Identification of Bacterial Blight Resistance Loci in Rice (Oryza sativa L.) against Diverse Xoo Thai Strains by Genome-Wide Association Study
Abstract
:1. Introduction
2. Results
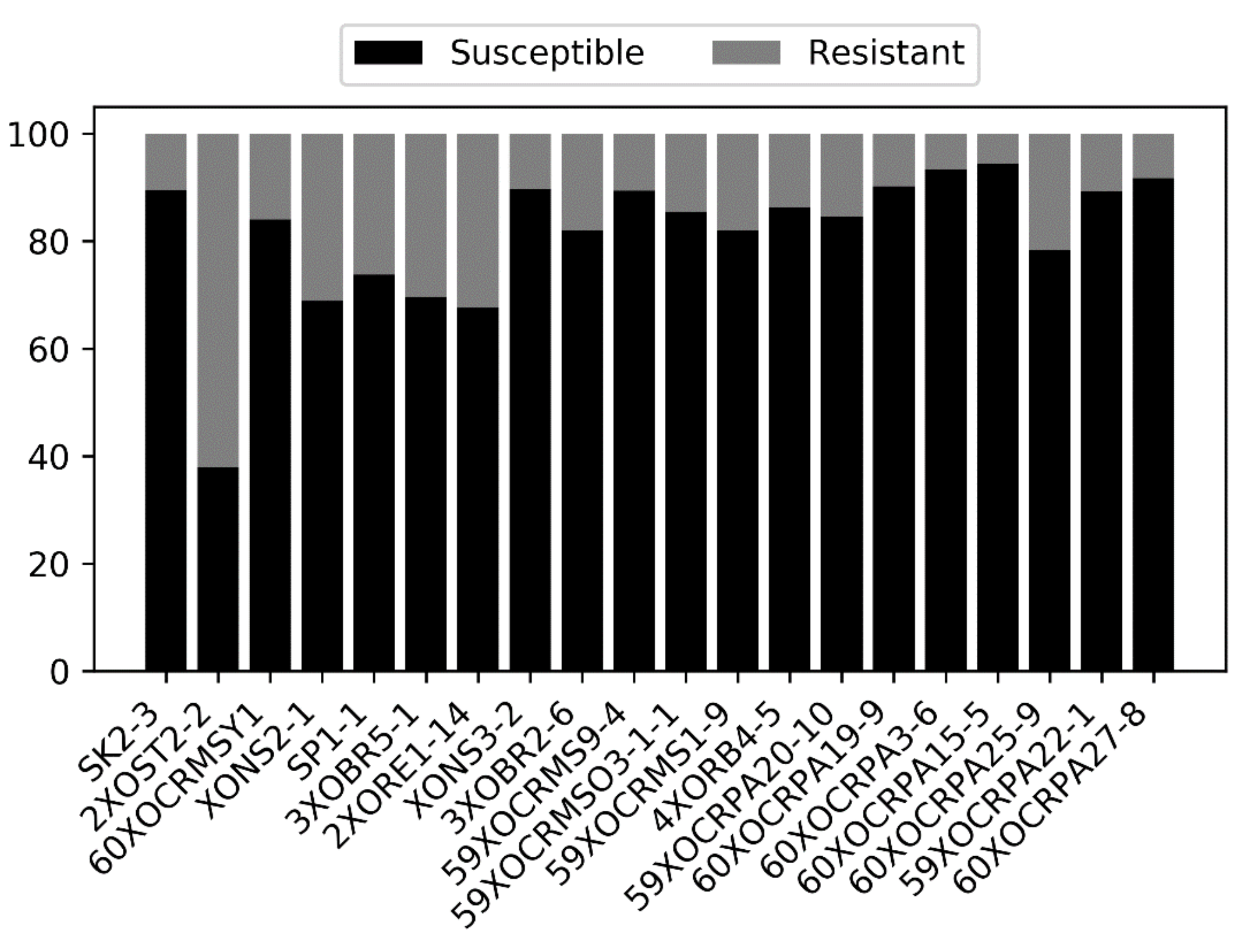
2.1. Phenotypic Screening
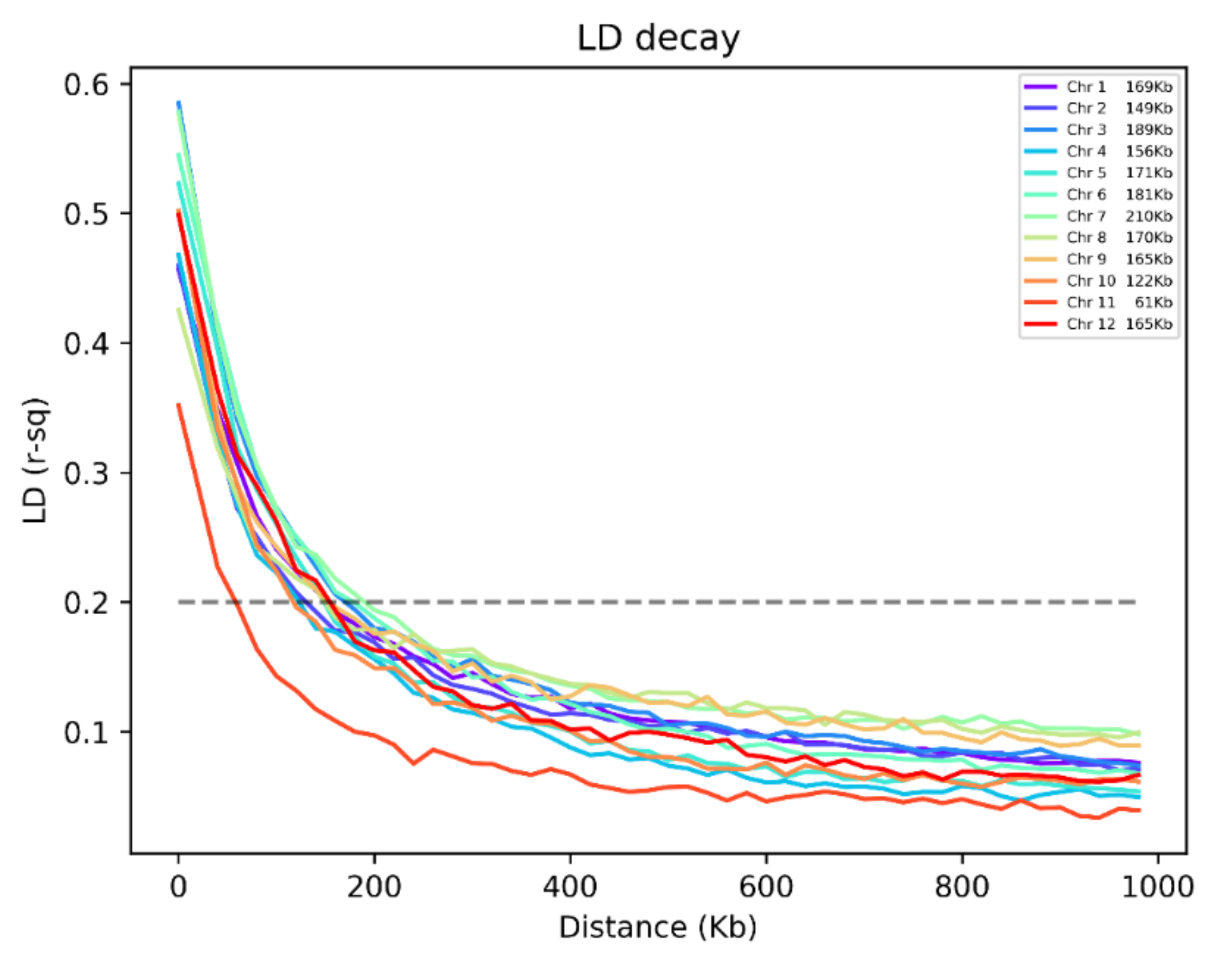
2.2. Population Structure of Rice Accessions
2.3. GWAS Analysis
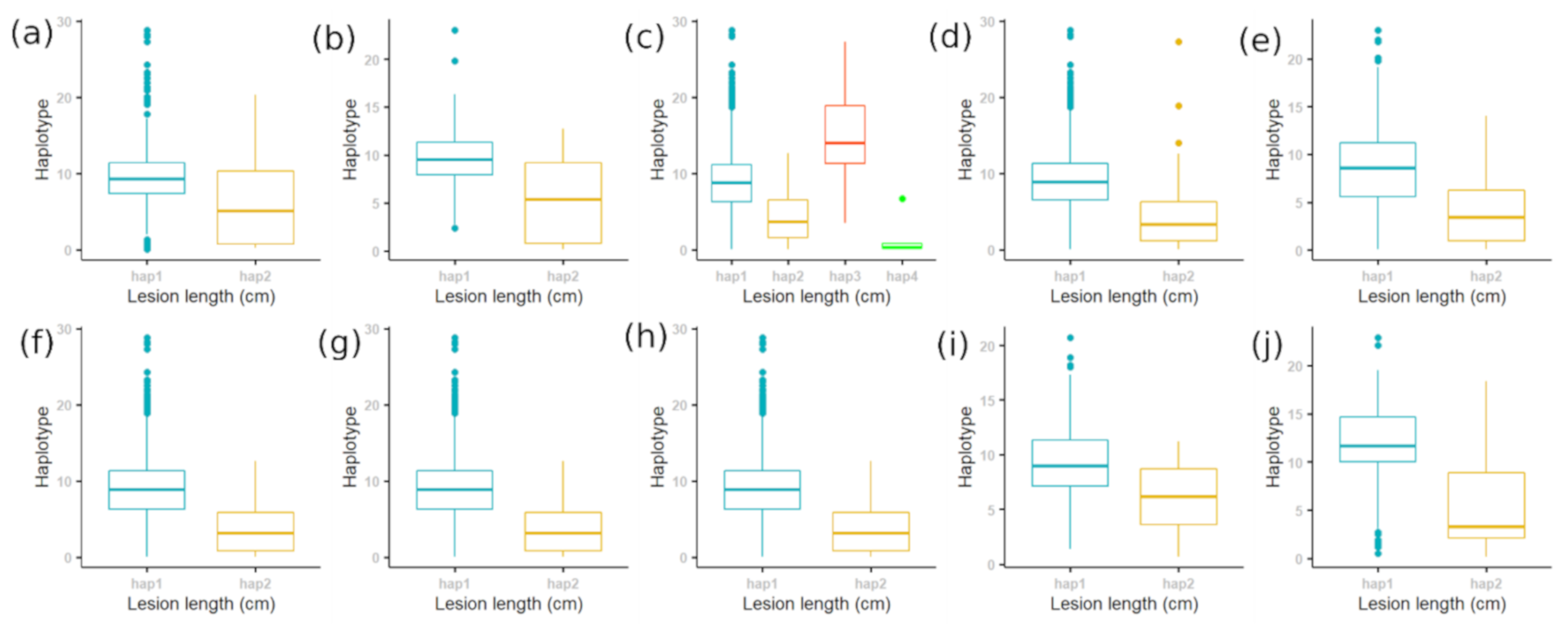
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. BLB Screening
4.3. Estimation of Population Parameters
4.4. Genome-Wide Association Study (GWAS) Methods
4.5. Additional Genomic Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huerta, A.I.; Delorean, E.E.; Bossa-Castro, A.M.; Tonnessen, B.W.; Raghavan, C.; Corral, R.; Pérez-Quintero, Á.L.; Leung, H.; Verdier, V.; Leach, J.E. Resistance and susceptibility QTL identified in a rice MAGIC population by screening with a minor-effect virulence factor from Xanthomonas oryzae pv. oryzae. Plant Biotechnol. J. 2021, 19, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Mew, T.W.; Cruz, C.V.; Medalla, E.S. Changes in race frequency of Xanthomonas oryzae pv. oryzae in response to rice cultivars planted in the Philippines. Plant Dis. 1992, 76, 1029–1032. [Google Scholar] [CrossRef]
- Ou, S.H. Rice Diseases; Commonwealth Mycological Institute: Kew, UK, 1985. [Google Scholar]
- Saha, S.; Garg, R.; Biswas, A.; Rai, A.B. Bacterial diseases of rice: An overview. J. Pure Appl. Microbiol. 2015, 9, 725–736. [Google Scholar]
- Srinivasan, B.; Gnanamanickam, S.S. Identification of a new source of resistance in wild rice, Oryza rufipogon to bacterial blight of rice caused by Indian strains of Xanthomonas oryzae pv. oryzae. Curr. Sci. 2005, 88, 1229–1231. [Google Scholar]
- Gautam, H.R.; Bhardwaj, M.L.; Kumar, R. Climate change and its impact on plant diseases. Curr. Sci. 2013, 105, 1685–1691. [Google Scholar]
- Reddy, A.P.K.; Mackenzie, D.R.; Rouse, D.I.; Rao, A.V. Relationship of bacterial leaf blight severity to grain yield of rice. Phytopathology 1979, 69, 967–969. [Google Scholar] [CrossRef]
- Schaad, N.W. Emerging plant pathogenic bacteria and global warming. In Pseudomonas syringae Pathovars and Related Pathogens—Identification, Epidemiology and Genomics; Fatmi, M., Collmer, A., Iacobellis, N.S., Mansfield, J.W., Murillo, J., Schaad, N.W., Ullrich, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 369–379. [Google Scholar]
- Chien, C.-C.; Chou, M.-Y.; Chen, C.-Y.; Shih, M.-C. Analysis of genetic diversity of Xanthomonas oryzae pv. oryzae populations in Taiwan. Sci. Rep. 2019, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.-Z.; Guan, Y.; Yang, R.; Qian, G.-L.; Yang, X.-H.; Wang, J.-S.; Jia, A.-Q. Growth inhibition and metabolomic analysis of Xanthomonas oryzae pv. oryzae treated with resveratrol. BMC Microbiol. 2020, 20, 117. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, H.; Inoue, Y.; Takeya, M.; Sasaki, A.; Kaku, H. Genome Sequence of Xanthomonas oryzae pv. oryzae Suggests Contribution of Large Numbers of Effector Genes and Insertion Sequences to Its Race Diversity. JARQ 2005, 39, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Kosawang, C.; Smitamana, P.; Toojinda, T.; Nilpanit, N.; Sirithunya, P. Amplified Fragment Length Polymorphism Fingerprinting Differentiates Genetic Diversity of Xanthomonas oryzae pv. oryzae from Northern Thailand. J. Phytopathol. 2006, 154, 550–555. [Google Scholar] [CrossRef]
- Sriprakorn, S. Identification and Geographical Distribution of Bacterial Leaf Blight Isolates (Xanthomonas oryzae pv. oryzae) and Tagging Resistance Genes in a Landrace. Master’s Thesis, Kasetsart University, Bangkok, Thailand, 2009. [Google Scholar]
- Apinyapanich, S. Studies on host range of Xanthomonas campestris Pv. Oryzae [rice] and chemical control. Master’s Thesis, Kasetsart University, Bangkok, Thailand, 1983. [Google Scholar]
- Adhikari, T.B.; Cruz, C.; Zhang, Q.; Nelson, R.J.; Skinner, D.Z.; Mew, T.W.; Leach, J.E. Genetic Diversity of Xanthomonas oryzae pv. oryzae in Asia. Appl. Environ. Microbiol. 1995, 61, 966–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, R.K.; Singh, P.K.; Sakthivel, K.; Srikumar, M.; Kumar, N.; Kumar, K.; Singh, A.K.; Roy, S.D. Analysis of Pathogenic Diversity of the Rice Bacterial Blight Pathogen (Xanthomonas oryzae pv. oryzae) in the Andaman Islands and Identification of Effective Resistance Genes. J. Phytopathol. 2015, 163, 423–432. [Google Scholar] [CrossRef]
- Gnanamanickam, S.S.; Priyadarisini, V.B.; Narayanan, N.N. An overview of bacterial blight disease of rice and strategies for its management. Curr. Sci. 1999, 77, 1435–1444. [Google Scholar]
- McDowell, J.M.; Woffenden, B.J. Plant disease resistance genes: Recent insights and potential applications. Trends Biotechnol. 2003, 21, 178–183. [Google Scholar] [CrossRef]
- Chen, X.L.; Yu, L.; Gao, L.L.; Jiang, T.; Li, Q.Y.; Huang, Q. Elevational Variation in Diversity of Xanthomonas oryzae pv. oryzae in South-West China. J. Phytopathol. 2012, 160, 261–268. [Google Scholar] [CrossRef]
- Nelson, R.; Wiesner-Hanks, T.; Wisser, R.; Balint-Kurti, P. Navigating complexity to breed disease-resistant crops. Nat. Rev. Genet. 2018, 19, 21–33. [Google Scholar] [CrossRef]
- Bhatia, D.; Lore, J.S.; Vikal, Y.; Gupta, V. Screening of Oryza species germplasm against a new variant of Xanthomonas oryzae pv. oryzae causing bacterial blight in Punjab. Plant Dis. 2010, 25, 44–47. [Google Scholar]
- Kumar, S.; Dwivedi, S.K.; Kumar, R.; Bhakta, N. Screening of different rice germplasm against multiple disease under submergence condition in middle Indo Gangetic Plain. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 335–339. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, F.M.; Masood, R.; Ahmad, H.; Khan, U. Molecular screening of Pakistani rice germplasm for xa5 gene resistance to bacterial blight. Afr. J. Biotechnol. 2011, 10, 2833–2837. [Google Scholar]
- Dilla-Ermita, C.J.; Tandayu, E.; Juanillas, V.M.; Detras, J.; Lozada, D.N.; Dwiyanti, M.S.; Vera Cruz, C.; Mbanjo, E.G.N.; Ardales, E.; Diaz, M.G.; et al. Genome-wide Association Analysis Tracks Bacterial Leaf Blight Resistance Loci In Rice Diverse Germplasm. Rice 2017, 10, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-M.; Reinke, R.F. A novel resistance gene for bacterial blight in rice, Xa43(t) identified by GWAS, confirmed by QTL mapping using a bi-parental population. PLoS ONE 2019, 14, e0211775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.B.; Zhang, D.P.; Lin, X.H. Identification and mapping of a novel bacterial blight resistance gene Xa35 (t) originated from Oryza minuta. Sci. Agric. Sin. 2010, 43, 2611–2618. [Google Scholar]
- Bhasin, H.; Bhatia, D.; Raghuvanshi, S.; Lore, J.S.; Sahi, G.K.; Kaur, B.; Vikal, Y.; Singh, K. New PCR-based sequence-tagged site marker for bacterial blight resistance gene Xa38 of rice. Mol. Breed. 2012, 30, 607–611. [Google Scholar] [CrossRef]
- Ellur, R.K.; Khanna, A.; Bhowmick, P.K.; Vinod, K.K.; Nagarajan, M.; Mondal, K.K.; Singh, N.K.; Singh, K.; Prabhu, K.V.; Singh, A.K.; et al. Marker-aided Incorporation of Xa38, a Novel Bacterial Blight Resistance Gene, in PB1121 and Comparison of its Resistance Spectrum with xa13 + Xa21. Sci. Rep. 2016, 6, 29188. [Google Scholar] [CrossRef]
- Kim, S.-M.; Suh, J.-P.; Qin, Y.; Noh, T.-H.; Reinke, R.F.; Jena, K.K. Identification and fine-mapping of a new resistance gene, Xa40, conferring resistance to bacterial blight races in rice (Oryza sativa L.). Appl. Genet. 2015, 128, 1933–1943. [Google Scholar] [CrossRef]
- Zhang, F.; Zhuo, D.L.; Zhang, F.; Huang, L.Y.; Wang, W.S.; Xu, J.L.; Vera Cruz, C.; Li, Z.K.; Zhou, Y.L. Xa39, a novel dominant gene conferring broad-spectrum resistance to Xanthomonas oryzae pv. oryzae in rice. Plant Pathol. 2015, 64, 568–575. [Google Scholar] [CrossRef]
- Liang, L.Q.; Wang, C.Y.; Zeng, L.X.; Wang, W.J.; Feng, J.Q.; Chen, B.; Su, J.; Chen, S.; Shang, F.D.; Zhu, X.Y.; et al. The rice cultivar Baixiangzhan harbours a recessive gene xa42 (t) determining resistance against Xanthomonas oryzae pv. oryzae. Plant Breed. 2017, 136, 603–609. [Google Scholar] [CrossRef]
- Miao, L.L.; Wang, C.L.; Zheng, C.K.; Che, J.Y.; Gao, Y. Molecular mapping of a new gene for resistance to rice bacterial blight. Sci. Agric. 2010, 43, 3051–3058. [Google Scholar]
- Busungu, C.; Taura, S.; Sakagami, J.-I.; Ichitani, K. Identification and linkage analysis of a new rice bacterial blight resistance gene from XM14, a mutant line from IR24. Breed. Sci. 2016, 66, 636–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Wang, C.; Yang, J.; Chen, B.; Wang, W.; Su, J.; Feng, A.; Zeng, L.; Zhu, X. Identification of the novel bacterial blight resistance gene Xa46(t) by mapping and expression analysis of the rice mutant H120. Sci. Rep. 2020, 10, 12642. [Google Scholar] [CrossRef] [PubMed]
- Chukwu, S.C.; Rafii, M.Y.; Ramlee, S.I.; Ismail, S.I.; Hasan, M.M.; Oladosu, Y.A.; Magaji, U.G.; Akos, I.; Olalekan, K.K. Bacterial leaf blight resistance in rice: A review of conventional breeding to molecular approach. Mol. Biol. Rep. 2019, 46, 1519–1532. [Google Scholar] [CrossRef]
- Win, K.M.; Korinsak, S.; Sirithunya, P.; Lanceras-Siangliw, J.; Jamboonsri, W.; Da, T.; Patarapuwadol, S.; Toojinda, T. Marker assisted introgression of multiple genes for bacterial blight resistance into aromatic Myanmar rice MK-75. Field Crop. Res. 2013, 154, 164–171. [Google Scholar] [CrossRef]
- Vikal, Y.; Bhatia, D. Geneticsand genomics of bacterial blight resistance in rice. In Advances in International Rice Research; Jin, Q.L., Ed.; Intech: Rijeka, Croatia, 2017. [Google Scholar]
- Liu, Q.; Yuan, M.; Zhou, Y.; Li, X.; Xiao, J.; Wang, S. A paralog of the MtN3/saliva family recessively confers race-specific resistance to Xanthomonas oryzae in rice. Plant Cell Environ. 2011, 34, 1958–1969. [Google Scholar] [CrossRef]
- Song, W.Y.; Wang, G.L.; Chen, L.L.; Kim, H.S.; Pi, L.Y.; Holsten, T.; Gardner, J.; Wang, B.; Zhai, W.X.; Zhu, L.H.; et al. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 1995, 270, 1804–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, A.S.; McCouch, S.R. The rice bacterial blight resistance gene xa5 encodes a novel form of disease resistance. Mol. Plant Microbe Interact. 2004, 17, 1348–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, D.; Wang, J.; Zeng, X.; Gu, K.; Qiu, C.; Yang, X.; Zhou, Z.; Goh, M.; Luo, Y.; Murata-Hori, M.; et al. The rice TAL effector-dependent resistance protein XA10 triggers cell death and calcium depletion in the endoplasmic reticulum. Plant Cell 2014, 26, 497–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Cao, Y.; Yang, Z.; Xu, C.; Li, X.; Wang, S.; Zhang, Q. Xa26, a gene conferring resistance to Xanthomonas oryzae pv. oryzae in rice, encodes an LRR receptor kinase-like protein. Plant J. 2004, 37, 517–527. [Google Scholar] [CrossRef]
- Chu, Z.; Fu, B.; Yang, H.; Xu, C.; Li, Z.; Sanchez, A.; Park, Y.J.; Bennetzen, J.L.; Zhang, Q.; Wang, S. Targeting xa13, a recessive gene for bacterial blight resistance in rice. Appl. Genet. 2006, 112, 455–461. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Fan, Y.; Gao, Y.; Zhu, Q.; Zheng, C.; Qin, T.; Li, Y.; Che, J.; Zhang, M.; et al. XA23 is an executor R protein and confers broad-spectrum disease resistance in rice. Mol. Plant 2014, 8, 290–302. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, S.; Yamanouchi, U.; Katayose, Y.; Toki, S.; Wang, Z.X.; Kono, I.; Kurata, N.; Yano, M.; Iwata, N.; Sasaki, T. Expression of Xa1, a bacterial blight-resistance gene in rice, is induced by bacterial inoculation. Proc. Natl. Acad. Sci. USA 1998, 95, 1663–1668. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Li, X.; Xu, C.; Wang, S. Fine genetic mapping of xa24, a recessive gene for resistance against Xanthomonas oryzae pv. oryzae in rice. Theor. Appl. Genet. 2008, 118, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Goh, M.L.; Sreekala, C.; Yin, Z. XA27 depends on an amino-terminal signal-anchor-like sequence to localize to the apoplast for resistance to Xanthomonas oryzae pv oryzae. Plant Physiol. 2008, 148, 1497–1509. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.; Cao, Y.; Xu, C.; Li, X.; Wang, S. Xa3, conferring resistance for rice bacterial blight and encoding a receptor kinase-like protein, is the same as Xa26. Theor. Appl. Genet. 2006, 113, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Kraakman, A.T.W.; Niks, R.E.; Van den Berg, P.M.M.M.; Stam, P.; Van Eeuwijk, F.A. Linkage disequilibrium mapping of yield and yield stability in modern spring barley cultivars. Genetics 2004, 168, 435–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brachi, B.; Morris, G.P.; Borevitz, J.O. Genome-wide association studies in plants: The missing heritability is in the field. Genome Biol. 2011, 12, 232. [Google Scholar] [CrossRef] [Green Version]
- Racedo, J.; Gutiérrez, L.; Perera, M.F.; Ostengo, S.; Pardo, E.M.; Cuenya, M.I.; Welin, B.; Castagnaro, A.P. Genome-wide association mapping of quantitative traits in a breeding population of sugarcane. BMC Plant Biol. 2016, 16, 142. [Google Scholar] [CrossRef] [Green Version]
- Nakano, Y.; Kobayashi, Y. Genome-wide Association Studies of Agronomic Traits Consisting of Field- and Molecular-based Phenotypes. Rev. Agric. Sci. 2020, 8, 28–45. [Google Scholar] [CrossRef] [Green Version]
- Verdeprado, H.; Kretzschmar, T.; Begum, H.; Raghavan, C.; Joyce, P.; Lakshmanan, P.; Cobb, J.N.; Collard, B.C.Y. Association mapping in rice: Basic concepts and perspectives for molecular breeding. Plant Prod. Sci. 2018, 21, 159–176. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Wu, Z.-C.; Wang, M.-M.; Zhang, F.; Dingkuhn, M.; Xu, J.-L.; Zhou, Y.-L.; Li, Z.-K. Genome-wide association analysis identifies resistance loci for bacterial blight in a diverse collection of indica rice germplasm. PLoS ONE 2017, 12, e0174598. [Google Scholar] [CrossRef] [Green Version]
- Bossa-Castro, A.M.; Tekete, C.; Raghavan, C.; Delorean, E.E.; Dereeper, A.; Dagno, K.; Koita, O.; Mosquera, G.; Leung, H.; Verdier, V.; et al. Allelic variation for broad-spectrum resistance and susceptibility to bacterial pathogens identified in a rice MAGIC population. Plant Biotechnol. J. 2018, 16, 1559–1568. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki-Tanaka, A.; Fukuta, Y. Genetic variation in resistance to blast disease (Pyricularia oryzae Cavara) in Japanese rice (Oryza sativa L.), as determined using a differential system. Breed. Sci. 2014, 64, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Descalsota, G.I.L.; Swamy, B.P.M.; Zaw, H.; Inabangan-Asilo, M.A.; Amparado, A.; Mauleon, R.; Chadha-Mohanty, P.; Arocena, E.C.; Raghavan, C.; Leung, H.; et al. Genome-Wide Association Mapping in a Rice MAGIC Plus Population Detects QTLs and Genes Useful for Biofortification. Front. Plant Sci. 2018, 9, 1347. [Google Scholar] [CrossRef]
- Chen, Z.; Feng, Z.; Kang, H.; Zhao, J.; Chen, T.; Li, Q.; Gong, H.; Zhang, Y.; Chen, X.; Pan, X.; et al. Identification of New Resistance Loci Against Sheath Blight Disease in Rice Through Genome-Wide Association Study. Rice Sci. 2019, 26, 21–31. [Google Scholar] [CrossRef]
- Huang, X.; Wei, X.; Sang, T.; Zhao, Q.; Feng, Q.; Zhao, Y.; Li, C.; Zhu, C.; Lu, T.; Zhang, Z.; et al. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat. Genet. 2010, 42, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Hur, Y.-J.; Cho, J.-H.; Park, H.-S.; Noh, T.-H.; Park, D.-S.; Lee, J.Y.; Sohn, Y.-B.; Shin, D.; Song, Y.C.; Kwon, Y.-U.; et al. Pyramiding of two rice bacterial blight resistance genes, Xa3 and Xa4, and a closely linked cold-tolerance QTL on chromosome 11. Appl. Genet. 2016, 129, 1861–1871. [Google Scholar] [CrossRef]
- Petpisit, V.; Khush, G.S.; Kauffman, H.E. Inheritance of resistance to bacterial blight in rice1. Crop Sci. 1977, 17, 551–554. [Google Scholar] [CrossRef]
- Streubel, J.; Pesce, C.; Hutin, M.; Koebnik, R.; Boch, J.; Szurek, B. Five phylogenetically close rice SWEET genes confer TAL effector-mediated susceptibility to Xanthomonas oryzae pv. oryzae. New Phytol. 2013, 200, 808–819. [Google Scholar] [CrossRef]
- NIÑO-LIU, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.K.; Arif, M.; Zhong, D.B.; Fu, B.Y.; Xu, J.L.; Domingo-Rey, J.; Ali, J.; Vijayakumar, C.H.M.; Yu, S.B.; Khush, G.S. Complex genetic networks underlying the defensive system of rice (Oryza sativa L.) to Xanthomonas oryzae pv. oryzae. Proc. Natl. Acad. Sci. USA 2006, 103, 7994–7999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kauffman, H.E. An improved technique for evaluat-ing resistance of rice varieties to Xanthomonas oryzae. Plant Dis. Rep. 1973, 57, 537–541. [Google Scholar]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. 1000 Genomes Project Analysis Group The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Shin, J.H.; Blay, S.; McNeney, B. LDheatmap: An R function for graphical display of pairwise linkage disequilibria between single nucleotide polymorphisms. J. Stat. Softw. 2006, 16, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Pressoir, G.; Briggs, W.H.; Vroh Bi, I.; Yamasaki, M.; Doebley, J.F.; McMullen, M.D.; Gaut, B.S.; Nielsen, D.M.; Holland, J.B.; et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 2006, 38, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Blom, G. Statistical Estimates and Transformed beta-Variables. Ph.D. Thesis, Stockholm College, Stockholm, Sweden, 1958. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Rom, D.M. A sequentially rejective test procedure based on a modified Bonferroni inequality. Biometrika 1990, 77, 663–665. [Google Scholar] [CrossRef]
- Ohyanagi, H.; Tanaka, T.; Sakai, H.; Shigemoto, Y.; Yamaguchi, K.; Habara, T.; Fujii, Y.; Antonio, B.A.; Nagamura, Y.; Imanishi, T.; et al. The Rice Annotation Project Database (RAP-DB): Hub for Oryza sativa ssp. japonica genome information. Nucleic Acids Res. 2006, 34, D741–D744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]


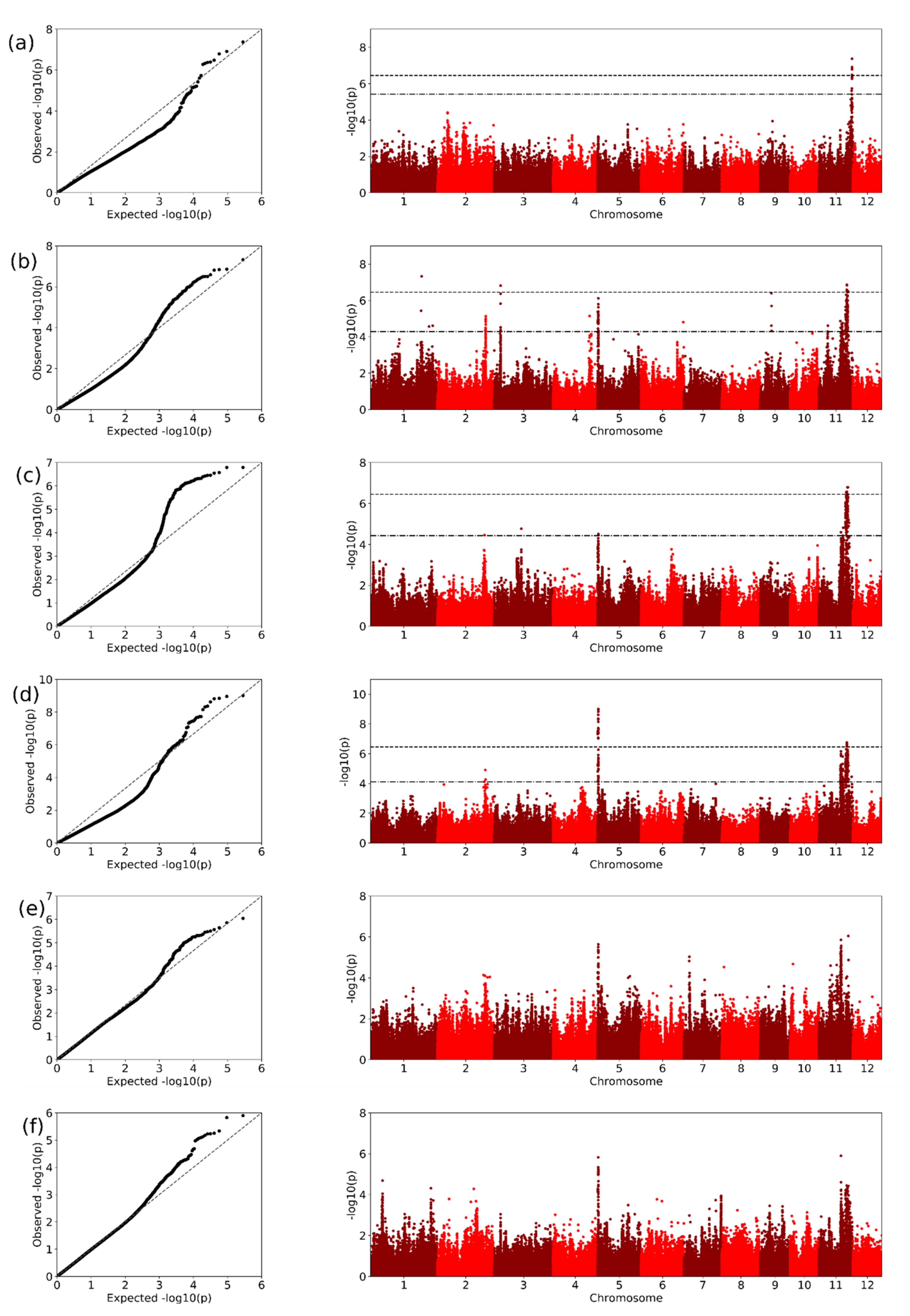
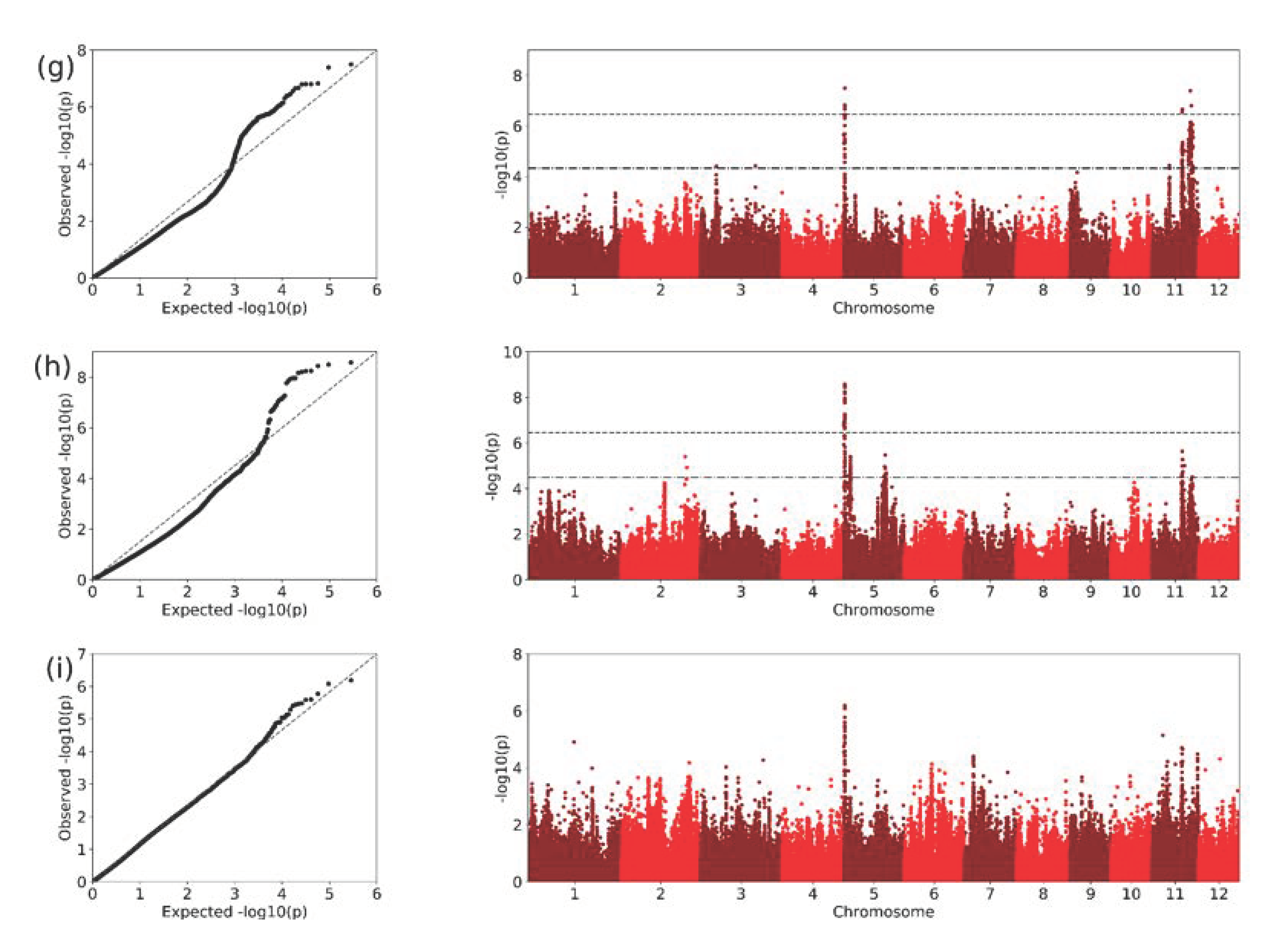


| Xoo Strain | Chr | Position (bp) | −log10 (p-Value) | SNP Genotypes | MAF (%) | Marker r2 | Genetic Var | Residual Var | Heritability |
|---|---|---|---|---|---|---|---|---|---|
| SP1-1 | 2 | 28895260 | 5.4 | G,T | 11.11 | 0.12 | 3.77 | 7.15 | 0.35 |
| 5 | 372359 | 8.58 | A,C | 8.78 | 0.19 | 3.77 | 7.15 | 0.35 | |
| 11 | 17828845 | 5.63 | C,T | 7.22 | 0.14 | 3.77 | 7.15 | 0.35 | |
| 2XOST2-2 | 1 | 35320544 | 7.33 | C,A | 5.91 | 0.16 | 4.5 | 5.44 | 0.45 |
| 2 | 30161196 | 5.13 | T,G | 13.44 | 0.13 | 4.5 | 5.44 | 0.45 | |
| 3 | 3379824 | 6.81 | A,G | 5.52 | 0.16 | 4.5 | 5.44 | 0.45 | |
| 4 | 30623393 | 5.15 | T,C | 6.15 | 0.11 | 4.5 | 5.44 | 0.45 | |
| 5 | 457765 | 6.12 | T,G | 4.59 | 0.13 | 4.5 | 5.44 | 0.45 | |
| 6 | 30380545 | 4.8 | C,A | 6.19 | 0.1 | 4.5 | 5.44 | 0.45 | |
| 9 | 10748870 | 6.4 | T,G | 7.04 | 0.14 | 4.5 | 5.44 | 0.45 | |
| 11 | 22311354 | 6.86 | A,G | 8.72 | 0.14 | 4.5 | 5.44 | 0.45 | |
| 3XOBR2-6 | 2 | 29390164 | 4.46 | C,T | 23.53 | 0.1 | 2.13 | 10.46 | 0.17 |
| 3 | 16299521 | 4.77 | G,C | 5.97 | 0.1 | 2.13 | 10.46 | 0.17 | |
| 5 | 463077 | 4.51 | A,G | 7.43 | 0.09 | 2.13 | 10.46 | 0.17 | |
| 11 | 23204208 | 6.8 | C,G | 6.82 | 0.17 | 2.13 | 10.46 | 0.17 | |
| 2XORE1-14 | 11 | 27582018 | 7.37 | A,G | 30.48 | 0.17 | 7.03 | 12.16 | 0.37 |
| 4XORB4-5 | 2 | 29791617 | 4.91 | C,A | 16.48 | 0.11 | 3.55 | 7.82 | 0.31 |
| 5 | 461986 | 9.01 | G,A | 4.5 | 0.2 | 3.55 | 7.82 | 0.31 | |
| 11 | 22258758 | 6.75 | G,A | 5.82 | 0.16 | 3.55 | 7.82 | 0.31 | |
| 59XOCRPA20-10 | 3 | 26795267 | 4.43 | T,C | 43.85 | 0.1 | 5.31 | 10.88 | 0.33 |
| 5 | 461986 | 7.5 | G,A | 4.5 | 0.17 | 5.31 | 10.88 | 0.33 | |
| 11 | 22311354 | 7.39 | A,G | 8.72 | 0.17 | 5.31 | 10.88 | 0.33 | |
| 60XOCRMSY1 | 1 | 33302431 | 5.17 | T,C | 6.53 | 0.1 | 10.28 | 9.19 | 0.53 |
| 3 | 16327075 | 4.54 | T,G | 7.27 | 0.11 | 10.28 | 9.19 | 0.53 | |
| 5 | 461986 | 7.31 | G,A | 4.5 | 0.16 | 10.28 | 9.19 | 0.53 | |
| 8 | 4006324 | 4.53 | G,A | 11.56 | 0.09 | 10.28 | 9.19 | 0.53 | |
| 10 | 15740836 | 4.7 | A,G | 5.83 | 0.1 | 10.28 | 9.19 | 0.53 | |
| 11 | 23617456 | 6.85 | A,T | 9.9 | 0.15 | 10.28 | 9.19 | 0.53 |
| Chromosome | Representative SNP Position (bp) | Xoo Strain | No. of Linkage Blocks | MSU ID: Annotation |
|---|---|---|---|---|
| 1 | 35320544 | 2XOST2-2; 60XOCRMSY1 | 5 | LOC_Os01g57082: insulin-degrading enzyme, putative, expressed; LOC_Os01g57590: expressed protein; LOC_Os01g59450: ZOS1-13-C2H2 zinc finger protein, expressed; LOC_Os01g60440: HEAT repeat family protein, putative, expressed; LOC_Os01g60660: methionyl-tRNA synthetase, putative, expressed; LOC_Os01g61110: ulp1 protease family, C-terminal catalytic domain containing protein, expressed; LOC_Os01g61120: expressed protein; LOC_Os01g61335: expressed protein; LOC_Os01g61440: expressed protein; LOC_Os01g61590: CAMK_CAMK_like.1—CAMK includes calcium/calmodulin dependent protein kinases, expressed; |
| 1 | 35320544 | 2XOST2-2; 60XOCRMSY1 | 4 | LOC_Os01g66490: no apical meristem protein, putative, expressed; LOC_Os01g66860: serine/threonine protein kinase, putative, expressed; LOC_Os01g70340: expressed protein; LOC_Os01g71960: endonuclease, putative, expressed; |
| 2 | 28895260 | SP1-1; 2XOST2-2; 3XOBR2-6; 4XORB4-5 | 3 | LOC_Os02g47310: Cyclopropane-fatty-acyl-phospholipid synthase, putative, expressed; LOC_Os02g48690: expressed protein; LOC_Os02g48730: rho GDP-dissociation inhibitor 1, putative, expressed; LOC_Os02g48830: microtubule associated protein, putative, expressed; LOC_Os02g48990: phosphatidylinositol transfer, putative, expressed; LOC_Os02g49140: glycosyltransferase, putative, expressed; LOC_Os02g49180: RNA polymerase subunit, putative, expressed; LOC_Os02g49230: CCT/B-box zinc finger protein, putative, expressed; LOC_Os02g49360: RNA methyltransferase domain-containing protein 2, putative, expressed; |
| 3 | 3379824 | 2XOST2-2; 59XOCRPA20-10 | 2 | LOC_Os03g06680: plant-specific domain TIGR01615 family protein, expressed; LOC_Os03g06710: PPR repeat domain containing protein, putative, expressed; LOC_Os03g12760: helix-loop-helix DNA-binding domain containing protein, expressed; |
| 3 | 3379824 | 3XOBR2-6; 60XOCRMSY1 | 1 | |
| 3 | 3379824 | 59XOCRPA20-10 | 1 | LOC_Os03g47380: expressed protein; |
| 4 | 30623393 | 2XOST2-2 | 1 | LOC_Os04g51690: glycosyl hydrolase family 47 domain contain protein, expressed; |
| 5 | 461986 | SP1-1; 2XOST2-2; 3XOBR2-6; 4XORB4-5; 59XOCRPA20-10; 60XOCRMSY1 | 3 | LOC_Os05g01030: phospholipid-transporting ATPase, putative, expressed; LOC_Os05g01060: expressed protein; LOC_Os05g01490: ras-related protein, putative, expressed; LOC_Os05g01500: tubulin-specific chaperone E, putative, expressed; LOC_Os05g01590: heat shock protein DnaJ, putative, expressed; LOC_Os05g01610: FYVE zinc finger domain containing protein, expressed; LOC_Os05g01620: OsFBX155—F-box domain containing protein, expressed; LOC_Os05g01690: expressed protein; LOC_Os05g01700: ABC transporter, ATP-binding protein, putative, expressed; LOC_Os05g01710: transcription initiation factor IIA gamma chain, putative, expressed; LOC_Os05g01730: drought induced 19 protein, putative, expressed; LOC_Os05g01750: TruB family pseudouridylate synthase, putative, expressed; LOC_Os05g01760: lysine ketoglutarate reductase trans-splicing related 1, putative, expressed; LOC_Os05g01780: STE_PAK_Ste20++TranslationKinase_Slob_Wnk.1 - STE kinases include homologs to sterile 7, sterile 11 and sterile 20 from yeast, expressed; LOC_Os05g01790: expressed protein; LOC_Os05g01810: xylem cysteine proteinase 2 precursor, putative, expressed; LOC_Os05g01910: pumilio-family RNA binding protein, putative, expressed; LOC_Os05g05700: cullin, putative, expressed; LOC_Os05g05770: hypothetical protein; LOC_Os05g05790: double-stranded RNA binding motif containing protein, expressed; LOC_Os05g05800: OsFBL21—F-box domain and LRR containing protein, expressed; LOC_Os05g05840: tRNA synthetase class II core domain containing protein, expressed; LOC_Os05g05880: retrotransposon protein, putative, unclassified, expressed; LOC_Os05g05910: retrotransposon protein, putative, unclassified, expressed; LOC_Os05g05950: TOC159, putative, expressed; LOC_Os05g06014: expressed protein; Xa5_Os05g01120: cytochrome P450, putative, expressed; |
| 5 | 461986 | SP1-1 | 3 | LOC_Os05g37500: expressed protein; LOC_Os05g37830: expressed protein; LOC_Os05g38270: regulator of chromosome condensation, putative, expressed; |
| 5 | 461986 | 60XOCRMSY1 | 1 | |
| 6 | 30380545 | 2XOST2-2 | 1 | LOC_Os06g50170: BRE, putative, expressed; |
| 8 | 4006324 | 60XOCRMSY1 | 1 | LOC_Os08g07170: cytokinin-O-glucosyltransferase 2, putative, expressed; LOC_Os08g07380: retrotransposon protein, putative, unclassified, expressed; |
| 9 | 10748870 | 2XOST2-2 | 1 | LOC_Os09g17650: expressed protein; |
| 10 | 15740836 | 60XOCRMSY1 | 1 | |
| 11 | 22311354 | 2XOST2-2; 59XOCRPA20-10 | 3 | LOC_Os11g13750: expressed protein; |
| 11 | 22311354 | SP1-1; 2XOST2-2; 3XOBR2-6; 4XORB4-5; 59XOCRPA20-10; 60XOCRMSY1 | 17 | LOC_Os11g30370: OsSPL19 - SBP-box gene family member, expressed; LOC_Os11g30560: dehydrogenase/reductase, putative, expressed; LOC_Os11g30600: hypothetical protein; LOC_Os11g30620: expressed protein; LOC_Os11g30740: transposon protein, putative, CACTA, En/Spm sub-class, expressed; LOC_Os11g30770: expressed protein; LOC_Os11g30790: expressed protein; LOC_Os11g30860: retrotransposon protein, putative, Ty3-gypsy subclass, expressed; LOC_Os11g30930: expressed protein; LOC_Os11g30940: retrotransposon protein, putative, unclassified, expressed; LOC_Os11g30960: retrotransposon protein, putative, unclassified, expressed; LOC_Os11g31050: retrotransposon protein, putative, unclassified, expressed; LOC_Os11g31090: transferase family protein, putative, expressed; LOC_Os11g31500: ATP binding protein, putative, expressed; LOC_Os11g31620: OsFBL55—F-box domain and LRR containing protein, expressed; LOC_Os11g31650: expressed protein; LOC_Os11g31670: retrotransposon protein, putative, unclassified, expressed; LOC_Os11g31690: expressed protein; LOC_Os11g31950: expressed protein; LOC_Os11g32210: jacalin-like lectin domain containing protein, expressed; LOC_Os11g32320: CCB1, putative, expressed; LOC_Os11g32340: hypothetical protein; LOC_Os11g32360: expressed protein; LOC_Os11g32369: expressed protein; LOC_Os11g32390: expressed protein; LOC_Os11g32410: expressed protein; LOC_Os11g32530: retrotransposon protein, putative, unclassified, expressed; LOC_Os11g32570: expressed protein; LOC_Os11g33190: OsFBX422 - F-box domain containing protein, expressed; LOC_Os11g35870: RWD domain containing protein, expressed; LOC_Os11g36050: prefoldin subunit, putative, expressed; LOC_Os11g36060: THUMP domain-containing protein, putative, expressed; LOC_Os11g36070: expressed protein; LOC_Os11g36090: receptor kinase, putative, expressed; LOC_Os11g36140: receptor-like protein kinase 2 precursor, putative, expressed; LOC_Os11g36180: receptor kinase, putative, expressed; LOC_Os11g36340: lymphoid organ expressed yellow head virus receptor protein, putative, expressed; LOC_Os11g36350: OsFBDUF50—F-box and DUF domain containing protein, expressed; LOC_Os11g36390: RFC1 - Putative clamp loader of PCNA, replication factor C subunit 1, expressed; LOC_Os11g37000: heat shock protein DnaJ, putative, expressed; LOC_Os11g37090: pumilio-family RNA binding repeat domain containing protein, expressed; LOC_Os11g37100: expressed protein; LOC_Os11g37130: mttA/Hcf106 family protein, putative, expressed; LOC_Os11g37140: expressed protein; LOC_Os11g37260: SEY1, putative, expressed; LOC_Os11g37300: OsFBDUF53—F-box and DUF domain containing protein, expressed; LOC_Os11g37330: pentatricopeptide repeat domain containing protein, putative, expressed; LOC_Os11g37510: ribosomal protein L4, putative, expressed; LOC_Os11g37670: expressed protein; LOC_Os11g37680: expressed protein; LOC_Os11g37690: TBC domain containing protein, expressed; LOC_Os11g37700: pleiotropic drug resistance protein, putative, expressed; LOC_Os11g37730: glutathione S-transferase, N-terminal domain containing protein, expressed; LOC_Os11g37740: stripe rust resistance protein Yr10, putative, expressed; |
| 11 | 22311354 | SP1-1; 2XOST2-2; 3XOBR2-6; 2XORE1-14; 4XORB4-5; 59XOCRPA20-10; 60XOCRMSY1 | 12 | LOC_Os11g37860: stripe rust resistance protein Yr10, putative, expressed; LOC_Os11g37870: stripe rust resistance protein Yr10, putative, expressed; LOC_Os11g37890: NAD dependent epimerase/dehydratase family protein, putative, expressed; LOC_Os11g37960: WIP4—Wound-induced protein precursor, expressed; LOC_Os11g38010: targeting protein for Xklp2, putative, expressed; LOC_Os11g38020: GTPase of unknown function domain containing protein, putative, expressed; LOC_Os11g38040: expressed protein; LOC_Os11g38050: phosphoesterase family protein, putative, expressed; LOC_Os11g38140: OsFBDUF58—F-box and DUF domain containing protein, expressed; LOC_Os11g38270: hypothetical protein; LOC_Os11g38620: expressed protein; LOC_Os11g38630: expressed protein; LOC_Os11g38640: expressed protein; LOC_Os11g38670: DEAD-box ATP-dependent RNA helicase, putative, expressed; LOC_Os11g38800: zinc finger family protein, putative, expressed; LOC_Os11g38810: mannose-6-phosphate isomerase, putative, expressed; LOC_Os11g38870: helix-loop-helix DNA-binding domain containing protein, expressed; LOC_Os11g38900: histone-lysine N-methyltransferase, H3 lysine-9 specific SUVH1, putative, expressed; LOC_Os11g38970: expressed protein; LOC_Os11g39360: pentatricopeptide repeat domain containing protein, putative, expressed; LOC_Os11g39540: 14-3-3 protein, putative, expressed; LOC_Os11g39650: WD domain, G-beta repeat domain containing protein, expressed; LOC_Os11g40200: expressed protein; LOC_Os11g40590: DUF1399 containing protein, putative, expressed; LOC_Os11g40840: receptor-like protein kinase 2 precursor, putative, expressed; LOC_Os11g44910: DEAD-box ATP-dependent RNA helicase, putative, expressed; |
| 11 | 22311354 | 2XORE1-14 | 3 | LOC_Os11g45290: retrotransposon protein, putative, unclassified, expressed; LOC_Os11g45580: embryogenesis transmembrane protein, putative, expressed; LOC_Os11g45590: transposon protein, putative, CACTA, En/Spm sub-class, expressed; LOC_Os11g45620: rust-resistance protein Lr21, putative, expressed. |
| MSU Locus | Haplotype Comparison | Mean Diffs | Lower CI (95%) | Upper CI (95%) | Adjusted P |
|---|---|---|---|---|---|
| LOC_Os11g31620 | Hap1-Hap2 | −3.803 | −5.44 | −2.167353 | 6.40E-06 *** |
| LOC_Os11g32210 | Hap1-Hap2 | −4.750 | −6.037 | −3.462886 | 0 *** |
| LOC_Os11g36390 | Hap1-Hap2 | −4.44 | −5.858 | −3.021265 | 0 *** |
| Hap1-Hap3 | 5.879 | 1.129 | 10.62905 | 0.008 ** | |
| Hap1-Hap4 | −7.501 | −12.251 | −2.75095 | 3E-04 *** | |
| Hap2-Hap3 | 10.319 | 5.383 | 15.253991 | 5E-07 *** | |
| Hap2-Hap4 | −3.061 | −7.997 | 1.873991 | 0.3812906 | |
| Hap3-Hap4 | −13.38 | −20.081 | −6.678551 | 2E-06 *** | |
| LOC_Os11g37740 | Hap1-Hap2 | −4.866 | −5.726 | −4.00688 | 0 *** |
| LOC_Os11g37860 | Hap1-Hap2 | −4.467 | −5.671 | −3.263031 | 0 *** |
| LOC_Os11g37870 | Hap1-Hap2 | −5.283 | −6.363 | −4.202503 | 0 *** |
| LOC_Os11g37960 | Hap1-Hap2 | −5.283 | −6.363 | −4.202503 | 0 *** |
| LOC_Os11g38870 | Hap1-Hap2 | −5.283 | −6.363 | −4.202503 | 0 *** |
| Xoo Strain | No. of MSU Genes Affected | Min. No. SNPs Per Gene | Max. No. SNPs Per Gene |
|---|---|---|---|
| SP1-1 | 33 | 1 | 4 |
| 2XOST2-2 | 62 | 1 | 4 |
| 3XOBR2-6 | 44 | 1 | 5 |
| 2XORE1-14 | 4 | 1 | 3 |
| 4XORB4-5 | 79 | 1 | 4 |
| 59XOCRPA20-10 | 54 | 1 | 3 |
| 60XOCRMSY1 | 66 | 1 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korinsak, S.; Darwell, C.T.; Wanchana, S.; Praphaisal, L.; Korinsak, S.; Thunnom, B.; Patarapuwadol, S.; Toojinda, T. Identification of Bacterial Blight Resistance Loci in Rice (Oryza sativa L.) against Diverse Xoo Thai Strains by Genome-Wide Association Study. Plants 2021, 10, 518. https://doi.org/10.3390/plants10030518
Korinsak S, Darwell CT, Wanchana S, Praphaisal L, Korinsak S, Thunnom B, Patarapuwadol S, Toojinda T. Identification of Bacterial Blight Resistance Loci in Rice (Oryza sativa L.) against Diverse Xoo Thai Strains by Genome-Wide Association Study. Plants. 2021; 10(3):518. https://doi.org/10.3390/plants10030518
Chicago/Turabian StyleKorinsak, Siriporn, Clive T. Darwell, Samart Wanchana, Lawan Praphaisal, Siripar Korinsak, Burin Thunnom, Sujin Patarapuwadol, and Theerayut Toojinda. 2021. "Identification of Bacterial Blight Resistance Loci in Rice (Oryza sativa L.) against Diverse Xoo Thai Strains by Genome-Wide Association Study" Plants 10, no. 3: 518. https://doi.org/10.3390/plants10030518





