How to Protect Natural Habitats of Rare Terrestrial Orchids Effectively: A Comparative Case Study of Cypripedium calceolus in Different Geographical Regions of Europe
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Conservation Status and Threats that Cypripedium calceolus Faces in Europe
2.3. Effectiveness of the Natura 2000 Network in Conserving Cypripedium calceolus
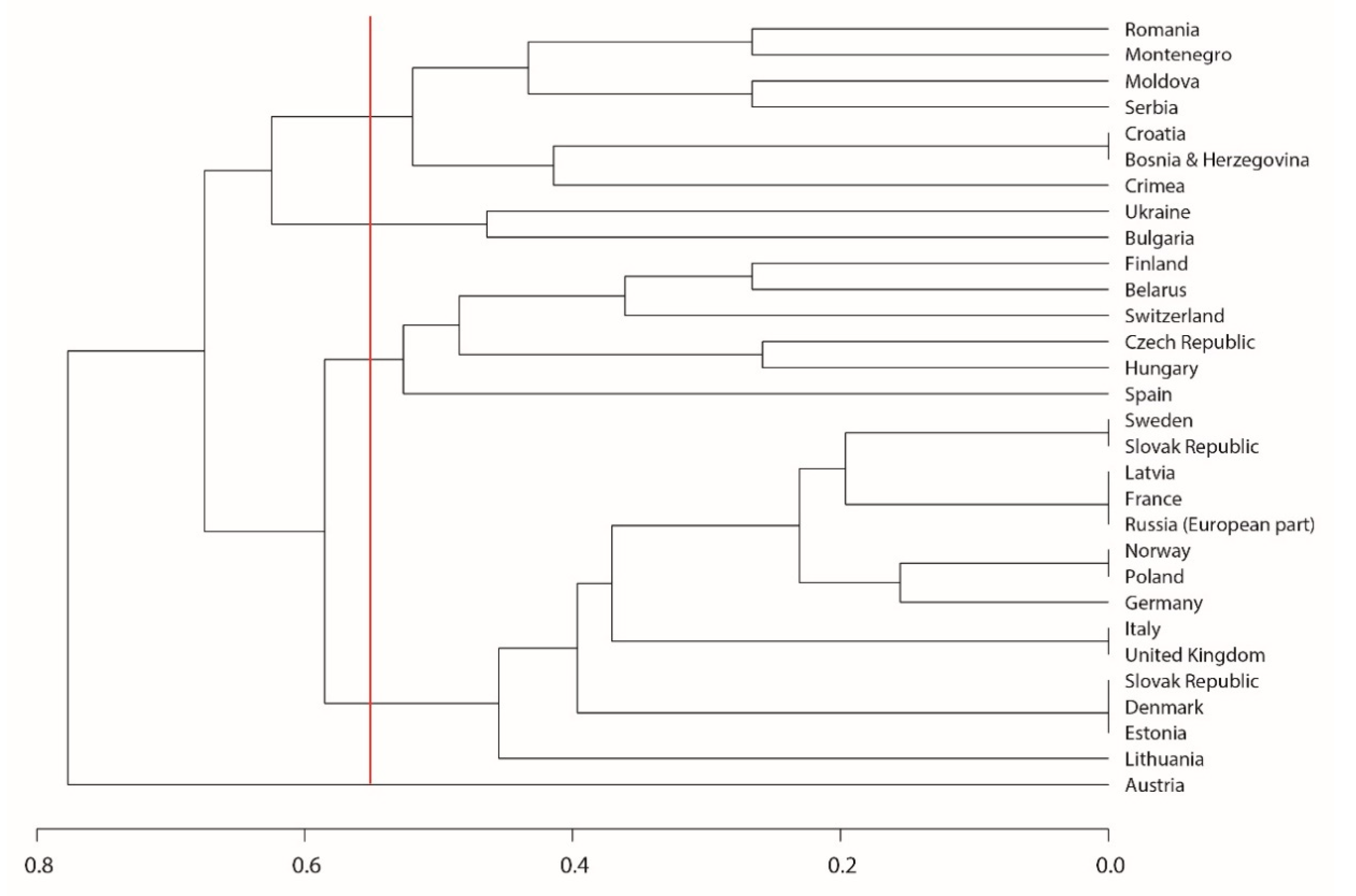
3. Results
4. Discussion
4.1. Conservation Status
4.2. Threats
4.3. Conservation Strategy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Bulgaria
Appendix A.2. Crimea
Appendix A.3. Croatia
Appendix A.4. Czech Republic
Appendix A.5. Germany
Appendix A.6. Poland
Appendix A.7. Romania
Appendix A.8. Russian Federation
Appendix A.9. Serbia and Montenegro
Appendix A.10. Slovenia
Appendix B
| Country | IUCN category | IUCN Criteria | References |
|---|---|---|---|
| Greece | EX | - | [92,93] |
| Bulgaria | CR | B1ab(v)c+2ab(v); C1+2a(i,ii); D | [94] |
| Serbia | CR | B2dC2b | [89] |
| Croatia | EN | A4a, A4d | [24,61,63] |
| Bosnia and Herzegovina | CR | Not evaluated | [95] |
| Montenegro | EN | D | [96] |
| Romania | VU | B2ab(i,ii,iii) | [97,98,99] |
| Poland | VU | B2ab(i, ii, iii, iv) | [14,26] |
| France | VU | A4acd | [100,101] |
| Italy | LC | - | [102,103,104] |
| Estonia | NT | Not evaluated | [105] |
| UK | CR | D | [106,107] |
| Spain | EN | C1 | [108,109] |
| Russia (only for C. calceolus) | LC | - | [110] |
| Crimea | CR | Evaluation B1ab(i,ii,iv,v) + 2ab(i,ii,iv,v); C2a(i,ii); D. | [111,112] |
| Germany | VU | Evaluation A2a+B2ab(i, ii, iii, iv) | [113] |
| Czech Republic | VU | C1 | [114,115,116] |
| Slovak Republic | NT | - | [117,118] |
| Belgium | EX | - | [6] |
| Luxemburg | EX | - | [6] |
| Ukraine | EN | Not estimated | [10,28,29] |
| Switzerland | VU | B2ab(iii) | [119] |
| Austria | NT | - | [120] |
| Slovenia | VU | Not estimated | [10,121,122] |
| Hungary | EN | Not estimated | [123,124] |
| Moldova | CR | D- | [10,125] |
| Belarus | VU | Not estimated | [10] |
| Lithuania | VU | Not estimated | [10,126] |
| Norway | NT | - | [127] |
| Latvia | VU | Not estimated | [128,129] |
| Sweden | LC | - | [130] |
| Finland | NT | A2ab, B2b(iii) | [131] |
| Denmark | VU | D2 | [132,133] |
| Liechtenstein | EX | - | [10] |
| The Netherlands | EX | - | [8] |
References
- Sundseth, K.; Creed, P. Natura 2000: Protecting Europe’s Biodiversity. Office for Official Publications of the European Communities; European Commission, Directorate General for the Environment. Information Press: Oxford, UK, 2008; p. 296. [Google Scholar]
- Del Prete, C.; Mazzola, P. Endemism and speciation in the orchids of Mediterranean Islands. Ecol. Mediterr. 1995, 21, 119–134. [Google Scholar] [CrossRef]
- Bilz, M.; Kell, S.P.; Maxted, N.; Lansdown, R.V. European Red List of Vascular Plants; Publications Office of the European Union: Luxembourg, 2011; p. 144. [Google Scholar]
- Hultén, E.; Fries, M. Atlas of North European Vascular Plants: North of the Tropic of Cancer I-III; Koeltz Scientific Books: Oberreifenberg, Germany, 1986; p. 1172. [Google Scholar]
- Averyanov, L.V. The genus Cypripedium (Orchidaceae) in Russia. Lindleyana 2000, 15, 197–221. [Google Scholar]
- Kreutz, C.A.J. The Orchids of Europe, North Africa and the Near East. A Fieldguide; Kreutz Publishers: Landgraaf, The Netherlands, 2021. [Google Scholar]
- Nemer, W.; Rebbas, K.; Krouchi, F. Découverte de Cypripedium calceolus (Orchidaceae) au Djurdjura (Algérie), nouvelle pour l’Afrique du Nord. Fl. Medit. 2019, 29, 207–214. [Google Scholar] [CrossRef]
- Terschuren, J. Action Plan for Cypripedium calceolus in Europe; Council of Europe Publications: Strasbourg, Germany, 1999; p. 27, Nature and environment, No 100. [Google Scholar]
- Rankou, H.; Bilz, M. The IUCN Red List of Threatened Species. Available online: http://dx.doi.org/10.2305/IUCN.UK.2014-1.RLTS.T162021A43316125.en. (accessed on 15 February 2021).
- Kull, T.; Selgis, U.; Pecinã, M.V.; Metsare, M.; Ilves, A.; Tali, K.; Sepp, K.; Kull, K.; Shefferson, R.P. Factors influencing IUCN threat levels to orchids across Europe on the basis of national red lists. Ecol. Evol. 2016, 6, 6245–6265. [Google Scholar] [CrossRef]
- Govaerts, R.; Dransfield, J.; Zona, S.F.; Hodel, D.R.; Henderson, A. World Checklist of Selected Plant Families. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet. Available online: http://wcsp.science.kew.org/ (accessed on 3 December 2020).
- Kreutz, C.A.J.; Fateryga, A.V.; Ivanov, S.P. Orchids of the Crimea; Kreutz Publishers: Landgraaf, The Netherlands, 2018; p. 576. [Google Scholar]
- Nicolè, F.; Brzosko, E.; Till-Botraud, I. Population viability analysis of Cypripedium calceolus in a protected area: Longevity, stability and persistence. J. Ecol. 2005, 93, 716–726. [Google Scholar] [CrossRef]
- Kucharczyk, M. Cypripedium calceolus L.; Obuwik pospolity. In Gatunki Roślin. Poradniki Ochrony Siedlisk i Gatunków Natura 2000-podręcznik Metodyczny; Sudnik-Wojciechowska, B., Werblan-Jakubiec, H., Eds.; Tom 9; Ministerstwo Środowiska: Warszawa, Poland, 2004; pp. 107–111. [Google Scholar]
- Kolanowska, M.; Jakubska-Busse, A. Is the lady’s-slipper orchid (Cypripedium calceolus) likely to shortly become extinct in Europe? -Insights based on ecological niche modelling. PLoS ONE 2020, 15, e0228420. [Google Scholar] [CrossRef]
- Walther, G.R. Plants in a warmer world. Perspect. Plant Ecol. Evol. Syst. 2004, 6, 169–185. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.-C.; Marquet, P.A.; de Ruffay, P. A Significant Upward Shift in Plant Species Optimum Elevation During the 20th Century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef]
- Hoffmann, S.; Irl, S.D.H.; Beierkuhnlein, C. Predicted climate shifts within terrestrial protected areas worldwide. Nat. Commun. 2019, 10, 4787. Available online: https://www.nature.com/articles/s41467-019-12603-w (accessed on 18 January 2021). [CrossRef]
- Araújo, M.B.; Alagador, D.; Cabeza, M.; Nogués-Bravo, D.; Thuiller, W. Climate change threatens European conservation areas. Ecol. Lett. 2011, 14, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Likens, G.E.; Lindenmayer, D.B. Integrating approaches leads to more effective conservation of biodiversity. Biodivers. Conserv. 2012, 21, 3323–3341. [Google Scholar] [CrossRef]
- Kull, T. Fruit-set and recruitment in populations of Cypripedium calceolus L. in Estonia. Bot. J. Linn. Soc. 1998, 126, 27–38. [Google Scholar] [CrossRef]
- Kull, T. Cypripedium calceolus L. J. Ecol. 1999, 87, 913–924. [Google Scholar] [CrossRef]
- Delforge, P. Orchids of Europe, North Africa and the Middle East; Timber Press: Portland, Oregon, 2006; p. 640. [Google Scholar]
- Justić, M.; Bučar, M.; Vizec, P.; Vukres, A.; Šegota, V.; Vuković, N. The rare and endangered orchid Cypripedium calceolus L. in Croatia-refound in Gorski Kotar (West Croatia) after 126 years. Nat. Croat. 2020, 29, 55–62. [Google Scholar] [CrossRef]
- Baumann, H.; Künkele, S.; Lorenz, R. Die Orchideen Europas. Mit angrenzenden Gebieten; Eugen Ulmer KG: Stuttgart, Germany, 2006; p. 336. [Google Scholar]
- Kaźmierczakowa, R. Polish Red List of Pteridophytes and Flowering Plants; Institute of Nature Conservation Polish Academy of Sciences: Krakow, Poland, 2016; 44p. [Google Scholar]
- IUCN Species Survival Commmission (SSC). IUCN Red list Categories and Criteria, Version 3.1 IUCN Species Survival Commission; IUCN: Gland, Switzerland; Cambridge, UK, 2001. [Google Scholar]
- Melnyk, V.I.; Shynder, O.I.; Nesyn, J.D. Distribution of Cypripedium calceolus (Orchidaceae) in Ukraine. Ukraiskij Bot. J. 2018, 75, 20–32. [Google Scholar] [CrossRef]
- Melnyk, V.I.; Shynder, O.I.; Nesyn, J.D. Habitats and current state of populations of Cypripedium calceolus (Orchidaceae) in Ukraine. IBID 2018, 75, 160–168. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kidt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5–6. 2019. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 18 January 2021).
- Phillips, S.J.; Dudík, M.; Schapire, R.E. A maximum entropy approach to species distribution modeling. In ICML ‘04. In Proceedings of the Twenty-First International Conference on Machine Learning, New York, NY, USA, 4–8 July 2004; pp. 655–662. [Google Scholar]
- Phillips, S.J.; Anderson, R.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Szczęśniak, E.; Jakubska-Busse, A.; Śliwiński, M. Zróżnicowanie i rozmieszczenie zbiorowisk z udziałem Cypripedium calceolus L. (Orchidaceae) na Dolnym Śląsku. Acta Bot. Sil. 2012, 8, 97–128. [Google Scholar]
- Wild, J.; Kaplan, Z.; Danihelka, J.; Petřík, P.; Chytrý, M.; Novotný, P.; Rohn, M.; Šulc, V.; Brůna, J.; Chobot, K.; et al. Plant distribution data for the Czech Republic integrated in the Pladias database. Preslia 2019, 91, 1–24. [Google Scholar] [CrossRef]
- Heinrich, W.; Lorenz, R. Frauenschuh (Cypripedium calceolus L.)-Die Orchidee des Jahres 1996. Ber. Arbeitskrs. Heim. Orchid. 1996, 13, 61–93. [Google Scholar]
- Pop, A. Studiul Populaţiei de Cypripedium calceolus L. din Împrejurimile Oraşului Sovata, Judeţ Mures, Lucrare de Licenţă/Population Study of Cypripedium calceolus L. from the Surroundings of Sovata City; Mures county, license Thesis; Universitatea “Babeş-Bolyai”: Cluj-Napoca, Romania, 2006; p. 80. [Google Scholar]
- Voronkina, N.V. Cypripedium calceolus L. In Krasnaya Kniga Kaluzhskoy Oblasti; Antokhina, V.A., Ed.; OOO Vash Dom: Kaluga, Russia, 2015; pp. 225–226. [Google Scholar]
- Dakskobler, I.; Vreš, B. Ecological characteristics, distribution, and preservation conditions of pteridophytes and spermathophytes of European conservational importance, growing in Slovenian forests. Gozdarski Vestn. 2014, 72, 440–451. [Google Scholar]
- Perrino, E.V.; Musarella, C.M.; Magazzini, P. Management of grazing Italian river buffalo to preserve habitats defined by Directive 92/43/EEC in a protected wetland area on the Mediterranean coast: Palude Frattarolo, Apulia, Italy. Euro-Mediterr. J. Environ. Integr. 2020, 13, 1682. [Google Scholar] [CrossRef]
- Bilz, M. Cypripedium calceolus, the IUCN Red List of Threatened Species. 2011. Available online: https://www.iucnredlist.org/species/162021/5532694 (accessed on 18 January 2021).
- Jackowiak, B.; Celka, Z.; Chmiel, J.; Latowski, K.; Żukowski, W. Red list of vascular flora of Wielkopolska (Poland). Biodiv. Res. Conserv. 2007, 5–8, 95–127. [Google Scholar]
- Nowak, A.; Nowak, S.; Spałek, K. Red list of vascular plants of Opole province-2008. Opole Sci. Soc. Nat. J. 2008, 41, 141–158. [Google Scholar]
- Jakubska-Busse, A.; Szczęśniak, E.; Śliwiński, M.; Narkiewicz, C. Zanikanie stanowisk obuwika pospolitego Cypripedium calceolus L. 1753 (Orchidaceae) w Sudetach. Przyr. Sudet. 2010, 13, 43–52. [Google Scholar]
- Czerepko, J.; Gawryś, R.; Cieśla, A.; Sokołowski, K. Warunki środowiska wpływające na stan zachowania obuwika pospolitego Cypripedium calceolus L. w lasach gospodarczych. Sylwan 2014, 158, 867–874. [Google Scholar]
- Blinova, I. Populations of orchids at the northern limit of their distribution (Murmansk Oblast): Effect of climate. Russ. J. Ecol. 2008, 39, 26–33. [Google Scholar] [CrossRef]
- Corkhill, P. Raising Cypripedium calceolus from flask. Orchid. Rev. 1996, 104, 348–352. [Google Scholar]
- Shefferson, R.P. Survival cost of adult dormancy and the confounding influence of size in Lady’s slipper orchids, genus Cypripedium. Oikos 2006, 115, 253–262. [Google Scholar] [CrossRef]
- Eu Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. A new EU Forest Strategy: For Forests and the Forest-Based Sector. SWD (2013) 342 Final}, SWD (2013) 343 Final. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:21b27c38-21fb-11e3-8d1c-01aa75ed71a1.0022.01/DOC_1&format=PDF (accessed on 22 January 2021).
- Druart, P. Etat initial et plan d’action pour Cypripedium calceolus L. Sabot de Vénus (Orchidées) dans le canton de Neuchâtel. Conservatoire Botanique National Alpin, Rapport d’étude. 2007. [Google Scholar]
- Pearson, R.G.; Dawson, T.P.; Liu, C. Modelling species distributions in Britain: A hierarchical integration of climate and land-cover data. Ecography 2004, 27, 285–298. [Google Scholar] [CrossRef]
- Thuiller, W.; Araújo, M.B.; Lavorel, S. Do we need land-cover data to model species distributions in Europe? J. Biogeogr. 2004, 31, 353–361. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Djordjević, V.; Tsiripidis, I. Neottia cordata (Orchidaceae) at its southernmost distribution border in Europe: Threat status and effectiveness of Natura 2000 Network for its conservation. J. Nat. Conserv. 2019, 48, 27–35. [Google Scholar] [CrossRef]
- Pearson, R.G. Species’ Distribution Modeling for Conservation Educators and Practitioners. Synthesis. Am. Mus. Nat. Hist. Lessons Conserv. 2009, 3, 54–89. [Google Scholar]
- Tsiftsis, S.; Djordjević, V. Modelling sexually deceptive orchid species distributions under future climates: The importance of plant-pollinator interactions. Sci. Rep. 2020, 10, 10623. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jacquemyn, H.; He, X.; Chen, W.; Huang, Y.; Yu, S.; Lu, Y.; Zhang, Y. The Impact of Human Pressure and Climate Change on the Habitat Availability and Protection of Cypripedium (Orchidaceae) in Northeast China. Plants 2021, 10, 84. [Google Scholar] [CrossRef]
- Allan, C.; Stankey, G.H. Adaptive Environmental Management: A Practitioner’s Guide; Springer Science & Business Media: New York, NY, USA, 2011; Volume 351. [Google Scholar]
- Wagensommer, R.P.; Medagli, P.; Turco, A.; Perrino, E.V. IUCN Red List evaluation of the Orchidaceae endemic to Apulia (Italy) and considerations on the application of the IUCN protocol to rare species. Nat. Conserv. Res. 2020, 5, 90–101. [Google Scholar] [CrossRef]
- Tashev, A.; Kuchtev, D. New floristic records in the Balkans. 40. Phytol. Balc. 2019, 25, 323. [Google Scholar]
- Denisova, L.V.; Vakhrameeva, M.G. Rod Bashmachok (venerin bashmachok)–Cypripedium L. In Biological Flora of the Moscow Region; Rabotnov, T.A., Ed.; Part 4; Moscow State University: Moscow, Russia, 1978; pp. 62–70. [Google Scholar]
- Kranjčev, R. Hrvatske orhideje. AKD, Zagreb. Bucureşti 2005, 38, 45–56. [Google Scholar]
- Randić, M.; Brkljačić, A.; Lukač, G.; Kremer, D. New localities of rare NATURA 2000 species: Pulsatilla grandis Wender, Genista holopetala (Koch) Bald and Cypripedium calceolus L. in the NW Dinarides in Croatia. Nat. Croat. Period. Musei Hist. Nat. Croat. 2013, 22, 95–109. [Google Scholar]
- Nikolić, T.; Topić, J. Red Book of Vascular Flora of Croatia. Ministry of Culture; State Institute for Nature Protection: Zagreb, Republic of Croatia, 2005. [Google Scholar]
- Procházka, F.; Velísek, V. Orchideje Naší Přírody, 1st ed.; Academia Publishing House of the Czech Academy of Sciences: Prague, Czech Republic, 1983; p. 279. [Google Scholar]
- Jersáková, J.; Kindlmann, P. Zásady péče o Orchidejová Stanoviště. 1. Vydání. KOPP nakladatelství: České Budějovice, 2004; p. 151.
- Zimmermann, F. Die Orchideen Brandenburgs-Verbreitung, Gefährdung, Schutz. Ber. Arbeitskrs. Heim. Orchid. 2019, 35, 4–147. [Google Scholar]
- Kretzschmar, H. Die statistische Gefährdung des Frauenschuhs im mittleren Deutschland. Ber. Arbeitskrs. Heim. Orchid. 1996, 13, 39–40. [Google Scholar]
- Lohr, M.; Beinlich, B.; Grawe, F.; Härtl, K.-H. Förderung von Orchideen im Kreis Höxter durch das Life-Projekt, “Vielfalt auf Kalk“. In Arbeitskreis Heimische Orchideen Nordrhein-Westfalen: Die Orchideen Nordrhein-Westfalens; Kuhn, W., Kupper, D., Lohr, M., Loos, G.-H., Luwe, M., Margenburg, B., Wenker, D., Westphal, G., Wolbeck, D., Eds.; LWL-Museum für Naturkunde: Münster, Germany, 2018; pp. 86–93. [Google Scholar]
- Matuszkiewicz, W. The systematic position of thermophilous beechwoods (Cephalanthero-Fagenion) in Poland. Fragm. Flor. Geobot. 2000, 45, 393–412. [Google Scholar]
- Matuszkiewicz, J.M.; Kozłowska, A. Orchidaceous” beech forests in the Góry Krowiarki Range (Eastern Sudety Mountains). Fragm. Flor. Geobot. 2000, 45, 373–391. [Google Scholar]
- Kucharczyk, M. Obuwik pospolity Cypripedium calceolus. In Monitoring Gatunków Roślin, Przewodnik Metodyczny; Perzanowska, J., Ed.; Część I, GIOŚ: Warszawa, Poland, 2010; pp. 83–98. [Google Scholar]
- Szeląg, Z. Koeleria pyramidata (Poaceae) kommt in Polen vor. Fragm. Flor. Geobot. 1995, 40, 749–753. [Google Scholar]
- Săvulescu, T. Flora Republicii Socialiste Romania, XII; Academia Republicii Socialiste Romania: Bucuresti, Romania, 1972. [Google Scholar]
- Balázs, Z.R.; Roman, A.; Balazs, H.E.; Căpraş, D.; Podar, D. Rediscovery of Cypripedium calceolus L. in the Vicinity of Cluj-Napoca (Romania) after 80 Years. Contribuţii Botanice 2016, 51, 43–53. [Google Scholar]
- Tomescu, C.V. Cypripedium calceolus L. în Pădurea Dragomirna – judeţul Suceava. Horticultură, Viticultură şi vinificaţie, Silvicultură şi grădini publice, Protecţia plantelor 2018, 47, 421–427. [Google Scholar]
- Mihãilescu, S.; Strat, D.; Cristea, I.; Honciuc, V. Raportul sintetic privind starea de conservare a speciilor si habitatelor de interes comunitar din România; S.C. Cuget Liber S.A.: Constanta, Romania, 2015; p. 282. [Google Scholar]
- Marinescu, V.M.; Neblea, M.; Marian, M.; Dinuță, C. Land survey and cadastral measurement in horticulture. Curr. Trends Nat. Sci. 2012, 1, 2. [Google Scholar]
- Vakhrameeva, M.G.; Varlygina, T.I.; Tatarenko, I.V. Orchids of Russia (Biology, Ecology and Protection); KMK Scientific Press: Moscow, Russia, 2014. [Google Scholar]
- Blinova, I. A Northernmost Population of Cypripedium calceolus L. (Orchidaceae): Demography, Flowering, and Pollination. Selbyana 2002, 23, 111–120. [Google Scholar]
- Teteryuk, L.; Kirillova, I. Rare and protected Orchids of the Komi Republic. Ber. Arb. Heim. Orchid. 2011, 28, 133–179. [Google Scholar]
- Kravchenko, A.V. Compendium of Karelian flora (vascular plants). Karelian Research Centre of the RAS, Petrosavodsk. 2007. (In Russian) [Google Scholar]
- Efimov, P.G. Orchids of North-West European Russia (in the Limits of Leningrad, Pskov and Novgorod Regions), 2nd ed.; KMK Scientific Press: Moscow, Russia, 2012. [Google Scholar]
- Seregin, A.P.; Borovichev, E.A.; Glazunova, K.P.; Kokoshnikova, Y.S.; Sennikov, A.N. Flora of Vladimir Oblast, Russia: checklist and Atlas; Grif & K Publisher: Tula, Russia, 2012; p. 620, (In Russian, with English abstract). [Google Scholar]
- Sukhorukov, A.P. A Manual of Vascular Plants of Tambov Region; Grif & K Publisher: Tula, Russia, 2010. [Google Scholar]
- Saksonov, S.V.; Koneva, N.V. The abstract of Orchidaceae family [of] the Samara Area. Vestnik of Udmurt State University. Ser. Biol. 2006, 10, 43–50. [Google Scholar]
- Shibanova, N.L. Demographic and ecological characteristics of Orchidaceae in Permskii Krai. Permsk. Agrar. Vestn. 2016, 2, 113–128. [Google Scholar]
- Suyundukov, I.V. Cypripedium calceolus L. In Krasnaya Kniga Respubliki Bashkortostan; Mirkin, B.M., Ed.; Media Print: Ufa, Russia, 2011; Volume 1, p. 76. [Google Scholar]
- Stevanović, V.; Niketić, M.; Lakušić, D. Chorological additions to the flora of eastern Yugoslavia. Flora Mediterr. 1991, 1, 121–142. [Google Scholar]
- Niketić, M.; Stevanović, V. Cypripedium calceolus L. In Crvena knjiga flore Srbije 1: Iščezli i krajnje ugroženi taksoni, Zavod za zaštitu prirode republike Srbije, Beograd; Stevanović, V., Ed.; Ministarstvo za životnu sredinu republike Srbije, Biološki fakultet Univerziteta u Beogradu: Beograd, Belgrade, 1999; pp. 222–225. [Google Scholar]
- Dakskobler, I.; Rozman, A.; Franz, W.R. Betula pubescens Ehrh. Subsp. carpatica (Willd.) Ascherson & Graebner, a new taxon in the flora of the Julian Alps and Slovenia and its new association Rhododendron hirsuti-Betuletum carpaticae ass. nov. Folia Biol. Geol. 2012, 53, 5–23. [Google Scholar]
- Dakskobler, I.; Anderle, B.; Zupan, B.; Vreš, B. Novosti v flori Slovenije. Hladnikia 2014, 33, 3–30. [Google Scholar]
- Tsiftsis, S.; Tsiripidis, I. Threat categories of the Greek orchids (Orchidaceae). Bot. Chron. 2016, 21, 43–74. [Google Scholar]
- Tsiftsis, S.; Antonopoulos, Z. Atlas of the Greek orchids; Mediterraneo Editions: Réthymno, Greece, 2017. [Google Scholar]
- Available online: http://e-ecodb.bas.bg/rdb/en/vol1/Cypcalce.html (accessed on 18 January 2021).
- Šilić, Č. Spisak biljnih vrsta (Pteridophyta i Spermatophyta) za Crvenu knjigu Bosne i Hercegovine (in Bosnian). Glasnik zemaljskog Muzeja Bosne i Hercegovine u Sarajevu. Nova Ser. 1996, 31, 323–367. [Google Scholar]
- Petrović, D.; Stešević, D.; Vuksanović, S. Materials for the Red book of Montenegro. Nat. Montenegrina 2008, 7, 605–631. [Google Scholar]
- Boscaiu, N.; Coldea, G.; Roreanu, C. Lista rosie aplantelor vasculare disparute, periclitate, vulnerabile si rare din flora Romaniei. Ocrotirea Nat. Mediu. Înconjurător Bucureşti 1994, 38, 45–56. [Google Scholar]
- Oltean, M.; Neagrean, G.; Popescu, A.; Roman, N.; Dihoru, G.; Sanda, V.; Mihailescu, S. Lista Roşie a plantelor superioare din România. Inst. Biol. Stud. Sint. Doc. Ecol. Bucur. 1994, 1, 1–52. [Google Scholar]
- Oprea, A. Lista Critică a Plantelor Vasculare din România; Editura Universităţii “Alexandru Ioan Cuza”: Iaşi, Romania, 2005; p. 668. [Google Scholar]
- IUCN France, MNHN, FCBN, SFO. La Liste rouge des espèces menacées en France-Chapitre Orchidées de France Métropolitaine; Chapitre Orchidées de France métropolitaine: Paris, France, 2010. [Google Scholar]
- Dusak, F.; Prat, D. Atlas des Orchidees de France. Biotope, Meze (Collection Parthenope); Museum National d’Histoire Natural: Paris, France, 2010. [Google Scholar]
- Rossi, G.; Montagnani, C.; Gargano, D.; Peruzzi, L.; Abeli, T.; Ravera, S.; Cogoni, A.; Fenu, G.; Magrini, S.; Gennai, M.; et al. Lista Rossa della Flora Italiana. 1. Policy Species e altre specie minacciate; Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare; Stampera Romana: Roma, Italy, 2013; p. 54. [Google Scholar]
- Brusa, G.; Armiraglio, S.; Ceriani, R.M. Monitoraggio delle specie vegetali della Direttiva 92/43/CEE presenti in Lombardia, a supporto della redazione del IV rapporto ex art. 17. Società Botanica Italaiana onlus - Sez. Lombarda, CFA-Regione Lombardia; Varese-Brescia, 2018.
- Perazza, G.; Perazza, M.D. Cartografia Orchidee Tridentine (COT): Cypripedium calceolus L. e Liparis loeselii (L.) Rich., specie citate nella Direttiva Habitat della CEE. Atti Acc. Rov. Agiati. Ser. 2002, 8, 129–210. [Google Scholar]
- eElurikkus, Eesti Ohustatud Liikide Punane Nimestik. 2008. Available online: http://elurikkus.ut.ee/prmt.php?lang=est (accessed on 29 October 2020).
- Cheffings, C.M.; Farrell, L.; Dines, T.D.; Jones, R.A.; Leach, S.J.; McKean, D.R.; Pearman, D.A.; Preston, C.D.; Rumsey, F.J.; Taylor, I. The Vascular Plant Red Data List for Great Britain; Joint Nature Conservation Committee, Peterborough. Species Status 2005, 7, 1–116. [Google Scholar]
- Foley, M.; Clarke, S. Orchids of the British Isles; Griffin Press Publishing Limited: Maidenhead, UK, 2005. [Google Scholar]
- Bañares, Á.; Blanca, G.; Güemes, J.; Moreno, J.C.; Ortiz, S. Atlas y Libro Rojo de la Flora Vascular Amenazada de España. Adenda 2010; Dirección General de Medio Natural y Política Forestal (Ministerio de Medio Ambiente y Medio Rural y Marino); Sociedad Española de Biología de la Conservatión de Plantas: Madrid, Spain, 2010; p. 170. [Google Scholar]
- Adenda, Dirección General de Medio Natural y Política Forestal (Ministerio de Medio Ambiente, y Medio Rural y Marino)-Sociedad Española de Biología de la Conservación de Plantas, Madrid. 2010. Available online: https://www.miteco.gob.es/fr/biodiversidad/temas/inventarios-nacionales/921_tcm36-99367.pdf (accessed on 18 January 2021).
- Averyanov, L.V. Cypripedium calceolus. In Red Data Book of Russian Federation (Plants and Fungi); Bardunov, L.V., Novikov, V.S., Eds.; KMK Scientific Press: Moscow, Russia, 2008; pp. 363–364. [Google Scholar]
- Fateryga, A.V.; Efimov, P.G.; Svirin, S.A. Orchids of the Crimean Peninsula; PP “ARIAL” LLC: Simferopol, Crimea, 2019; p. 224. [Google Scholar]
- Fateryga, V.V. Modern state of the coenopopulation of Cypripedium calceolus (Orchidaceae) in the Crimea. Ekosistemy 2019, 20, 76–81. [Google Scholar]
- Available online: https://www.rote-liste-zentrum.de/de/Detailseite.html?species_id=35993&q=Frauenschuh (accessed on 18 January 2021).
- Gola, P. Ekobiologické nároky lesních populací střevíčníku pantoflíčku (Cypripedium calceolus L.); Palacký University Olomouc, Faculty of Science. Available online: https://theses.cz/id/ddiu4j/ (accessed on 18 January 2020).
- Grulich, V. Červený seznam rostlin ČR. In Červený Seznam Ohrožených Druhů České Republiky, Příroda; Grulich, V., Chobot, K., Eds.; Agentura ochrany přírody a krajiny ČR; Tiskárna Bílý Slon: Plzeň, ČR, 2017; pp. 75–132. [Google Scholar]
- Available online: https://www.ochranaprirody.cz/pece-o-prirodu-a-krajinu/programy-eu/life/life-ceske-stredohori/cilove-druhy/strevicnik-pantoflicek/ (accessed on 18 January 2021).
- Elias, P.; Dítě, D.; Kliment, J.; Hrivnák, R.; Feráková, V. Red list of ferns and flowering plants of Slovakia, 5th edition (October 2014). Biologia 2015, 70, 218–228. [Google Scholar] [CrossRef]
- Vlčko, J.; Dítě, D.; Kolník, M. Orchids of Slovakia; ZO SZOPK Orchidea: Zvolen, Slovakia, 2003; p. 120. [Google Scholar]
- Bornand, C.; Gygax, A.; Juillerat, P.; Jutzi, M.; Möhl, A.; Rometsch, S.; Sager, L.; Santiago, H.; Eggenberg, S. Rote Liste Gefässpflanzen. Gefährdete Arten der Schweiz. Bundesamt für Umwelt, Bern und Info Flora, Genf. Umwelt-Vollzug 2016, 1621, 178. [Google Scholar]
- Niklfeld, H.; Schratt-Ehrendorfer, L. Rote Liste gefährdeter Farn- und Blütenpflanzen (Pteridophyta und Spermatophyta) Österreichs. 2. Fassung. In Rote Listen gefährdeter Pflanzen Österreichs. 2. Auflage. Grüne Reihe des Bundesministeriums für Umwelt; Jugend und Familie, Band 10; Austria Medien Service: Graz, Österreich, 1999; pp. 33–152. [Google Scholar]
- Pravilnik o uvrstitvi ogroženih rastlinskih in živalskih vrst v rdeči seznam; Uradni list RS 82/2002. Available online: http://www.pisrs.si/Pis.web/pregledPredpisa?id=ODRE1883 (accessed on 18 January 2021).
- Cŭšin, B. Natura 2000 v Sloveniji: Rastline, ZRC SAZU: Biološki inštitut Jovana Hadžija; Založba ZRC: Ljubljana, Slovenia, 2004. [Google Scholar]
- Király, G. Vörös Lista. A Magyarországi Edényes Flóra Veszélyeztetett fajai. Red list of the Vascular Flora of Hungary, Saját kiadás; Lővér Print: Sopron, Hungary, 2007; p. 73. [Google Scholar]
- Sulyok, J. Boldogasszony papucsa (Cypripedium calceolus). In Környezetvédelmi és Vízügyi Minisztérium Természetvédelmi Hivatal Fajmegőrzési Tervek; Természetvédelem: Budapest, Hungary, 2006; p. 66. [Google Scholar]
- Lozan, A.; Tofan-Dorofeev, E.; Cotofana, I.; Threatened plant species included in the Emerald Network in Moldova. In Conversation of Plant Diversity; Alexandru, T, ed.; international scientific symposium, 1–3 June 2017; pp. 41. Available online: https://ibn.idsi.md/sites/default/files/imag_file/Conversation%20of%20Plant%20Diversity_1-3_iunie_2017_0.pdf (accessed on 18 January 2021).
- Gudžinskas, Z.; Ryla, M. Lietuvos Gegužraibiniai (Orchidaceae), Botanikos Instituto Leidykla; Spausdino UAB, Petro Ofsetas: Žalgirio, 2006; p. 104. [Google Scholar]
- Bjørndalen, J.E. Protection of Norwegian orchids—A review of achievements and challenges. Eur. J. Environ. Sci. 2015, 5, 121–133. [Google Scholar] [CrossRef][Green Version]
- Ingelög, T.; Andersson, R.; Tjernberg, M. Red Data Book of the Baltic Region. Part 1: Lists of Threatened Vascular Plants and Vertebrates; Swedish Threatened Species Unit Uppsala: Riga, Latvia, 1993. [Google Scholar]
- Kļaviņa, D.; Grauda, D.; Priede, A.; Rashal, I. The habitat diversity and genetic variability of Cypripedium calceolus in Latvia. In Proceedings of the Actions for Wild Plants, Papers of the 6th Planta Europa Conference on the Conservation of Plants, Kraków, Poland, 23–27 May 2011; pp. 91–97. [Google Scholar]
- Gärdenfors, U. (Ed.) ; Rödlistade arter i Sverige 2010–The 2010 Red List of Swedish Species. ArtDatabanken, SLU, Uppsala; Elanders Sverige AB: Mölnlycke, Sweden, 2010. [Google Scholar]
- Hyvärinen, E.; Juslén, A.; Kemppainen, E.; Uddström, A.; Liukko, U.M. The 2019 Red List of Finnish Species; Ympäristöministeriö & Suomen ympäristökeskus: Helsinki, Finland, 2019; p. 704. [Google Scholar]
- Available online: https://bios.au.dk/forskningraadgivning/temasider/redlistframe/soeg-en-art/#10414 (accessed on 18 January 2021).
- Daamgard, C.; Moeslund, J.E.; Wind, P. Changes in the Abundance of Danish Orchids over the Past 30 Years. Diversity 2020, 12, 244. [Google Scholar] [CrossRef]



| Country/Area | IUCN Category | IUCN Criteria | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | ||
| Belgium, Greece, Liechtenstein, Luxemburg, The Netherlands | EX | - | - | - | - | - |
| Bulgaria, Crimea | CR | - | 1 | 1 | 1 | - |
| Serbia | CR | - | 1 | 1 | - | - |
| Moldova | CR | - | 1 | - | 1 | - |
| Bosnia and Herzegovina | CR | 1 | - | - | - | - |
| United Kingdom | CR | - | - | - | 1 | - |
| Croatia, Hungary | EN | 1 | - | - | - | - |
| Montenegro | EN | - | - | - | 1 | - |
| Ukraine | EN | - | 1 | - | - | - |
| Spain | EN | - | - | 1 | - | - |
| Germany | VU | 1 | 1 | - | - | - |
| Belarus, France, Latvia, Lithuania, Slovenia | VU | 1 | - | - | - | - |
| Poland, Romania, Switzerland | VU | - | 1 | - | - | - |
| Czech Republic | VU | - | - | 1 | - | - |
| Denmark | VU | - | - | - | 1 | - |
| Austria, Estonia, Finland, Slovak Republic, Norway | NT | - | - | - | - | - |
| Italy, Russia (European part), Sweden | LC | - | - | - | - | - |
| EX: Extinct; CR: Critically Endangered; EN: Endangered; VU: Vulnerable; NT: Near Threatened; LC: Least Concern | ||||||
| Countries where C. calceolus is absent: Albania, Cyprus, Iceland, Ireland, Malta, North Macedonia, Portugal | ||||||
| Collecting | Vegetation Succession | Clear Cutting of the Woods | Grazing, Damage by Rodents | Changes in the Dominant Treespecies (e.g., Reforestation with Pines or Spruce) | Habitat Destruction | Trampling | Road Construction | Global Heating | Habitats Drainage | Forest and Grassland Fires | Eutrophication | Animal Pests (Insects, Smails) | Grassland Mowing | Other Stochastic Events | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | ● | ● | ● | ||||||||||||
| Belarus | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||||||
| Bosnia and Herzegovina | ● | ||||||||||||||
| Bulgaria | ● | ● | ● | ● | |||||||||||
| Crimea | ● | ● | |||||||||||||
| Croatia | ● | ||||||||||||||
| Czech Republic | ● | ● | ● | ● | ● | ● | |||||||||
| Denmark | ● | ● | |||||||||||||
| Estonia | ● | ● | |||||||||||||
| Finland | ● | ● | ● | ● | ● | ● | |||||||||
| France | ● | ● | ● | ||||||||||||
| Germany | ● | ● | ● | ● | ● | ||||||||||
| Hungary | ● | ● | ● | ● | ● | ● | ● | ||||||||
| Italy | ● | ● | |||||||||||||
| Latvia | ● | ● | ● | ||||||||||||
| Lithuania | ● | ● | ● | ● | |||||||||||
| Moldova | ● | ● | ● | ||||||||||||
| Montenegro | ● | ● | |||||||||||||
| Norway | ● | ● | ● | ● | |||||||||||
| Poland | ● | ● | ● | ● | ● | ● | |||||||||
| Romania | ● | ● | ● | ||||||||||||
| Russia (European part) | ● | ● | ● | ||||||||||||
| Serbia | ● | ● | |||||||||||||
| Slovak Republic | ● | ● | ● | ● | |||||||||||
| Slovenia | ● | ● | |||||||||||||
| Spain | ● | ● | ● | ● | |||||||||||
| Sweden | ● | ● | ● | ● | |||||||||||
| Switzerland | ● | ● | ● | ● | ● | ● | |||||||||
| Ukraine | ● | ● | ● | ||||||||||||
| United Kingdom | ● | ● | |||||||||||||
| TOTAL | 25 | 20 | 19 | 7 | 6 | 6 | 5 | 5 | 4 | 3 | 2 | 1 | 1 | 1 | 1 |
| Country | Grid Cells of the Natura 2000 Network | Potentially Suitable Area (% of the Natura 2000 Network) | ||||
|---|---|---|---|---|---|---|
| Current Climate | Future Climatic Conditions (CCSM4) | |||||
| RCP2.6 | RCP4.5 | RCP6.0 | RCP8.5 | |||
| Austria | 8638 | 89.65 | 55.14 | 43.45 | 43.49 | 29.88 |
| Belgium | 2023 | 5.88 | 4.60 | 4.40 | 0.99 | 0 |
| Bulgaria | 48,054 | 15.57 | 3.03 | 1.93 | 1.47 | 0.02 |
| Croatia | 26,356 | 29.85 | 5.46 | 0.63 | 1.75 | 0 |
| Cyprus | 1217 | 0 | 0 | 0 | 0 | 0 |
| Czech Republic | 14,230 | 98.31 | 66.85 | 44.43 | 26.48 | 12.35 |
| Denmark | 5868 | 74.63 | 61.71 | 34.99 | 54.93 | 19.07 |
| Estonia | 9325 | 16.85 | 10.43 | 0.46 | 0 | 0 |
| Finland | 53,579 | 2.61 | 4.90 | 2.85 | 2.47 | 4.97 |
| France | 80,612 | 24.62 | 22.24 | 20.11 | 18.43 | 12.17 |
| Germany | 59,599 | 65.65 | 34.95 | 26.84 | 27.13 | 10.29 |
| Greece | 29,836 | 0.42 | 0.28 | 0.07 | 0.07 | 0 |
| Hungary | 21,865 | 8.82 | 0.03 | 0 | 0 | 0 |
| Ireland | 13,986 | 0 | 0 | 0 | 0 | 0 |
| Italy | 47,610 | 23.84 | 18.13 | 16.56 | 17.46 | 13.49 |
| Latvia | 1779 | 29.62 | 13.04 | 0.06 | 0 | 0 |
| Lithuania | 11,170 | 59.30 | 20.67 | 13.05 | 0 | 0 |
| Luxembourg | 738 | 89.02 | 57.32 | 70.05 | 58.67 | 12.87 |
| Malta | 43 | 0 | 0 | 0 | 0 | 0 |
| The Netherlands | 4813 | 9.27 | 26.01 | 15.62 | 11.68 | 0 |
| Poland | 58,730 | 57.01 | 12.92 | 5.50 | 6.73 | 2.66 |
| Portugal | 21,279 | 0 | 0 | 0 | 0 | 0 |
| Romania | 67,318 | 47.65 | 30.95 | 22.23 | 21.25 | 8.61 |
| Slovak Republic | 10,838 | 86.81 | 54.92 | 31.03 | 38.91 | 20.04 |
| Slovenia | 11,137 | 62.37 | 28.64 | 21.06 | 14.62 | 9.26 |
| Spain | 115,180 | 1.48 | 1.90 | 1.71 | 1.87 | 1.35 |
| Sweden | 83,859 | 23.07 | 33.56 | 26.14 | 24.70 | 22.93 |
| United Kingdom | 26,962 | 3.53 | 2.82 | 1.34 | 4.61 | 4.41 |
| Habitat Type | Alpine and Subalpine Grasslands | Montane and Xerothermic Grasslands | Alternately Wet Meadows | Wet Screes | Beech Forests (Fertile, Calcified) | Oak-hornbeam Forests | Ravine Forests | Riparian Forests | Thermofilous Oak Forests | |
|---|---|---|---|---|---|---|---|---|---|---|
| Natura 2000 Code | 6170 | 6210 6520 | 6410 | 8160 | 9130 9150 | 9170 | 9180 | 91E0 | 91I0 | |
| Country | Crimea | ● | ||||||||
| Croatia | ● | |||||||||
| Czech Republic | ● | ● | ● | ● | ● | ● | ||||
| Germany | ● | ● | ● | |||||||
| Poland | ● | ● | ● | ● | ● | |||||
| Romania | ● | ● | ● | |||||||
| Russian Federation (Europaean Regions) | ● | |||||||||
| Slovenia | ● | ● | ● | ● | ||||||
| Protective Actions for Natural Populations of Cypripedium calceolus |
|---|
| Recommended |
|
| Inappropriate |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubska-Busse, A.; Tsiftsis, S.; Śliwiński, M.; Křenová, Z.; Djordjević, V.; Steiu, C.; Kolanowska, M.; Efimov, P.; Hennigs, S.; Lustyk, P.; et al. How to Protect Natural Habitats of Rare Terrestrial Orchids Effectively: A Comparative Case Study of Cypripedium calceolus in Different Geographical Regions of Europe. Plants 2021, 10, 404. https://doi.org/10.3390/plants10020404
Jakubska-Busse A, Tsiftsis S, Śliwiński M, Křenová Z, Djordjević V, Steiu C, Kolanowska M, Efimov P, Hennigs S, Lustyk P, et al. How to Protect Natural Habitats of Rare Terrestrial Orchids Effectively: A Comparative Case Study of Cypripedium calceolus in Different Geographical Regions of Europe. Plants. 2021; 10(2):404. https://doi.org/10.3390/plants10020404
Chicago/Turabian StyleJakubska-Busse, Anna, Spyros Tsiftsis, Michał Śliwiński, Zdenka Křenová, Vladan Djordjević, Corina Steiu, Marta Kolanowska, Petr Efimov, Sebastian Hennigs, Pavel Lustyk, and et al. 2021. "How to Protect Natural Habitats of Rare Terrestrial Orchids Effectively: A Comparative Case Study of Cypripedium calceolus in Different Geographical Regions of Europe" Plants 10, no. 2: 404. https://doi.org/10.3390/plants10020404
APA StyleJakubska-Busse, A., Tsiftsis, S., Śliwiński, M., Křenová, Z., Djordjević, V., Steiu, C., Kolanowska, M., Efimov, P., Hennigs, S., Lustyk, P., & Kreutz, K. (2021). How to Protect Natural Habitats of Rare Terrestrial Orchids Effectively: A Comparative Case Study of Cypripedium calceolus in Different Geographical Regions of Europe. Plants, 10(2), 404. https://doi.org/10.3390/plants10020404








