L.; Cakmak, I. Biofortification of wheat with iron through soil and foliar application of nitrogen and iron fertilizers. Plant Soil. 2011, 349, 215–225. [Google Scholar] [CrossRef]
- Zou, C.Q.; Zhang, Y.Q.; Rashid, A.; Ram, H.; Savasli, E.; Arisoy, R.Z.; Ortiz-Monasterio, I.; Simunji, S.; Wang, Z.H.; Sohu, V. Biofortification of wheat with zinc through zinc fertilization in seven countries. Plant Soil. 2012, 361, 119–130. [Google Scholar] [CrossRef]
- Phattarakul, N.; Rerkasem, B.; Li, L.J.; Wu, L.H.; Zou, C.Q.; Ram, H.; Sohu, V.S.; Kang, B.S.; Surek, H.; Kalayci, M. Biofortification of rice grain with zinc through zinc fertilization in different countries. Plant Soil. 2012, 361, 131–141. [Google Scholar] [CrossRef]
- Zou, C.; Du, Y.; Rashid, A.; Ram, H.; Savasli, E.; Pieterse, P.J.; Ortiz Monasterio, I.; Yazici, A.; Kaur, C.; Mahmood, K.; et al. Simultaneous biofortification of wheat with zinc, iodine, selenium, and iron through foliar treatment of a micronutrient cocktail in six countries. J. Agric. Food Chem. 2019, 67, 8096–8106. [Google Scholar] [CrossRef] [Green Version]
- Prom-u-thai, C.; Rashid, A.; Ram, H.; Zou, C.; Guilherme, L.; Roberto, G.; Corguinha, A.P.B.; Guo, S.; Kaur, C.; Naeem, A.; et al. Simultaneous Biofortification of Rice With Zinc, Iodine, Iron and Selenium Through Foliar Treatment of a Micronutrient Cocktail in Five Countries. Front Plant Sci. 2020, 11, 15–16. [Google Scholar] [CrossRef]
- Cakmak, I.; Pfeiffer, W.H.; McClafferty, B. Biofortification of durum wheat with zinc and iron. Cereal Chem. 2010, 87, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Kutman, U.B.; Yildiz, B.; Ozturk, L.; Cakmak, I. Biofortification of durum wheat with zinc through soil and foliar applications of nitrogen. Cereal Chem. 2010, 87, 1–9. [Google Scholar] [CrossRef]
- Erenoglu, E.B.; Kutman, U.B.; Ceylan, Y.; Yildiz, B.; Cakmak, I. Improved nitrogen nutrition enhances root uptake, root-to-shoot translocation and remobilization of zinc (65Zn) in wheat. New Phytol. 2011, 189, 438–448. [Google Scholar] [CrossRef]
- LeBlanc, P.V.; Gupta, U.C.; Christie, B.R. Zinc nutrition of silage corn grown on acid podzols. J. Plant Nutr. 1997, 20, 345–353. [Google Scholar] [CrossRef]
- Losak, T.; Hlusek, J.; Martinec, J.; Jandak, J.; Szostkova, M.; Filipcik, R.; Manasek, J.; Prokes, K.; Peterka, J.; Varga, L. Nitrogen fertilization does not affect micronutrient uptake in grain maize (Zea mays L.). Acta Agric. Scand. B Soil. Plant Sci. 2011, 61, 543–550. [Google Scholar]
- Wang, J.; Mao, H.; Zhao, H.; Huang, D.; Wang, Z. Different increases in maize and wheat grain zinc concentrations caused by soil and foliar applications of zinc in Loess Plateau, China. Field Crops Res. 2012, 135, 89–96. [Google Scholar] [CrossRef]
- Xue, Y.; Yue, S.; Zhang, W.; Liu, D.; Cui, Z.; Chen, X.; Ye, Y.; Zou, C. Zinc, iron, manganese and copper uptake requirement in response to nitrogen supply and the increased grain yield of summer maize. PLoS ONE. 2014, 9, e93895. [Google Scholar] [CrossRef]
- Randjelovic, V.; Prodanovic, S.; Tomic, Z.; Simic, A. Genotype x year effect on grain yield and nutritive values of maize (Zea mays L.). J. Anim. Vet. Adv. 2011, 10, 835–840. [Google Scholar]
- Amrani, M.; Westfall, D.G.; Peterson, G.A. Influence of water solubility of granular zinc fertilizers on plant uptake and growth. J. Plant Nutr. 1999, 22, 1815–1827. [Google Scholar] [CrossRef]
- Shaver, T.M.; Westfall, D.G.; Ronaghi, M. Zinc fertilizer solubility and its effects on zinc bioavailability over time. J. Plant Nutr. 2007, 30, 123–133. [Google Scholar] [CrossRef]
- ZINC. Available online: https://crops.zinc.org/ (accessed on 18 January 2021).
- Degryse, F.; da Silva, R.C.; Baird, R.; Cakmak, I.; Yazici, M.A.; McLaughlin, M.J. Comparison and modelling of extraction methods to assess agronomic effectiveness of fertilizer zinc. J. Plant Nutr. Soil Sci. 2020, 183, 248–259. [Google Scholar] [CrossRef] [Green Version]
- Monreal, C.M.; DeRosa, M.; Mallubhotla, S.C.; Bindraban, P.S.; Dimkpa, C. Nanotechnologies for increasing the crop use efficiency of fertilizer-micronutrients. Biol. Fertil. Soils 2016, 52, 423–437. [Google Scholar] [CrossRef]
- Kopittke, P.; Lombi, E.; Wang, P.; Schjørring, J.K.; Husted, S. Nanomaterials as fertilizers for improving plant mineral nutrition and environmental outcomes. Environ. Sci. Nano 2019, 6, 3513–3524. [Google Scholar] [CrossRef]
- Milani, N.; McLaughlin, M.J.; Stacey, S.P.; Kirby, J.K.; Hettiarachchi, G.M.; Beak, D.G.; Cornelis, G. Dissolution kinetics of macronutrient fertilizers coated with manufactured zinc oxide nanoparticles. J. Agric. Food Chem. 2012, 60, 3991–3998. [Google Scholar] [CrossRef]
- Cakmak, I.; Kalayci, M.; Kaya, Y.; Torun, A.A.; Aydin, N.; Wang, Y.; Arisoy, Z.; Erdem, H.; Yazici, A.; Gokmen, O.; et al. Biofortification and localization of zinc in wheat grain. J. Agric. Food Chem. 2010, 58, 9092–9102. [Google Scholar] [CrossRef] [PubMed]
- Kutman, U.B.; Yildiz, B.; Cakmak, I. Effect of nitrogen on uptake, remobilization and partitioning of zinc and iron throughout the development of durum wheat. Plant Soil. 2011, 342, 149–164. [Google Scholar] [CrossRef]
- Aciksoz, S.B.; Ozturk, L.; Gokmen, O.O.; Romheld, V.; Cakmak, I. Effect of nitrogen on root release of phytosiderophores and root uptake of Fe(III)- phytosiderophore in Fe-deficient wheat plants. Physiol. Plant 2011, 142, 287–296. [Google Scholar] [CrossRef]
- Römheld, V.; Marschner, H. Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol. 1986, 80, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Tsukamoto, T.; Inoue, H.; Watanabe, S.; Matsuhashi, S.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Deoxymugineic acid increases Zn translocation in Zn-deficient rice plants. Plant Mol. Biol. 2008, 66, 609–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, H.; Suzuki, M.; Morikawa, K.C.; Kobayashi, T.; Nakanishi, H.; Takahashi, M.; Saigusa, M.; Mori, S.; Nishizawa, N.K. Increase in iron and zinc concentrations in rice grains via the introduction of barley genes involved in phytosiderophore synthesis. Rice 2008, 1, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Banuelos, G.S.; Min, J.; Shi, W. Effect of continuous application of inorganic nitrogen fertilizer on selenium concentration in vegetables grown in the Taihu Lake region of China. Plant Soil. 2015, 393, 351–360. [Google Scholar] [CrossRef]
- Chilimba, A.D.C.; Young, S.D.; Black, C.R.; Meacham, M.C.; Lammel, J.; Broadley, M.R. Agronomic biofortification of maize with selenium (Se) in Malawi. Field Crop Res. 2012, 125, 118–128. [Google Scholar] [CrossRef]
- Mao, H.; Wang, J.; Zan, Y.; Lyons, G.; Zou, C. Using agronomic biofortification to boost zinc, selenium, and iodine concentrations of food crops grown on the loess plateau in China. J. Soil. Sci. Plant Nutr. 2014, 14, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Ngigi, P.B.; Lachat, C.; Masinde, P.W.; Du Laing, G. Agronomic biofortification of maize and beans in Kenya through selenium fertilization. Environ. Geochem. Health 2019, 41, 2577–2591. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, S.J.; Laven, R.A. Selenium requirements in grazing dairy cows: A review. N. Z. Vetj. 2020, 68, 13–22. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Dairy Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2001; p. 405. [Google Scholar]

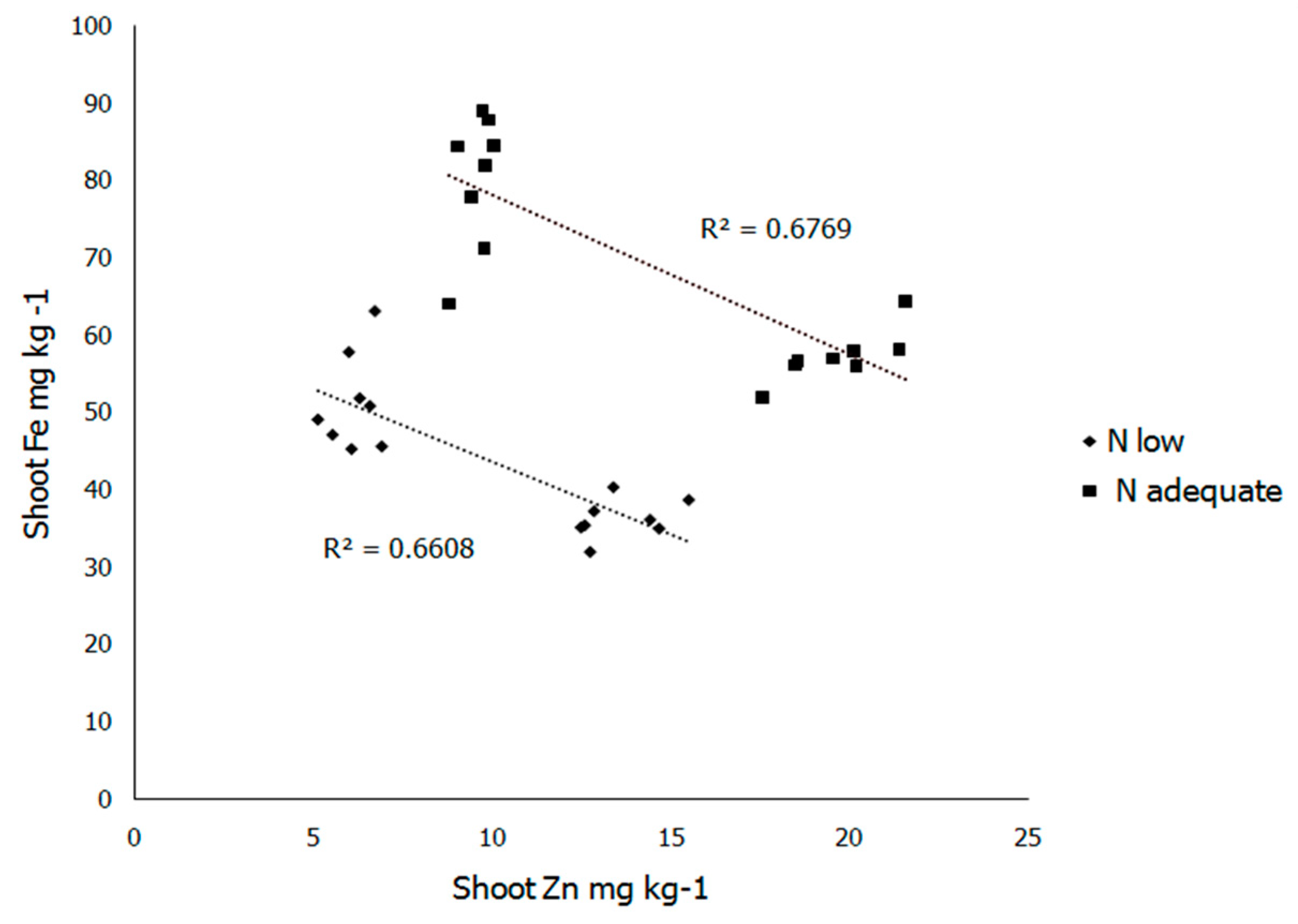
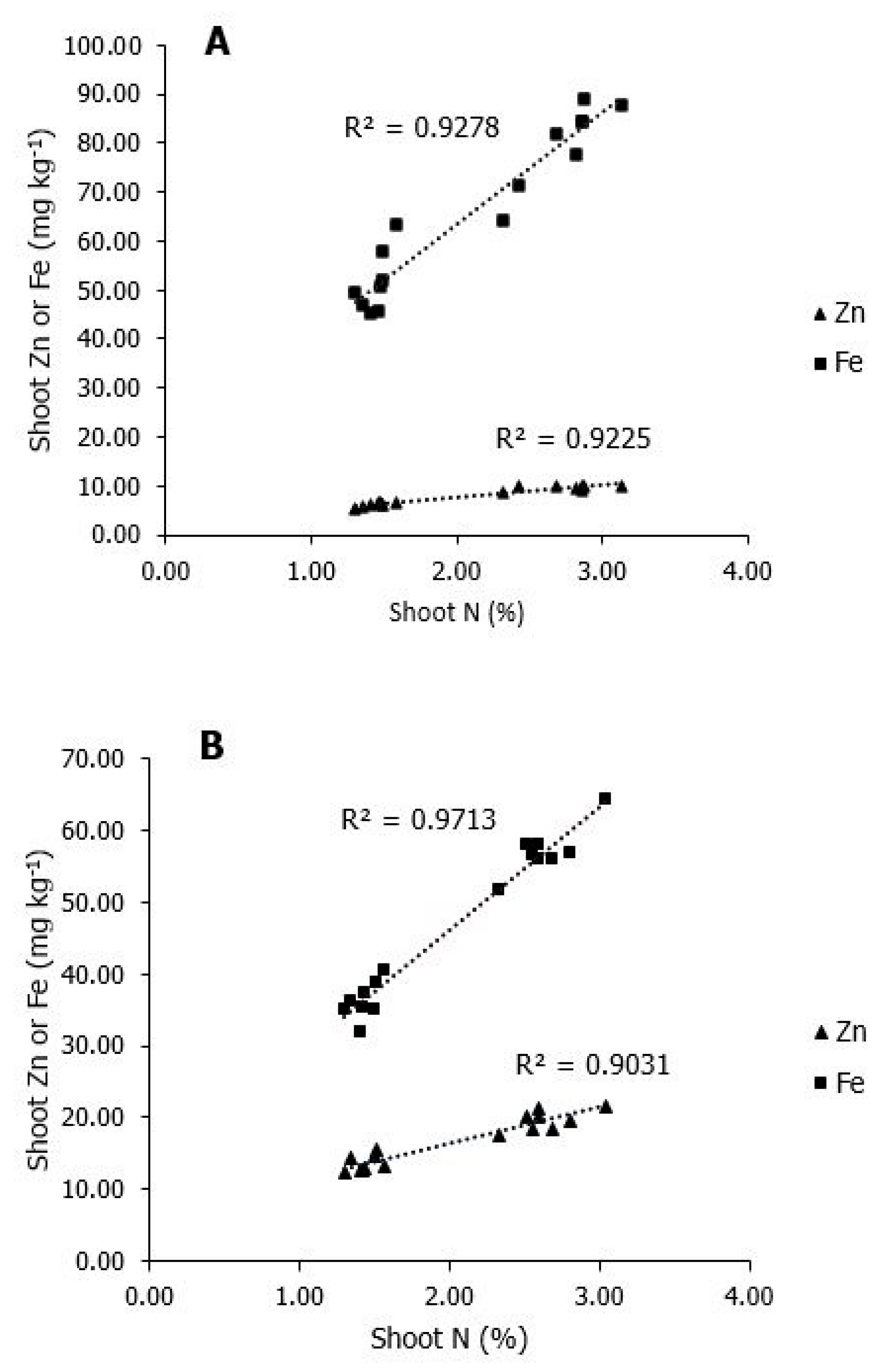

| Source of Variation (Treatments) | df | Shoot Dry Matter | Shoot Zn Concentration | Shoot Zn Uptake | Shoot Fe Concentration | Shoot Fe Uptake | Shoot Se Concentration | Shoot Se Uptake | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | F Pr | SS | F Pr | SS | F Pr | SS | F Pr | SS | F Pr | SS | F Pr | SS | F Pr | ||
| Soil N (A) | 1 | 0.047 | 0.461 | 332.3 | <0.001 | 3802.4 | <0.001 | 6088.3 | <0.001 | 67,088 | <0.001 | 0.0463 | 0.586 | 0.221 | 0.703 |
| Soil Zn (B) | 1 | 1.981 | <0.001 | 1539.2 | <0.001 | 19,448.5 | <0.001 | 1838.8 | <0.001 | 6056 | 0.039 | 0.0019 | 0.911 | 0.192 | 0.722 |
| A × B | 1 | 0.058 | 0.749 | 34.2 | 0.172 | 564.7 | 0.093 | 41.5 | 0.576 | 32 | 0.878 | 0.0299 | 0.661 | 0.342 | 0.635 |
| Experimental error | 44 | 3.756 | 783.6 | 8472.5 | 5752.5 | 59400 | 6.7965 | 66.2 | |||||||
| N Treatment a | Shoot Dry Matter (g plant−1) b,c | |
|---|---|---|
| Low Zn | Adequate Zn | |
| Low | 2.99 ± 0.28 Aa | 3.27 ± 0.29 Ab |
| Adequate | 3.02 ± 0.12 Aa | 3.37 ± 0.41 Ab |
| Micronutrient | Zn Treatment a | Shoot Concentration c,e | Shoot Uptake d,e | ||
|---|---|---|---|---|---|
| Low N b | Adequate N b | Low N b | Adequate N b | ||
| Zn | Low | 7.2 ± 0.5 Aa | 10.8 ± 0.5 Ab | 21.4 ± 2.3 Aa | 32.5 ± 2.4 Ab |
| Adequate | 16.8 ± 1.1 Ba | 23.8 ± 1.2 Bb | 54.9 ± 4.3 Ba | 80.1 ± 11.8 Bb | |
| Fe | Low | 51.4 ± 5.5 Aa | 80.0 ± 7.3 Ab | 153.5 ± 16.1 Aa | 241.6 ± 25.3 Ab |
| Adequate | 36.3 ± 2.5 Ba | 57.2 ± 3.0 Bb | 118.3 ± 11.8 Ba | 192.8 ± 27.1 Bb | |
| Se | Low | 0.83 ± 0.06 Aa | 0.80 ± 0.12 Aa | 2.4 ± 0.30 Aa | 2.5 ± 0.32 Aa |
| Adequate | 0.88 ± 0.03 Aa | 0.71 ± 0.07 Aa | 2.8 ± 0.25 Aa | 2.4 ± 0.37 Aa | |
| Micronutrient | Micronutrient Treatment a | Shoot Concentration c,e | Shoot Uptake d,e | ||
|---|---|---|---|---|---|
| Low N b | Adequate N b | Low N b | Adequate N b | ||
| Fe | No treatment | 29.2 ± 2.8 Aa | 47.2 ± 3.7 Ab | 92.3 ± 4.4 Aa | 147.4 ± 17.9 Ab |
| Adequate | 42.6 ± 2.5 Ba | 71.0 ± 2.9 Bb | 137.7 ± 9.6 Ba | 222.3 ± 25.6 Bb | |
| Se | No treatment | 0.04 ± 0.01 Aa | 0.05 ± 0.01 Aa | 0.1 ± 0.0 Aa | 0.2 ± 0.0 Aa |
| Adequate | 0.85 ± 0.03 Ba | 0.88 ± 0.08 Ba | 2.7 ± 0.2 Ba | 2.8 ± 0.4 Ba | |
| Zn Source | Zn Supply mg/kg | Dry Matter (g plant−1) | Shoot Zn (mg kg−1) | Shoot Zn Uptake (µg plant−1) |
|---|---|---|---|---|
| Control | No Zn | 0.60 ± 0.02 | 5.65 ± 0.10 | 3.4 ± 0.1 |
| ZnO | 0.5 | 0.98 ± 0.11 | 5.01 ± 0.65 | 4.9 ± 1.1 |
| 1 | 1.28 ± 0.32 | 6.32 ± 0.29 | 8.1 ± 1.9 | |
| 2.5 | 2.52 ± 1.35 | 8.09 ± 0.36 | 20.7 ± 11.9 | |
| 7.5 | 4.64 ± 1.97 | 9.24 ± 1.10 | 44.5 ± 23.3 | |
| ZnSO4 | 0.5 | 2.21 ± 0.48 | 6.88 ± 0.77 | 15.3 ± 4.3 |
| 1 | 3.97 ± 0.60 | 7.24 ± 0.54 | 28.7 ± 4.6 | |
| 2.5 | 6.16 ± 0.24 | 8.36 ± 0.27 | 51.5 ± 2.7 | |
| 7.5 | 7.43 ± 0.30 | 13.55 ± 1.32 | 99.6 ± 8.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grujcic, D.; Yazici, A.M.; Tutus, Y.; Cakmak, I.; Singh, B.R. Biofortification of Silage Maize with Zinc, Iron and Selenium as Affected by Nitrogen Fertilization. Plants 2021, 10, 391. https://doi.org/10.3390/plants10020391
Grujcic D, Yazici AM, Tutus Y, Cakmak I, Singh BR. Biofortification of Silage Maize with Zinc, Iron and Selenium as Affected by Nitrogen Fertilization. Plants. 2021; 10(2):391. https://doi.org/10.3390/plants10020391
Chicago/Turabian StyleGrujcic, Djordje, Atilla Mustafa Yazici, Yusuf Tutus, Ismail Cakmak, and Bal Ram Singh. 2021. "Biofortification of Silage Maize with Zinc, Iron and Selenium as Affected by Nitrogen Fertilization" Plants 10, no. 2: 391. https://doi.org/10.3390/plants10020391







