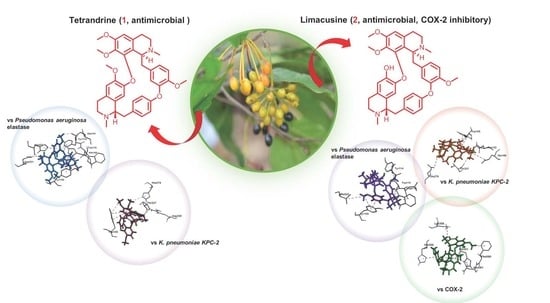
Antibacterial and COX-2 Inhibitory Tetrahydrobisbenzylisoquinoline Alkaloids from the Philippine Medicinal Plant Phaeanthus ophthalmicus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antibacterial Activity of Extracts and Alkaloids
2.2. Cyclooxygenase (COX) -1 and -2 Inhibitory Activity of Alkaloids
2.3. Molecular Docking of Alkaloids 1 and 2 against Disease Targets
2.4. Molecular Dynamics Simulation on Limacusine-COX-2 Docked Complex
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Extraction, Isolation and Structure Elucidation of Compounds 1 and 2
3.4. Biological Assays
3.4.1. Test Organisms and Growth Conditions
3.4.2. MIC Determination
3.4.3. MBC Determination
3.4.4. Anti-Cyclooxygenase (COX) -1 and -2 Assays
3.5. Computational Methods
3.5.1. Molecular Docking
3.5.2. All Atoms Molecular Dynamics Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fischbach, M.A.; Walsh, C.T. Antibiotics for Emerging Pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef]
- Walsh, C.T.; Fischbach, M.A. Inhibitors of Sterol Biosynthesis as Staphylococcus aureus Antibiotics. Angew. Chem. Int. Ed. 2008, 47, 5700–5702. [Google Scholar] [CrossRef]
- Nikaido, H. Multidrug Resistance in Bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [Green Version]
- Stryjewski, M.E.; Corey, G.R. Methicillin-resistant Staphylococcus aureus: An evolving pathogen. Clin. Infect. Dis. 2014, 58, S10–S19. [Google Scholar] [CrossRef] [Green Version]
- Kalle, A.M.; Rizvi, A. Inhibition of bacterial multidrug resistance by celecoxib, a cyclooxygenase-2 inhibitor. Antimicrob. Agents Chemother. 2010, 55, 439–442. [Google Scholar] [CrossRef] [Green Version]
- Thangamani, S.; Younis, W.; Seleem, M.N. Repurposing celecoxib as a topical antimicrobial agent. Front. Microbiol. 2015, 6, 750. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K.; Rather, M.A.; Jha, A.K.; Shashank, A.; Singhal, S.; Sharma, M.; Pathak, U.; Sharma, D.; Mastinu, A. Artocarpus Lakoocha Roxb. and Artocarpus heterophyllus Lam. Flowers: New sources of bioactive compounds. Plants 2020, 9, 1329. [Google Scholar] [CrossRef]
- Butnariu, M.; Coradini, C.Z. Evaluation of biologically active compounds from Calendula officinalis flowers using spectropho-tometry. Chem. Cent. J. 2012, 6, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Rath, C.C.; Devi, S.; Dash, S.K.; Mishra, R.K. Antibacterial potential assessment of jasmine essential oil against E. coli. Indian J. Pharm. Sci. 2008, 70, 238–241. [Google Scholar] [CrossRef] [Green Version]
- Mols, J.B.; Kebler, P.A. Revision of genus Phaeanthus (Annonaceae). Blumea 2000, 45, 205–233. [Google Scholar]
- Obico, J.A.; Ragragio, E.M. A survey of plants used as repellents against hematophagous insects by the Ayta people of Porac, Pampanga province, Philippines. Philipp. Sci. Lett. 2014, 7, 179–186. [Google Scholar]
- Lipp, F.J.; Perry, L.M.; Metzger, J. Medicinal Plants of East and Southeast Asia; Massachusetts Institute of Technology Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Tan, P.J.; Ong, C.Y.; Danial, A.; Yusof, H.M.; Neoh, B.K.; Lee, H.B. Cyclic Tetrapyrrolic photosensitisers from the leaves of Phaeanthus ophthalmicus. Chem. Cent. J. 2011, 5, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macabeo, A.P.G.; Flores, A.I.G.; Fernandez, R.A.T.; Budde, S.; Faderl, C.; Dahse, H.-M.; Franzblau, S.G. Antitubercular and cytotoxic polyoxygenated cyclohexane derivatives from Uvaria grandiflora. Nat. Prod. Res. 2020, 1–4. [Google Scholar] [CrossRef]
- Macabeo, A.P.G.; Letada, A.G.; Budde, S.; Faderl, C.; Dahse, H.-M.; Franzblau, S.G.; Alejandro, G.J.D.; Pierens, G.K.; Garson, M.J. Antitubercular and cytotoxic chlorinated seco-cyclohexenes from Uvaria alba. J. Nat. Prod. 2017, 80, 3319–3323. [Google Scholar] [CrossRef]
- Macabeo, A.P.G.; Lopez, A.D.C.; Schmidt, S.; Heilmann, J.; Dahse, H.-M.; Franzblau, S.G.; Alejandro, G.J.D. Antitubercular and cytotoxic constituents from Goniothalamus sibuyanensis. Rec. Nat. Prod. 2014, 8, 41–45. [Google Scholar]
- Macabeo, A.P.G.; Martinez, F.P.A.; Kurtán, T.; Tóth, L.; Mándi, A.; Schmidt, S.; Heilmann, J.; Alejandro, G.J.D.; Knorn, M.; Dahse, H.-M.; et al. Tetrahydroxanthene-1,3(2H)-dione derivatives from Uvaria valderramensis. J. Nat. Prod. 2014, 77, 2711–2715. [Google Scholar] [CrossRef]
- Macabeo, A.P.G.; Rubio, P.Y.M.; Higuchi, T.; Umezawa, N.; Faderl, C.; Budde, S.; Bangcaya, P.S.; Alejandro, G.J.D. Polyoxy-genated seco-cyclohexenes and other constituents from Uvaria valderramensis. Biochem. Syst. Ecol. 2017, 71, 200–204. [Google Scholar] [CrossRef]
- Macabeo, A.P.G.; Tudla, F.A.; Krohn, K.; Franzblau, S.G. Antitubercular activity of the semi–polar extractives of Uvaria rufa. Asian Pac. J. Trop. Med. 2012, 5, 777–780. [Google Scholar] [CrossRef] [Green Version]
- Thevand, A.; Stanculescu, I.; Mandravel, C.; Woisel, P.; Surpateanu, G. Total assignment and structure in solution of tetran-drine by NMR spectroscopy and molecular modeling. Spectrochim. Acta A 2004, 60, 1825–1830. [Google Scholar] [CrossRef]
- Damas, P.; Bruneton, J.; Fournet, A.; Guinaudeau, H. 2-Norlimacusine, new bisbenzylisoquinoline isolated from Sciadotenia eichleriana. J. Nat. Prod. 1985, 48, 69–71. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Genet. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Miller, S.I. Antibiotic resistance and regulation of the gram-negative bacterial outer membrane barrier by host innate immune molecules. MBio 2016, 7, e01541-16. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.; Opatz, T. Bisbenzylisoquinoline Alkaloids. In The Alkaloids: Chemistry and Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 81, pp. 1–114. [Google Scholar]
- Cho, H.S.; Chang, S.H.; Chung, Y.S.; Shin, J.Y.; Park, S.J.; Lee, E.S.; Hwang, S.K.; Kwon, J.T.; Tehrani, A.M.; Woo, M.; et al. Synergistic effect of ERK inhibition on tetrandrine-induced apoptosis in A549 human lung carcinoma cells. J. Vet. Sci. 2009, 10, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Bun, S.S.; Laget, M.; Chea, A.; Bun, H.; Ollivier, E.; Elias, R. Cytotoxic activity of alkaloids isolated from Stephania rotunda. Phytother. Res. 2009, 23, 587–590. [Google Scholar] [CrossRef]
- Beuria, T.K.; Santra, A.M.K.; Panda, D. Sanguinarine blocks cytokinesis in bacteria by inhibiting FtsZ assembly and bundling. Biochemistry 2005, 44, 16584–16593. [Google Scholar] [CrossRef]
- Domadia, P.N.; Bhunia, A.; Sivaraman, J.; Swarup, S.; Dasgupta, D. Berberine targets assembly of Escherichia coli cell division protein FtsZ. Biochemistry 2008, 47, 3225–3234. [Google Scholar] [CrossRef]
- Boberek, J.M.; Stach, J.; Good, L. Genetic evidence for inhibition of bacterial division protein FtsZ by berberine. PLoS ONE 2010, 5, e13745. [Google Scholar] [CrossRef]
- Casu, L.; Cottiglia, F.; Leonti, M.; De Logu, A.; Agus, E.; Tse-Dinh, Y.-C.; Lombardo, V.; Sissi, C. Ungeremine effectively targets mammalian as well as bacterial type I and type II topoisomerases. Bioorg. Med. Chem. Lett. 2011, 21, 7041–7044. [Google Scholar] [CrossRef] [Green Version]
- Atan, M.S.; Dzulkefly, K.A.; Mohd Aspollah, S.; Anuar, K.; Vijay, S. Isolation and antibacterial activity of alkaloids from Phaeanthus ophthalmicus. Asian J. Chem. 2011, 23, 3824–3826. [Google Scholar]
- Cerella, C.; Sobolewski, C.; Dicato, M.; Diederich, D. Targeting COX-2 expression by natural compounds: A promising alter-native strategy to synthetic COX-2 inhibitors for cancer chemoprevention and therapy. Biochem. Pharmacol. 2010, 80, 1801–1815. [Google Scholar] [CrossRef]
- Morihara, K.; Tsuzuki, H.; Oda, K. Protease and elastase of Pseudomonas aeruginosa: Inactivation of human plasma alpha 1-proteinase inhibitor. Infect. Immun. 1979, 24, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting virulence: A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Woodford, N.; Tierno, P.M.; Young, K.; Tysall, L.; Palepou, M.-F.I.; Ward, E.; Painter, R.E.; Suber, D.F.; Shungu, D.; Silver, L.L.; et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York Medical Center. Antimicrob. Agents Chemother. 2004, 48, 4793–4799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Fernández, A.; Villa, L.; Carta, C.; Venditti, C.; Giordano, A.; Venditti, M.; Mancini, C.; Carattoli, A. Klebsiella pneumoniae ST258 Producing KPC-3 Identified in Italy carries novel plasmids and OmpK36/OmpK35 porin variants. Antimicrob. Agents Chemother. 2012, 56, 2143–2145. [Google Scholar] [CrossRef] [Green Version]
- Zhanel, G.G.; Simor, A.E.; Vercaigne, L.; Mandell, L.; Canadian Carbapenem Discussion Group Imipenem and Meropenem. Comparison of in vitro activity, pharmacokinetics, clinical trials and adverse effects. Can. J. Infect. Dis. 1998, 9, 215–228. [Google Scholar] [CrossRef]
- Zarghi, A.; Arfaei, S. Selective COX-2 Inhibitors: A review of their structure-activity relationships. Iran. J. Pharm. Res. 2011, 10, 655–683. [Google Scholar]
- Reddy, K.K.; Rajan, V.K.V.; Gupta, A.; Aparoy, P.; Reddanna, P. Exploration of binding site pattern in arachidonic acid me-tabolizing enzymes, cyclooxygenases and lipoxygenases. BMC Res. Notes 2015, 8, 152–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quimque, M.T.J.; Notarte, K.I.R.; Fernandez, R.A.T.; Mendoza, M.A.O.; Liman, R.A.D.; Lim, J.A.K.; Pilapil, L.A.E.; Ong, J.K.H.; Pastrana, A.M.; Khan, A.; et al. Virtual screening-driven drug discovery of SARS-CoV2 enzyme inhibitors targeting viral attachment, replication, post-translational modification and host immunity evasion infection mechanisms. J. Biomol. Struct. Dyn. 2020. [Google Scholar] [CrossRef] [PubMed]
- Macabeo, A.P.G.; Vidar, W.S.; Wan, B.; Franzblau, S.G.; Chen, X.; Decker, M.; Heilmann, J.; Aguinaldo, M.A.M.; Cordell, G.A. Mycobacterium tuberculosis H37Rv and cholinesterase inhibitors from Voacanga globosa. Eur. J. Med. Chem. 2011, 46, 3118–3123. [Google Scholar] [CrossRef] [PubMed]
- Panlilio, B.G.; Macabeo, A.P.G.; Knorn, M.; Kohls, P.; Richomme, P.; Kouam, S.F.; Gehle, D.; Krohn, K.; Franzblau, S.G.; Zhang, Q.; et al. A lanostane aldehyde from Momordica charantia. Phytochem. Lett. 2012, 5, 682–684. [Google Scholar] [CrossRef] [Green Version]
- Macabeo, A.P.G.; Krohn, K.; Gehle, D.; Read, R.W.; Brophy, J.J.; Cordell, G.A.; Franzblau, S.G.; Aguinaldo, A.M. Indole alkaloids from the leaves of Philippine Alstonia scholaris. Phytochemistry 2005, 66, 1158–1162. [Google Scholar] [CrossRef]
- Clinic Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing, 26th ed.; CLSI: Wayne, PA, USA, 2016. [Google Scholar]
- Clinic Laboratory Standard Institute. Methods for dilution antimicrobial susceptibility testing for bacteria that grow aerobically: Approved standards. In CSLI, 8th ed.; CSLI: Wayne, PA, USA, 2009. [Google Scholar]
- Cos, P.; Vlietinck, A.; Berghe, D.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof of concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Irobi, O.; Daramola, S. Bactericidal properties of crude extracts of Mitracarpus villosus. J. Ethnopharmacol. 1994, 42, 39–43. [Google Scholar] [CrossRef]
- Farhadi, T.; Fakharian, A.; Ovchinnikov, R.S. Virtual Screening for Potential inhibitors of CTX-M-15 protein of Klebsiella pneumoniae. Interdiscip. Sci. Comput. Life Sci. 2018, 10, 694–703. [Google Scholar] [CrossRef]
- Geethalakshmi, R.; Sundaramurthi, J.C.; Sarada, D.V. Antibacterial activity of flavonoid isolated from Trianthema decandra against Pseudomonas aeruginosa and molecular docking study of FabZ. Microb. Pathog. 2018, 121, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Mizdal, C.R.; Stefanello, S.T.; Nogara, P.A.; Soares, F.A.A.; Marques, L.D.L.; De Campos, M.M.A. Molecular docking, and anti-biofilm activity of gold-complexed sulfonamides on Pseudomonas aeruginosa. Microb. Pathog. 2018, 125, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Malathi, K.; Anbarasu, A.; Ramaiah, S. Identification of potential inhibitors for Klebsiella pneumoniae carbapenemase-3: A mo-lecular docking and dynamics study. J. Biomol. Struct. Dyn. 2019, 37, 4601–4613. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Libera, J.L.; Murillo-López, J.A.; De La Torre, A.F.; Caballero, J. Structural requirements of n-alpha-mercaptoacetyl dipeptide (namdp) inhibitors of pseudomonas Aeruginosa Virulence factor LasB: 3D-QSAR, molecular docking, and interaction fingerprint studies. Int. J. Mol. Sci. 2019, 20, 6133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahfuz, A.M.U.B.; Opazo, F.S.; Aguilar, L.F.; Iqbal, M.N. Carfilzomib as a potential inhibitor of NADH-dependent enoyl-acyl carrier protein reductases of Klebsiella pneumoniae and Mycobacterium tuberculosis as a drug target enzyme: Insights from mo-lecular docking and molecular dynamics. J. Biomol. Struct. Dyn. 2020. [Google Scholar] [CrossRef] [PubMed]
- Leiris, S.; Davies, D.T.; Sprynski, N.; Castandet, J.; Beyria, L.; Bodnarchuk, M.S.; Sutton, J.M.; Mullins, T.M.G.; Jones, M.W.; Forrest, A.K.; et al. Virtual screening approach to identifying a novel and tractable series of Pseudomonas aeruginosa elastase inhibitors. ACS Med. Chem. Lett. 2021, 12, 217–227. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. Software news and update AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [PubMed] [Green Version]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical cal-culations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Macabeo, A.P.G.; Pilapil, L.A.E.; Garcia, K.Y.M.; Quimque, M.T.J.; Phukhamsakda, C.; Cruz, A.J.C.; Hyde, K.D.; Stadler, M. Alpha-glucosidase- and lipase-inhibitory phenalenones from a new species of Pseudolophiostoma originating from Thailand. Molecules 2020, 25, 965. [Google Scholar] [CrossRef] [Green Version]
- Salomon-Ferrer, R.; Case, D.A.; Walker, R.C. An overview of the Amber biomolecular simulation package. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3, 198–210. [Google Scholar] [CrossRef]
- Case, D.A.; Cheatham, T.E., III; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M., Jr.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Antechamber: An accessory software package for molecular mechanical cal-culations. J. Am. Chem. Soc. 2001, 222, U403. [Google Scholar]
- Vassetti, D.; Pagliai, M.; Procacci, P. Assessment of GAFF2 and OPLS-AA general force fields in combination with the water Models TIP3P, SPCE, and OPC3 for the solvation free energy of druglike organic molecules. J. Chem. Theory Comput. 2019, 15, 1983–1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidchack, R.L.; Handel, R.; Tretyakov, M.V. Langevin thermostat for rigid body dynamics. J. Chem. Phys. 2009, 130, 234101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salomon-Ferrer, R.; Götz, A.W.; Poole, D.; Le Grand, S.; Walker, R.C. Routine Microsecond molecular dynamics simulations with AMBER on GPUs. 2. explicit solvent particle mesh ewald. J. Chem. Theory Comput. 2013, 9, 3878–3888. [Google Scholar] [CrossRef] [PubMed]
- Petersen, H.G. Accuracy and efficiency of the particle mesh Ewald method. J. Chem. Phys. 1995, 103, 3668–3679. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef] [Green Version]
- Toukmaji, A.; Sagui, C.; Board, J.; Darden, T. Efficient particle-mesh Ewald based approach to fixed and induced dipolar interactions. J. Chem. Phys. 2000, 113, 10913–10927. [Google Scholar] [CrossRef]
- Kräutler, V.; Van Gunsteren, W.F.; Hünenberger, P.H. A fast SHAKE algorithm to solve distance constraint equations for small molecules in molecular dynamics simulations. J. Comput. Chem. 2001, 22, 501–508. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E., III. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Kaushik, A.C.; Ali, S.S.; Ahmad, N.; Wei, D.-Q. Deep-learning-based target screening and similarity search for the predicted inhibitors of the pathways in Parkinson’s disease. RSC Adv. 2019, 9, 10326–10339. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Saleem, S.; Idrees, M.; Ali, S.S.; Junaid, M.; Kaushik, A.C.; Wei, D.-Q. Allosteric ligands for the pharmacologically important Flavivirus target (NS5) from ZINC database based on pharmacophoric points, free energy calculations and dynamics correlation. J. Mol. Graph. Model. 2018, 82, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Sadikot, R.T.; Zeng, H.; Azim, A.C.; Joo, M.; Dey, S.K.; Breyer, R.M.; Peebles, R.S.; Blackwell, T.S.; Christman, J.W. Bacterial clearance of Pseudomonas aeruginosa is enhanced by the inhibition of COX-2. Eur. J. Immunol. 2007, 37, 1001–1009. [Google Scholar] [CrossRef] [PubMed]






| Tested Samples | MIC and MBC (μg/mL) | IC50 (μM) vs. COX | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MRSA | VRE | K. pneumoniae, + CRE | P. aeruginosa, + MBL | COX-1 | COX-2 | |||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |||
| Po | 275 | 275 | 137.5 | 275 | 137.5 | 137.5 | 137.5 | 137.5 | - | - |
| PoA | 137.5 | 275 | 137.5 | 275 | 137.5 | 137.5 | 68.75 | 137.5 | - | - |
| PoB | 137.5 | 137.5 | 137.5 | 137.5 | 68.75 | 137.5 | 68.75 | 68.75 | - | - |
| PoE | 137.5 | 137.5 | 137.5 | 137.5 | 137.5 | 137.5 | 68.75 | 68.75 | - | - |
| 1 | 137.5 | 137.5 | 137.5 | 137.5 | 68.75 | 68.75 | 68.75 | 68.75 | >100 | >100 |
| 2 | 137.5 | 137.5 | 137.5 | 137.5 | 68.75 | 68.75 | 68.75 | 68.75 | >100 | 68.8 |
| Penicillin | 14 | 14 | 12 | 12 | - | - | - | - | - | - |
| Meropenem | - | - | - | - | 43.75 | 43.5 | 12 | 12.5 | - | - |
| Celecoxib | - | - | - | - | - | - | - | - | 6.9 | 0.9 |
| Test Compounds | Binding Energy (kcal/mol) | ||||||
|---|---|---|---|---|---|---|---|
| P. aeruginosa Enzymes | K. pneumoniae Enzymes | Ovine Cyclooxygenase | |||||
| FabA | LasR | Elastase (LasB) | KPC-2 carbapenemase | CTX-M-15 | COX-1 | COX-2 | |
| 1 | −6.3 | −5.8 * | −8.1 | −7.6 | −6.5 * | −7.4 | −7.8 |
| 2 | −6.9 | −6.5 * | −7.2 | −9.0 | −6.6 * | −8.8 | −9.0 |
| Meropenem | −6.7 | −6.7 | −5.3 | −5.9 | −6.6 | - | - |
| Celecoxib | - | - | - | - | - | −9.9 | −11.9 |
| Docked Ligands | Ovine COX-2 | P. aeruginosa Elastase (LasB) | K. pneumoniae KPC-2 Carbapenemase | |||
|---|---|---|---|---|---|---|
| Hydrogen Bond | Other Interactions | Hydrogen Bond | Other Interactions | Hydrogen Bond | Other Interactions | |
| Tetrandrine (1) | Asn104 | Asn581, Glu346, Asp347, Lys97 (hydrophobic), Lys358 (pi-cation) | His144, Trp115 | Glu141, Tyr155 (hydrophobic), Asp221 (attractive charge), His223 (pi-pi stacked), Tyr114 (pi-alkyl) | Thr237, Arg220 | Trp105 (pi-pi T-shaped), His274 (pi-alkyl) |
| Limacusine (2) | None | His351, His356 (pi-pi T-shaped) | None | Val222, Tyr114 (pi-alkyl), Tyr155 (pi-pi stacked), Trp115, Asp116 (hydrophobic) | None | Glu276, Glu166 (attractive charge), Thr237 (hydrophobic), Trp105 (pi-pi stacked) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magpantay, H.D.; Malaluan, I.N.; Manzano, J.A.H.; Quimque, M.T.; Pueblos, K.R.; Moor, N.; Budde, S.; Bangcaya, P.S.; Lim-Valle, D.; Dahse, H.-M.; et al. Antibacterial and COX-2 Inhibitory Tetrahydrobisbenzylisoquinoline Alkaloids from the Philippine Medicinal Plant Phaeanthus ophthalmicus. Plants 2021, 10, 462. https://doi.org/10.3390/plants10030462
Magpantay HD, Malaluan IN, Manzano JAH, Quimque MT, Pueblos KR, Moor N, Budde S, Bangcaya PS, Lim-Valle D, Dahse H-M, et al. Antibacterial and COX-2 Inhibitory Tetrahydrobisbenzylisoquinoline Alkaloids from the Philippine Medicinal Plant Phaeanthus ophthalmicus. Plants. 2021; 10(3):462. https://doi.org/10.3390/plants10030462
Chicago/Turabian StyleMagpantay, Hilbert D., Ivane N. Malaluan, Joe Anthony H. Manzano, Mark Tristan Quimque, Kirstin Rhys Pueblos, Natalija Moor, Simon Budde, Porferio S. Bangcaya, Demi Lim-Valle, Hans-Martin Dahse, and et al. 2021. "Antibacterial and COX-2 Inhibitory Tetrahydrobisbenzylisoquinoline Alkaloids from the Philippine Medicinal Plant Phaeanthus ophthalmicus" Plants 10, no. 3: 462. https://doi.org/10.3390/plants10030462
APA StyleMagpantay, H. D., Malaluan, I. N., Manzano, J. A. H., Quimque, M. T., Pueblos, K. R., Moor, N., Budde, S., Bangcaya, P. S., Lim-Valle, D., Dahse, H.-M., Khan, A., Wei, D.-Q., Alejandro, G. J. D., & Macabeo, A. P. G. (2021). Antibacterial and COX-2 Inhibitory Tetrahydrobisbenzylisoquinoline Alkaloids from the Philippine Medicinal Plant Phaeanthus ophthalmicus. Plants, 10(3), 462. https://doi.org/10.3390/plants10030462