Sex-Linked Molecular Markers Identify Female Lines in Endosperm-Derived Kiwifruit Callus and in Regenerants
Abstract
:1. Introduction
2. Results
2.1. Callus Lines, Plant Regeneration, and Acclimatization
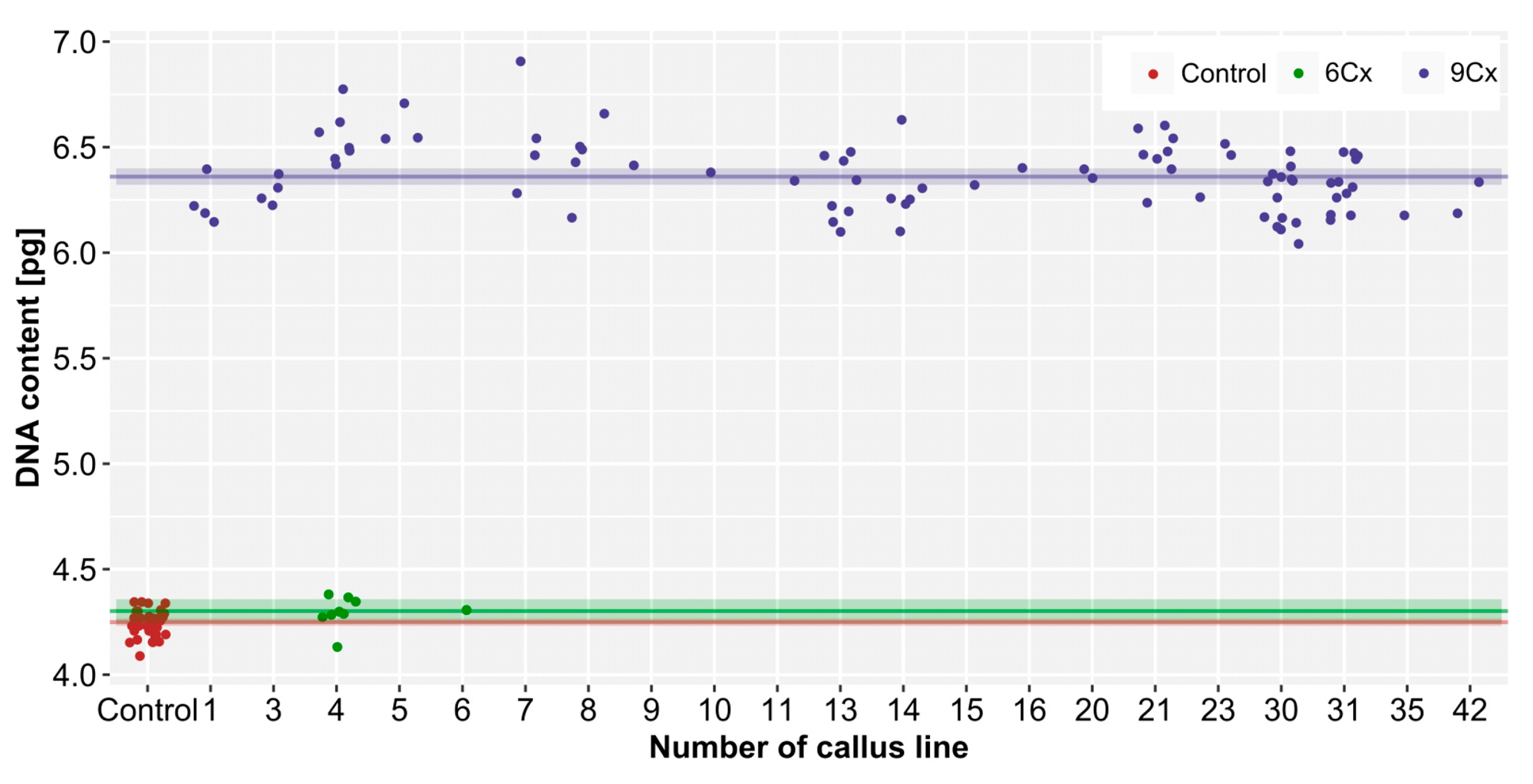
2.2. Nuclear DNA Content and Ploidy Range
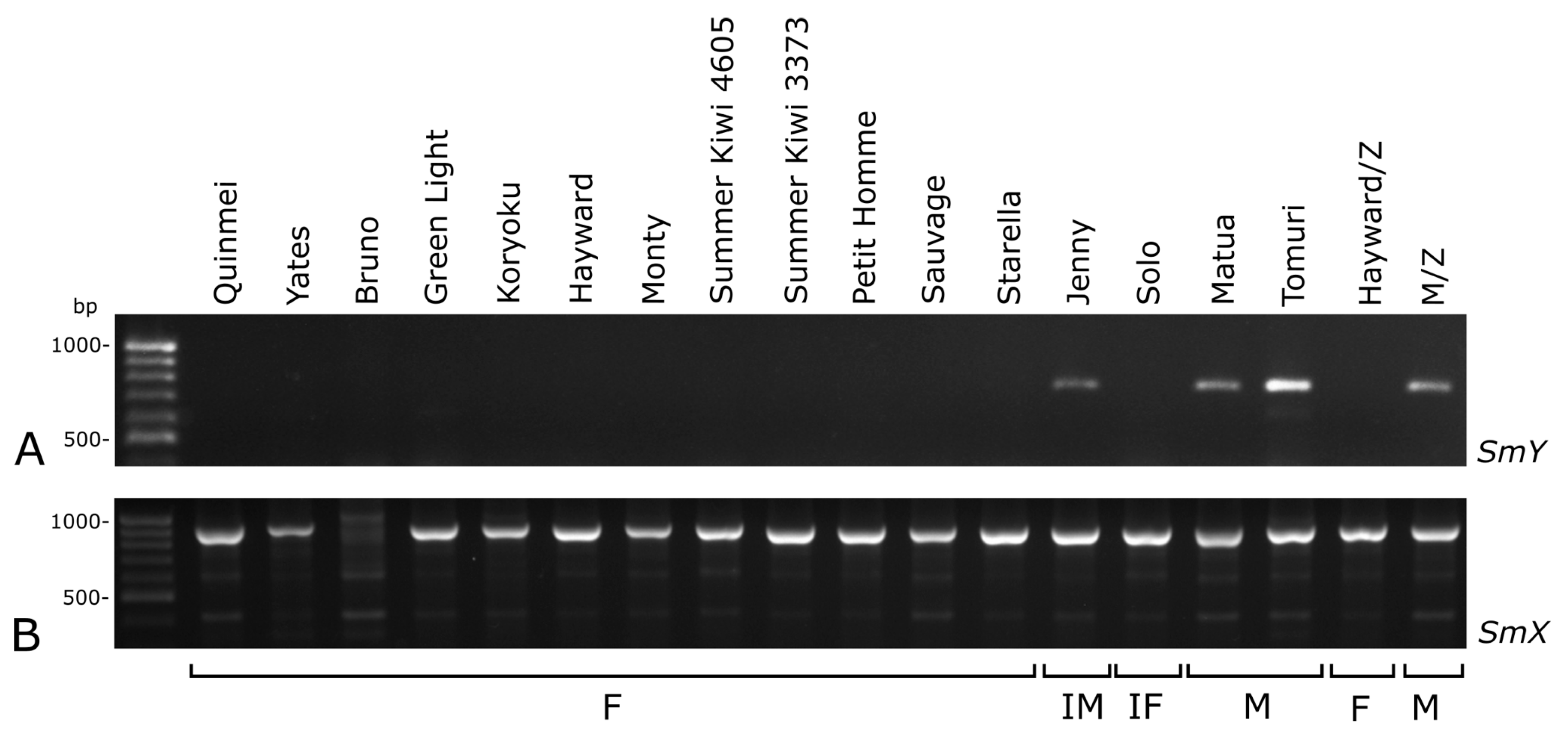
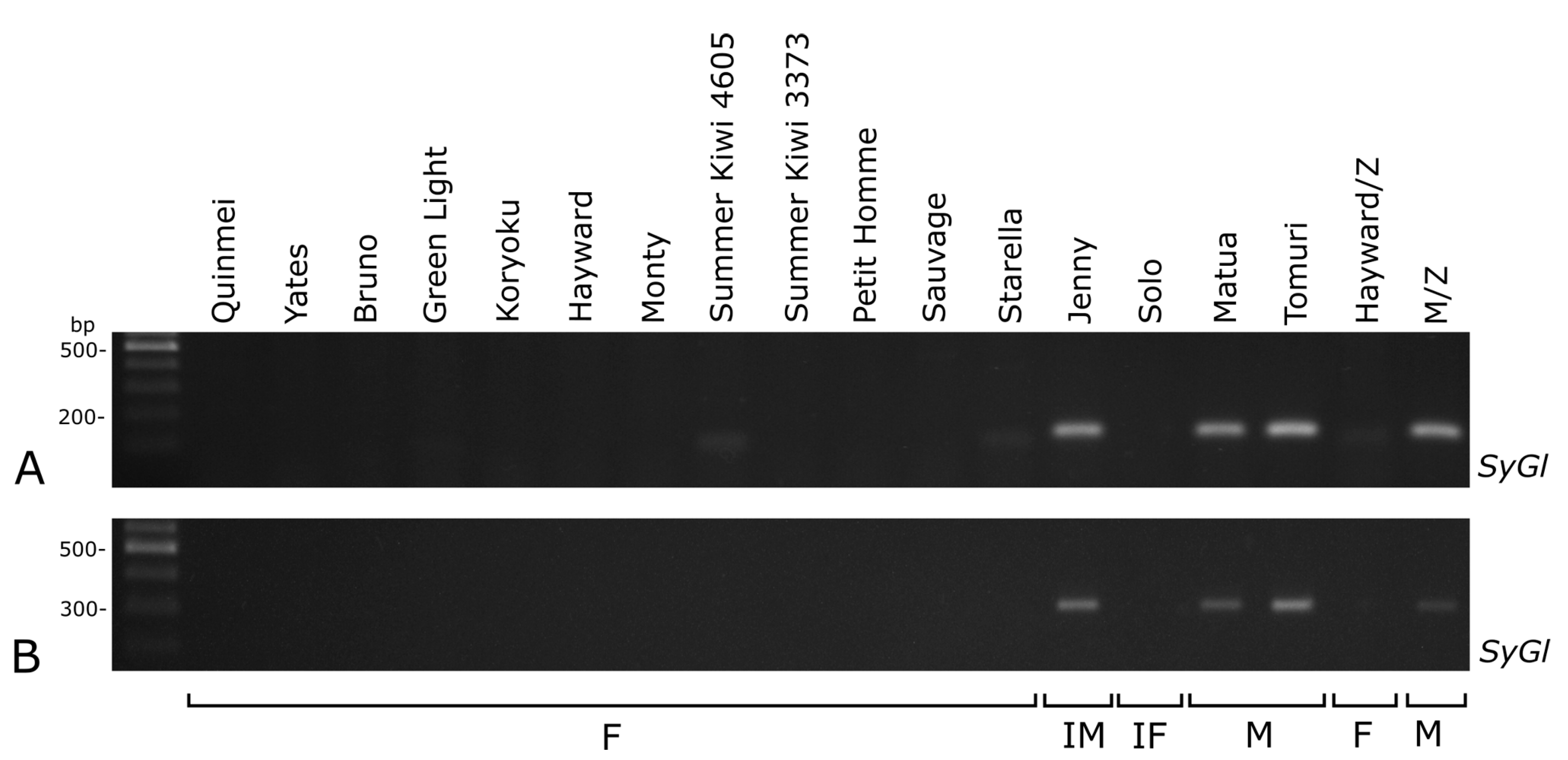
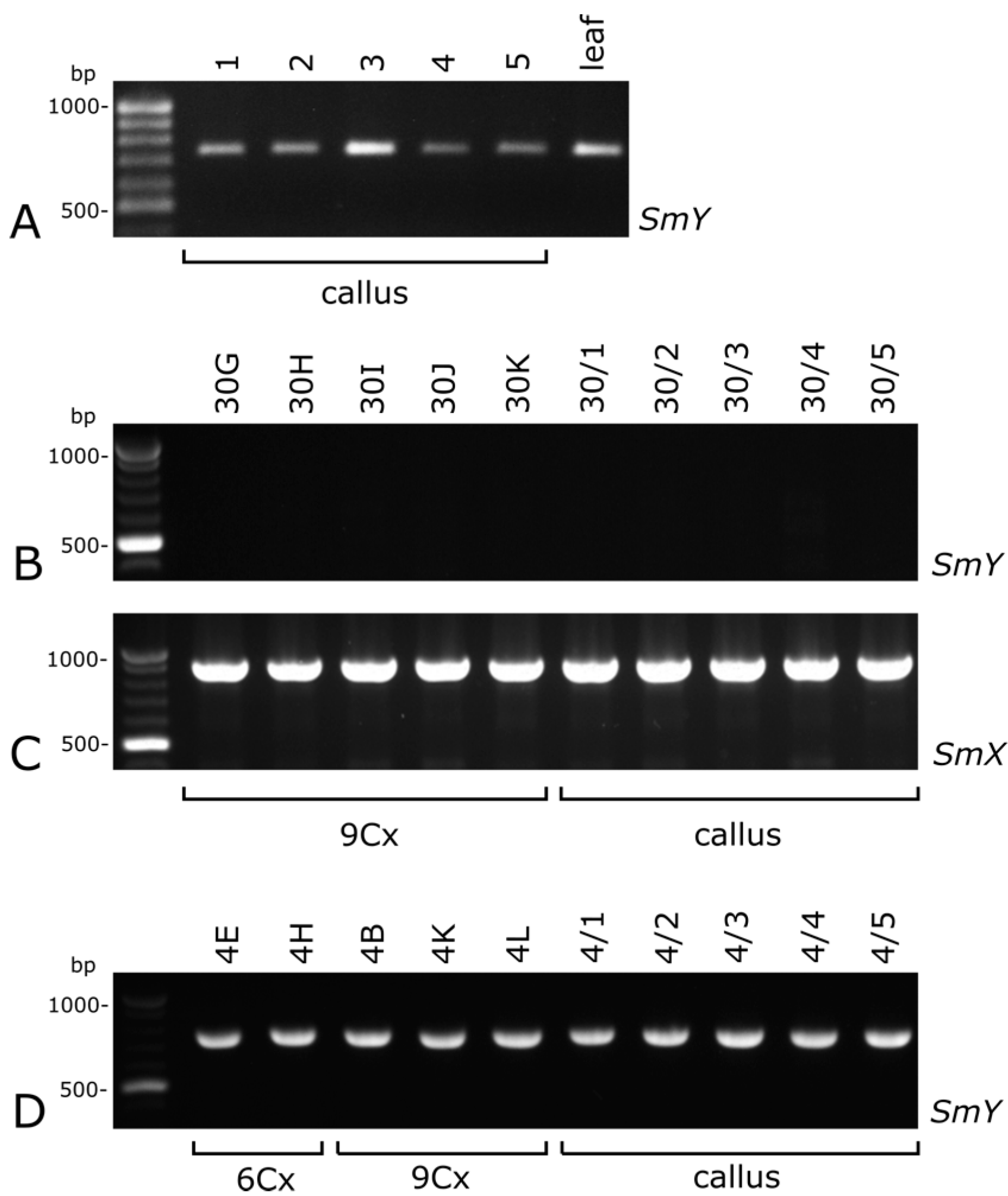
2.3. Sex-Linked Molecular Markers Useful in Sex Identification of Endosperm-derived Callus Lines and Regenerated Plants
3. Discussion
3.1. Callus Multiplication, Plants Regeneration, and Acclimatization to Ex Vitro Condition
3.2. Nuclear DNA Content and Ploidy of the Regenerants
3.3. Sex-Linked Molecular Markers Identify Sex of In Vitro and Ex Vitro Samples
4. Materials and Methods
4.1. Culture of Endosperm-Derived Callus, Plant Regeneration and Acclimation to Ex Vitro Conditions
4.2. Flow Cytometric Analyses
4.3. Sex-Linked Molecular Markers
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cailleau, A.; Cheptou, P.O.; Lenormand, T. Ploidy and the evolution of endosperm of flowering plants. Genetics 2010, 184, 439–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eng, W.H.; Ho, W.S. Polyploidization using colchicine in horticultural plants: A review. Sci. Hortic. 2019, 246, 604–617. [Google Scholar] [CrossRef]
- Hoshino, Y.; Miyashita, T.; Thomas, T.D. In vitro culture of endosperm and its application in plant breeding: Approaches to polyploidy breeding. Sci. Hortic. 2011, 130, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Cheng, Z.M.; Zhi, S.; Xu, F. Breeding triploid plants: A review. Czech. J. Genet. Plant Breed. 2016, 52, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Köhler, C.; Scheid, O.M.; Erilova, A. The impact of the triploid block on the origin and evolution of polyploid plants. Trends Genet. 2010, 26, 142–148. [Google Scholar] [CrossRef]
- Huang, H.W. Kiwifruit: The Genus ACTINIDIA; Elsevier Inc.: Saint Louis, MN, USA, 2016. [Google Scholar] [CrossRef]
- Ferguson, A.R. Botanical description. In The Kiwifruit Genome, Compendium of Plant Genomes; Testolin, R., Huang, H.W., Ferguson, A.R., Eds.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Fraser, L.G.; McNeilage, M.A. Reproductive biology. In The Kiwifruit Genome, Compendium of Plant Genomes; Testolin, R., Huang, H.W., Ferguson, A.R., Eds.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Przywara, L.; Pandey, K.K.; Sanders, P.M. Length of stomata as an indicator of ploidy level in Actinidia deliciosa. N. Z. J. Bot. 1988, 26, 179–182. [Google Scholar] [CrossRef]
- Boase, M.R.; Hopping, M.E. DNA dodecaploid plants detected among somaclonal of Actinidia deliciosa var. deliciosa cv. Hayward. Plant Cell Rep. 1995, 14, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.R.; Huang, H.W. Cytology, ploidy and ploidy manipulation. In The Kiwifruit Genome, Compendium of Plant Genomes; Testolin, R., Huang, H.W., Ferguson, A.R., Eds.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Seal, A.G.; Ferguson, A.R.; Silva, H.N.; Zhang, J.L. The effect of 2n gametes on sex ratios in Actinidia. Sex Plant Reprod. 2012, 25, 197–203. [Google Scholar] [CrossRef]
- Hirsch, A.M.; Testolin, R.; Brown, S.; Chat, J.; Fortune, D.; Bureau, J.M.; Nay, D. Embryo rescue from interspecific crosses in the genus Actinidia (kiwifruit). Plant Cell Rep. 2001, 20, 508–516. [Google Scholar] [CrossRef]
- Gui, Y.; Hong, S.; Ke, S.; Skirvin, R.M. Fruit and vegetative characteristic of endosperm-derived kiwifruit (Actinidia chinensis F) plants. Euphytica 1993, 71, 57–62. [Google Scholar] [CrossRef]
- Góralski, G.; Popielarska, M.; Ślesak, H.; Siwińska, D.; Batycka, M. Organogenesis in endosperm of Actinidia deliciosa cv. Hayward cultured in vitro. Acta Biol. Cracov. Ser. Bot. 2005, 47, 121–128. [Google Scholar]
- McNeilage, M.A. Progress in breeding hermaphrodite kiwifruit cultivars and understanding the genetics of sex determination. Acta Hortic. 1997, 444, 73–78. [Google Scholar] [CrossRef]
- Fraser, L.G.; Tsang, G.K.; Datson, P.; De Silva, H.N.; Harvey, C.F.; Gill, P.G.; Crowhurst, R.N.; McNeilage, M.A. A gene-rich linkage map in a dioecious species Actinidia chinensis (kiwifruit) reveals putative X/Y sex determining chromosomes. BMC Genom. 2009, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Testolin, R.; Cipriani, G.; Messina, R. Sex control in Actinidia is monofactorial and remains so in polyploids. In Sex Determination in Plants; Ainsworth, C.C., Ed.; BIOS Scientific Publishers Ltd.: Oxford, UK, 1999; pp. 173–181. [Google Scholar]
- Testolin, R.; Cipriani, G. Markers, maps, and marker-assisted selection. In The Kiwifruit Genome, Compendium of Plant Genomes; Testolin, R., Huang, H.W., Ferguson, A.R., Eds.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Gill, G.P.; Harvey, C.F.; Gardner, R.C.; Fraser, G. Development of sex-linked PCR markers for gender identification in Actinidia. Theor. Appl. Genet. 1998, 97, 439–445. [Google Scholar] [CrossRef]
- Shirkot, P.; Sharma, D.R.; Mohapatra, T. Molecular identification of sex in Actinidia deliciosa var. deliciosa by RAPD markers. Sci. Hortic. 2002, 94, 33–39. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, C.; Liu, Y.; VanBuren, R.; Yao, X.; Zhong, C.; Huang, H. High density interspecific genetic maps of kiwifruit and the identification of sex-specific markers. DNA Res. 2015, 22, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Akagi, T.; Pilkington, S.M.; Akagi, T.; Henry, I.M.; Ohtani, H.; Morimoto, T.; Beppu, K.; Kataoka, I.; Tao, R. A Y-encoded suppressor of feminization arose via lineage-specific duplication of a cytokinin response regulator in kiwifruit. Plant Cell 2018, 30, 780–795. [Google Scholar] [CrossRef]
- Akagi, T.; Pilkinton, S.M.; Varkonyi-Gasic, E.; Henry, I.M.; Sugano, S.S.; Sonoda, M.; Firl, A.; McNeilage, M.A.; Douglas, M.J.; Wang, T.; et al. Two Y-chromosome-encoded genes determine sex in kiwifruit. Nat. Plants 2019, 5, 801–809. [Google Scholar] [CrossRef]
- Ferguson, A.R. Kiwifruit: The wild and cultivated plants. In Nutritional Benefits of Kiwifruit; Boland, M., Moughan, P.J., Eds.; Adv Food Nutr Res; Elsevier Inc.: Waltham, MA, USA, 2013; Volume 68, pp. 15–32. [Google Scholar] [CrossRef]
- Gustafson, H. Marker Assisted Selection of Sex Determination in Kiwiberry (Actinidia arguta and Actinidia kolomikta). Master’s Thesis, University of New Hampshire, Durham, UK, 2016. Available online: https://scholars.unh.edu/thesis/1101 (accessed on 20 April 2019).
- Czernicka, M.; Chłosta, I.; Kęska, K.; Kozieradzka-Kiszkurno, M.; Abdullah, M.; Popielarska-Konieczna, M. Protuberances are organized distinct regions of long-term callus: Histological and transcriptomic analyses in kiwifruit. Plant Cell Rep. 2021. [Google Scholar] [CrossRef]
- Thomas, T.D.; Bhatnagar, A.K.; Bhojwani, S.S. Production of triploid plants of mulberry (Morus alba L) by endosperm culture. Plant Cell Rep. 2000, 19, 395–399. [Google Scholar] [CrossRef]
- Sun, D.Q.; Lu, X.H.; Liang, G.L.; Guo, Q.G.; Mo, Y.W.; Xie, J.H. Production of triploid plants of papaya by endosperm culture. Plant Cell Tiss. Org. Cult. 2011, 104, 23–29. [Google Scholar] [CrossRef]
- Thang, B.V.; Viet, N.V.; Nam, V.Q.; Tung, H.T.; Nhut, D.T. Triploid pant regeneration from immature endosperms of Melia azedazach. Plant Cell Tiss. Org. Cult. 2018, 133, 351–357. [Google Scholar] [CrossRef]
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Muñoz-Concha, D. Culture of tripoid tissue from the endosperm of an endangered Chilean tree species Gomortegakeule. J. Hortic. Sci. Biotech. 2016, 91, 79–86. [Google Scholar] [CrossRef]
- Lowe, A.J.; Hanotte, O.H.; Guarino, L. Standardization of molecular genetic techniques for the characterization of germplasm collections: The case of Random Amplified Polymorphic DNA (RAPD). Plant Genet. Resour. Newsl. 1996, 107, 50–54. Available online: https://hdl.handle.net/10568/29494 (accessed on 15 May 2000).
- Testolin, R.; Messina, R.; Lai, O.; Cipriani, G. A natural sex mutant in kiwifruit (Actinidia deliciosa). N. Zeal. J. Crop. Hort. Sci. 2004, 32, 179–183. [Google Scholar] [CrossRef]
- Popielarska-Konieczna, M.; Kozieradzka-Kiszkurno, M.; Bohdanowicz, J. Cutin plays a role in differentiation of endosperm-derived callis in kiwifruit. Plant Cell Rep. 2011, 30, 2143–2152. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Ducár, E.; Rewers, M.; Jedrzejczyk, I.; Mártonfi, P.; Sliwinska, E. Comparison of the genome size, endoreduplication, and ISSR marker polymorphism in eight Lotus (Fabaceae) species. Turk. J. Bot. 2018, 42, 1–14. [Google Scholar] [CrossRef]
- Marie, D.; Brown, S.C. A cytometric exercise in plant histograms, with 2C values for 70 species. Biol. Cell 1993, 78, 41–51. [Google Scholar] [CrossRef]
- Greilhuber, J.; Doležel, J.; Lysak, M.A.; Bennett, M.D. The origin, evolution and proposed stabilization of the terms ‘genome size’and ‘C-value’ to describe nuclear DNA contents. Ann. Bot. 2005, 95, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Gawel, N.J.; Jarret, R.L. A modified CTAB DNA extraction procedure for Musa and Ipomoea. Plant Mol. Biol. Rep. 1991, 9, 262–266. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 23 June 2019).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]

| Female | ||||||||||||
| Callus ID | 1 | 6 | 9 | 10 | 14 | 20 | 21 | 23 | 30 | 35 | 10 lines | |
| Number of Regenerants | 4 | 1 | 1 | 1 | 6 | 2 | 8 | 3 | 14 | 1 | Total Number: 41 | |
| Male | ||||||||||||
| Callus ID | 3 | 4 | 5 | 7 | 8 | 11 | 13 | 15 | 16 | 31 | 42 | 11 lines |
| Number of Regenerants | 4 | 15 | 3 | 4 | 5 | 1 | 8 | 1 | 1 | 12 | 2 | Total Number: 56 |
| Sex | Cultivar | Source | |
|---|---|---|---|
| Female | ‘Hayward’ | Private collection (Belgium) and Zespri International Ltd. (Italy) | |
| ‘Bruno’ ‘Koryoku’ ‘Petit Homme’ ‘Sauvage’ ‘Summer Kiwi 3373’‘Yates’ | ‘Green Light’ ‘Monty’ ‘Quinmei’ ‘Starella’ ‘Summer Kiwi 4605’ | Private collection (Belgium) | |
| Male | M * | Zespri International Ltd. (Italy) | |
| ‘Matua’ | Private collection (Belgium) | ||
| ‘Tomuri’ | Palm Center (Bulgaria) | ||
| Inconsistent Female | ‘Solo’ | Private collection (Belgium) | |
| Inconsistent Male | ‘Jenny’ | Private collection (Belgium) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chłosta, I.; Kwolek, D.; Sliwinska, E.; Góralski, G.; Popielarska-Konieczna, M. Sex-Linked Molecular Markers Identify Female Lines in Endosperm-Derived Kiwifruit Callus and in Regenerants. Plants 2021, 10, 526. https://doi.org/10.3390/plants10030526
Chłosta I, Kwolek D, Sliwinska E, Góralski G, Popielarska-Konieczna M. Sex-Linked Molecular Markers Identify Female Lines in Endosperm-Derived Kiwifruit Callus and in Regenerants. Plants. 2021; 10(3):526. https://doi.org/10.3390/plants10030526
Chicago/Turabian StyleChłosta, Iwona, Dagmara Kwolek, Elwira Sliwinska, Grzegorz Góralski, and Marzena Popielarska-Konieczna. 2021. "Sex-Linked Molecular Markers Identify Female Lines in Endosperm-Derived Kiwifruit Callus and in Regenerants" Plants 10, no. 3: 526. https://doi.org/10.3390/plants10030526
APA StyleChłosta, I., Kwolek, D., Sliwinska, E., Góralski, G., & Popielarska-Konieczna, M. (2021). Sex-Linked Molecular Markers Identify Female Lines in Endosperm-Derived Kiwifruit Callus and in Regenerants. Plants, 10(3), 526. https://doi.org/10.3390/plants10030526








