Phenolic Compounds and Biological Activity of Selected Mentha Species
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Analysis of Selected Mentha Species
2.2. Biological Activities of Selected Mentha Species
3. Materials and Methods
3.1. Plant Material
3.2. Isolations
3.3. Analysis of Mentha Extracts
3.4. Assessment of Biological Activities
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Riachi, L.G.; De Maria, A.B. Peppermint antioxidants revisited. Food Chem. 2015, 176, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Marten, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rice Evans, C.; Miler, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trend. Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 4th ed.; Council of Europe: Strasbourg, France, 2002.
- Tucker, A.O. Mentha: Economic uses. In Mint: The Genus Mentha; Lawrence, B.M., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 519–528. [Google Scholar]
- Wiersema, J.H.; León, B. World Economic Plants: A Standard Reference, 2nd ed.; CRC Press: London, UK, 2016. [Google Scholar]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharopov, F.; Antolak, H.; Kręgiel, D.; Sen, S.; Sharifi-Rad, M.; Acharya, K.; Sharifi-Rad, R.; et al. Plants of genus Mentha: From farm to food factory. Plants 2018, 7, 70. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, A.K. Prospective of essential oils of the genus Mentha as biopesticides: A review. Front. Plant Sci. 2018, 9, 1295. [Google Scholar] [CrossRef]
- Mimica-Dukic, N.; Bozin, B. Mentha L. species (Lamiaceae) as promising sources of bioactive secondary metabolites. Curr. Pharmaceut. Design 2008, 14, 3141–3150. [Google Scholar] [CrossRef]
- Bisset, N.G.; Wichtl, M. (Eds.) Herbal Drugs and Phytopharmaceuticals; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2001. [Google Scholar]
- Burzanska-Herman, Z. Isolation and identification of components of the flavonoid fraction of domestic species of Mentha L, section Verticillatae (M. arvensis L, M. sachalinensis Kudo, M. verticillata L., M. smithiana Graham, M. gentilis L.). Acta Poloniae Pharm. Drug Res. 1978, 3, 673–680. [Google Scholar]
- Dorman, H.J.D.; Koşar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J. Agric. Food Chem. 2003, 51, 4563–4569. [Google Scholar] [CrossRef] [PubMed]
- Khanuja, S.P.S.; Shasany, A.K.; Srivastava, A.; Kumar, S. Assessment of genetic relationships in Mentha species. Euphytica 2000, 111, 121–125. [Google Scholar] [CrossRef]
- Rösch, P.; Kiefer, W.; Popp, J. Chemotaxonomy of mints of genus Mentha by applying Raman spectroscopy. Biopolymers 2002, 67, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Shasany, A.K.; Darokar, M.P.; Dhawan, S.; Gupta, A.K.; Gupta, S.; Shukla, A.K.; Patra, N.K.; Khanuja, S.P.S. Use of RAPD and AFLP markers to identify inter- and intraspecific hybrids of Mentha. J. Hered. 2005, 96, 542–549. [Google Scholar] [CrossRef]
- Šarić-Kundalić, B.; Fialová, S.; Dobeš, C.; Ölzant, S.; Grančai, D.; Reznicek, G.; Saukel, J. Multivariate numerical taxonomy of Mentha species, hybrids, varieties and cultivars. Sci. Pharmaceut. 2009, 77, 851–876. [Google Scholar] [CrossRef]
- Gobert, V.; Moja, S.; Colson, M.; Taberlet, P. Hybridization in the section Mentha (Lamiaceae) inferred from AFLP markers. Am. J. Bot. 2002, 89, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.O.; Chambers, H.L. Mentha canadensis L. (Lamiaceae): A Relict amphidiploid from the lower tertiary. Taxon 2002, 51, 703–718. [Google Scholar] [CrossRef]
- Kokkini, S. Chemical races within the Genus Mentha L. In Essential Oils and Waxes. Modern Methods of Plant Analysis; Linskens, H.F., Jackson, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; Volume 12, pp. 63–78. [Google Scholar]
- Caballero, B. (Ed.) Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Bones, K.; Mills, S. Principles and Practice of Phytotherapy: Modern Herbal Medicine, 2nd ed.; Churchill Livingstone: London, UK, 2013. [Google Scholar]
- Lawrence, B.M. The composition of commercially important Mints. In Mint: The Genus Mentha; Lawrence, B.M., Ed.; CRC Press: Boca Raton, Fl, USA, 2007; pp. 519–528. [Google Scholar]
- Shah, S.; Gupta, L. Response of Mentha species to different harvesting intervals. Prog. Hort. 1989, 21, 148–150. [Google Scholar]
- Zielinski, A.A.F.; Haminiuk, C.W.I.; Alberti, A.; Nogueira, A.; Demiate, I.M.; Granato, D. A comparative study of the phenolic compounds and the in vitro antioxidant activity of different Brazilian teas using multivariate statistical techniques. Food Res. Int. 2014, 60, 246–254. [Google Scholar] [CrossRef]
- Stagos, D.; Portesis, N.; Spanou, C.; Mossialos, D.; Aligiannis, N.; Chaita, E.; Panagoulis, C.; Reri, E.; Skaltsounis, L.; Tsatsakis, A.M.; et al. Correlation of total polyphenolic content with antioxidant and antibacterial activity of 24 extracts from Greek domestic Lamiaceae species. Food Chem. Toxicol. 2012, 50, 4115–4124. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Ioele, G.; Statti, G.A.; Marrelli, M.; Ragno, G.; Menichini, F. Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food Chem. Toxicol. 2008, 46, 3325–3332. [Google Scholar] [CrossRef]
- Pereira, O.R.; Macias, R.I.R.; Domingues, M.R.; Marin, J.J.G.; Cardoso, S.M. Hepatoprotection of Mentha aquatica L., Lavandula dentata L. and Leonurus cardiaca L. Antioxidants 2019, 8, 267. [Google Scholar] [CrossRef]
- Gatea, F.; Teodor, E.D.; Matei, A.O.; Badea, G.I.; Radu, G.L. Capillary electrophoresis method for 20 polyphenols separation in propolis and plant extracts. Food Anal. Methods 2015, 8, 1197–1206. [Google Scholar] [CrossRef]
- Salin, O.; Tormakangas, L.; Leinonen, M.; Saario, M.; Hagstrom, M.; Ketola, R.A.; Saikku, P.; Vuorela, H.; Vuorela, P.M. Corn Mint (Mentha arvensis) extract diminishes acute Chlamydia pneumoniae infection in vitro and in vivo. J. Agric. Food Chem. 2011, 59, 12836–12842. [Google Scholar] [CrossRef]
- Miguel, M.; Barros, L.; Pereira, C.; Calhelha, R.C.; Garcia, P.A.; Castro, M.A.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical characterization and bioactive properties of two aromatic plants: Calendula officinalis L. (flowers) and Mentha cervina L. (leaves). Food Funct. 2016, 7, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Politi, M.; Rodrigues, C.L.; Gião, M.S.; Pintado, M.E.; Castro, P.M.L. Antioxidant principles and volatile constituents from the north-western Iberian mint “erva-peixeira”, Mentha cervina. Nat. Prod. Commun. 2008, 3, 2065–2068. [Google Scholar] [CrossRef]
- Vladimir-Knežević, S.; Blažeković, B.; Kindl, M.; Vladić, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the Lamiaceae family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Patonay, K.; Korózs, M.; Murányi, Z.; Kónya, E.P. Polyphenols in northern Hungarian Mentha longifolia (L.) L. treated with ultrasonic extraction for potential oenological uses. Turk. J. Agric. For. 2017, 41, 208–217. [Google Scholar] [CrossRef]
- Krzyzanowska, J.; Janda, B.; Pecio, L.; Stochmal, A.; Oleszek, A. Determination of polyphenols in Mentha longifolia and M. piperita field-grown and in vitro plant samples using UPLC-TQ-MS. J. AOAC Int. 2011, 94, 43–50. [Google Scholar] [CrossRef]
- Spiridon, I.; Bodirlau, R.; Teaca, C.-A. Total phenolic content and antioxidant activity of plants used in traditional Romanian herbal medicine. Cent. Eur. J. Biol. 2011, 6, 388–396. [Google Scholar] [CrossRef]
- Kulig, D.; Matysiak, M.; Baldovská, S.; Štefániková, J.; Maruniaková, N.; Mňahončáková, E.; Árvay, J.; Galbavý, D.; Kolesárová, A. Screening of polyphenolic compounds from traditional medicinal herbs. J. Microbiol. Biotech. Food Sci. 2019, 9, 487–491. [Google Scholar] [CrossRef]
- Bittner Fialová, S.; Kurin, E.; Trajcíková, E.; Jánošová, L.; Šušaníková, I.; Tekelová, D.; Nagy, M.; Mucaji, P. Mentha rhizomes as an alternative source of natural antioxidants. Molecules 2020, 25, 200. [Google Scholar] [CrossRef]
- Kogiannou, D.A.A.; Kalogeropoulos, N.; Kefalas, P.; Polissiou, M.G.; Kaliora, A.C. Herbal infusions; their phenolic profile, antioxidant and anti-inflammatory effects in HT29 and PC3 cells. Food Chem. Toxicol. 2013, 61, 152–159. [Google Scholar] [CrossRef]
- Ferreres, F.; Bernardo, J.; Andrade, P.B.; Sousa, C.; Gil-Izquierdoa, A.; Valentao, P. Pennyroyal and gastrointestinal cells: Multi-target protection of phenolic compounds against t-BHPinduced toxicity. RSC Adv. 2015, 5, 41576–41584. [Google Scholar] [CrossRef]
- Mata, A.T.; Proenca, C.; Ferreira, A.R.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Araujo, M.E.M. Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices. Food Chem. 2007, 103, 778–786. [Google Scholar] [CrossRef]
- Zugic, A.; Ðordevic, S.; Arsic, I.; Markovic, G.; Zivkovic, J.; Jovanovic, S.; Tadic, J. Antioxidant activity and phenolic compounds in 10 selected herbs from Vrujci Spa, Serbia. Ind. Crop. Prod. 2014, 52, 519–527. [Google Scholar] [CrossRef]
- Kratchanova, M.; Denev, P.; Ciz, M.; Lojek, A.; Mihailov, A. Evaluation of antioxidant activity of medicinal plants containing polyphenol compounds. Comparison of two extraction systems. Acta Biochim. Pol. 2010, 57, 229–234. [Google Scholar] [CrossRef]
- Petkova, N.; Ivanova, L.; Filova, G.; Ivanov, I.; Denev, P. Antioxidants and carbohydrate content in infusions and microwave extracts from eight medicinal plants. J. Appl. Pharm. Sci. 2017, 7, 55–61. [Google Scholar]
- Chrpová, D.; Kouřimsk, L.; Gordon, M.H.; Heřmanová, V.; Roubíčková, I.; Pánek, J. Antioxidant activity of selected phenols and herbs used in diets for medical conditions. Czech J. Food Sci. 2010, 28, 317–325. [Google Scholar] [CrossRef]
- Dorman, D.H.J.; Koşar, M.; Başer, K.H.C.; Hiltunen, R. phenolic profile and antioxidant evaluation of Mentha x piperita L. (peppermint) extracts. Nat. Prod. Commun. 2009, 4, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Reichling, J.; Nolkemper, S.; Stintzing, F.C.; Schnitzler, P. Impact of ethanolic Lamiaceae extracts on herpesvirus infectivity in cell culture. Forsch. Komplementmed. 2008, 15, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Fecka, I.; Turek, S. Determination of water-soluble polyphenolic compounds in commercial herbal teas from Lamiaceae: Peppermint, melissa, and sage. J. Agric. Food Chem. 2007, 55, 10908–10917. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, U.; Zabinski, A.; Szumny, A.; Dziadekc, K. An effect of peppermint herb (Mentha piperita L.) pressing onphysico-chemical parameters of the resulting product. Ind. Crop. Prod. 2016, 94, 909–919. [Google Scholar] [CrossRef]
- Pereira, E.; Pimenta, A.I.; Calhelha, R.C.; Antonio, A.L.; Cabo Verde, S.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Effects of gamma irradiation on cytotoxicity and phenolic compounds of Thymus vulgaris L. and Mentha x piperita L. LWT-Food Sci. Technol. 2016, 71, 370–377. [Google Scholar] [CrossRef]
- Pavlić, B.; Teslić, N.; Zengin, G.; Đurović, S.; Rakić, D.; Cvetanović, A.; Gunes, A.K.; Zeković, Z. Antioxidant and enzyme-inhibitory activity of peppermint extracts and essential oils obtained by conventional and emerging extraction techniques. Food Chem. 2021, 338, 127724. [Google Scholar] [CrossRef]
- Areias, F.M.; Valentao, P.; Andrade, P.B.; Ferreres, F.; Seabra, R.M. Phenolic fingerprint in pepermin leaves. Food Chem. 2001, 73, 307–311. [Google Scholar] [CrossRef]
- Orphanides, A.; Goulas, V.; Gekas, V. Effect of drying method on the phenolic content and antioxidant capacity of spearmint. Czech J. Food Sci. 2013, 31, 509–513. [Google Scholar] [CrossRef]
- Marrelli, M.; Cristaldi, B.; Menichini, F.; Conforti, F. Inhibitory effects of wild dietary plants on lipid peroxidation and on the proliferation of human cancer cells. Food Chem. Toxicol. 2015, 86, 16–24. [Google Scholar] [CrossRef]
- Rita, I.; Pereira, C.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Mentha spicata L. infusions as sources of antioxidant phenolic compounds: Emerging reserve lots with special harvest requirements. Food Funct. 2016, 7, 4188–4192. [Google Scholar] [CrossRef]
- Goncalves, S.; Moreira, E.; Grosso, C.; Andrade, P.B.; Valentao, P.; Romano, A. Phenolic profile, antioxidant activity and enzyme inhibitory activities of extracts from aromatic plants used in Mediterranean diet. J. Food Sci. Technol. 2017, 54, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef]
- Fialova, S.; Veizerova, L.; Nosalova, V.; Drabikova, K.; Tekelova, D.; Grancai, D.; Sotnikova, R. Water extract of Mentha × villosa: Phenolic fingerprint and effect on ischemia-reperfusion injury. Nat. Prod. Commun. 2015, 10, 937–940. [Google Scholar] [CrossRef]
- Fletcher, R.S.; Slimmon, T.; Kott, L.S. Environmental factors affecting the accumulation of rosmarinic acid in spearmint (Mentha spicata L.) and peppermint (Mentha piperita L.). Open Agri. J. 2010, 4, 10–16. [Google Scholar] [CrossRef]
- Petersen, M. Rosmarinic acid: New aspects. Phytochem. Rev. 2013, 12, 207–227. [Google Scholar] [CrossRef]
- Pavela, R.; Kaffková, K.; Kumšta, M. Chemical composition and larvicidal activity of essential oils from different Mentha L. and Pulegium species against Culex quinquefasciatus Say (Diptera: Culicidae). Plant Protect. Sci. 2014, 50, 36–42. [Google Scholar] [CrossRef]
- Fancello, F.; Zara, S.; Petretto, G.L.; Chessa, M.; Addis, R.; Rourke, J.P.; Pintore, G. Essential oils from three species of Mentha harvested in Sardinia: Chemical characterization and evaluation of their biological activity. Int. J. Food Prop. 2017, 20, 1751–1761. [Google Scholar] [CrossRef]
- Rodrigues, L.; Póvo, O.; van den Berg, C.; Figueiredo, A.C.; Moldão, M.; Monteiro, A. Genetic diversity in Mentha cervina based on morphological traits, essential oils profile and ISSRs markers. Biochem. Syst. Ecol. 2013, 51, 50–59. [Google Scholar] [CrossRef]
- Mogosan, C.; Vostinaru, O.; Oprean, R.; Heghes, C.; Filip, L.; Balica, G.; Moldovan, R.I. A comparative analysis of the chemical composition, anti-inflammatory, and antinociceptive effects of the essential oils from three species of Mentha cultivated in Romania. Molecules 2017, 22, 263. [Google Scholar] [CrossRef]
- Bokić, B.S.; Rat, M.M.; Kladar, N.V.; Anačkov, G.T.; Božin, B.N. Chemical diversity of volatile compounds of mints from southern part of Pannonian Plain and Balkan Peninsula—new data. Chem. Biodivers. 2020, 17, e2000211. [Google Scholar] [CrossRef]
- Tomei, P.E.; Manganelli, R.E.U. Composition of the essential oil of Mentha microphylla from the Gennargentu Mountains (Sardinia, Italy). J. Agric. Food Chem. 2003, 51, 3614–3617. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Wojtunik, K.A.; Ciesla, L.M.; Waksmundzka-Hajnos, M. Model studies on the antioxidant activity of common terpenoid constituents of essential oils by means of the 2,2-diphenyl-1- picrylhydrazyl method. J. Agric. Food Chem. 2014, 62, 9088–9094. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.B.; Zengin, G.; Bahadori, S.; Dinparast, L.; Movahhedin, N. Phenolic composition and functional properties of wild mint (Mentha longifolia var. calliantha (Stapf) Briq.). Int. J. Food Prop. 2018, 21, 198–208. [Google Scholar]
- Fiocco, D.; Fiorentino, D.; Frabboni, L.; Benvenuti, S.; Orlandini, G.; Pellati, F.; Gallone, A. Lavender and peppermint essential oils as effective mushroom tyrosinase inhibitors: A basic study. Flavour Fragr. J. 2011, 26, 441–446. [Google Scholar] [CrossRef]
- Ćavar Zeljković, S.; Komzáková, K.; Šišková, J.; Karalija, E.; Smékalová, K.; Tarkowski, P. Phytochemical variability of selected basil genotypes. Ind. Crop. Prod. 2020, 157, 112910. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rosi, J.A. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Nagy, M.; Grancai, D. Colorimetric determination of flavanones in propolis. Pharmazie 1996, 51, 100–101. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Saghaie, L.; Pourfarzam, M.; Fassihi, A.; Sartippour, B. Synthesis and tyrosinase inhibitory properties of some novel derivates of kojic acid. PMC Res. Pharm. Sci. 2013, 8, 233–242. [Google Scholar]

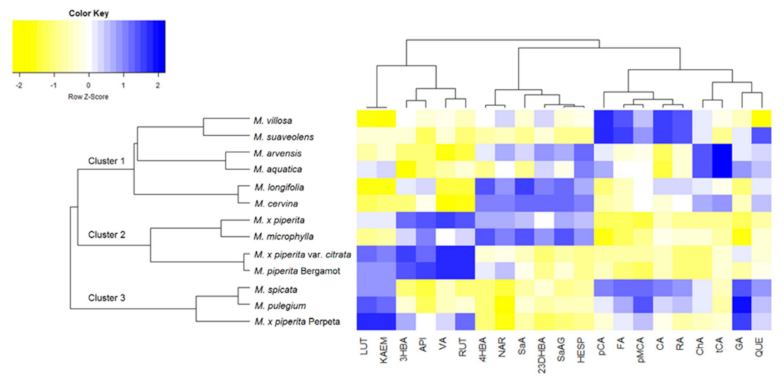
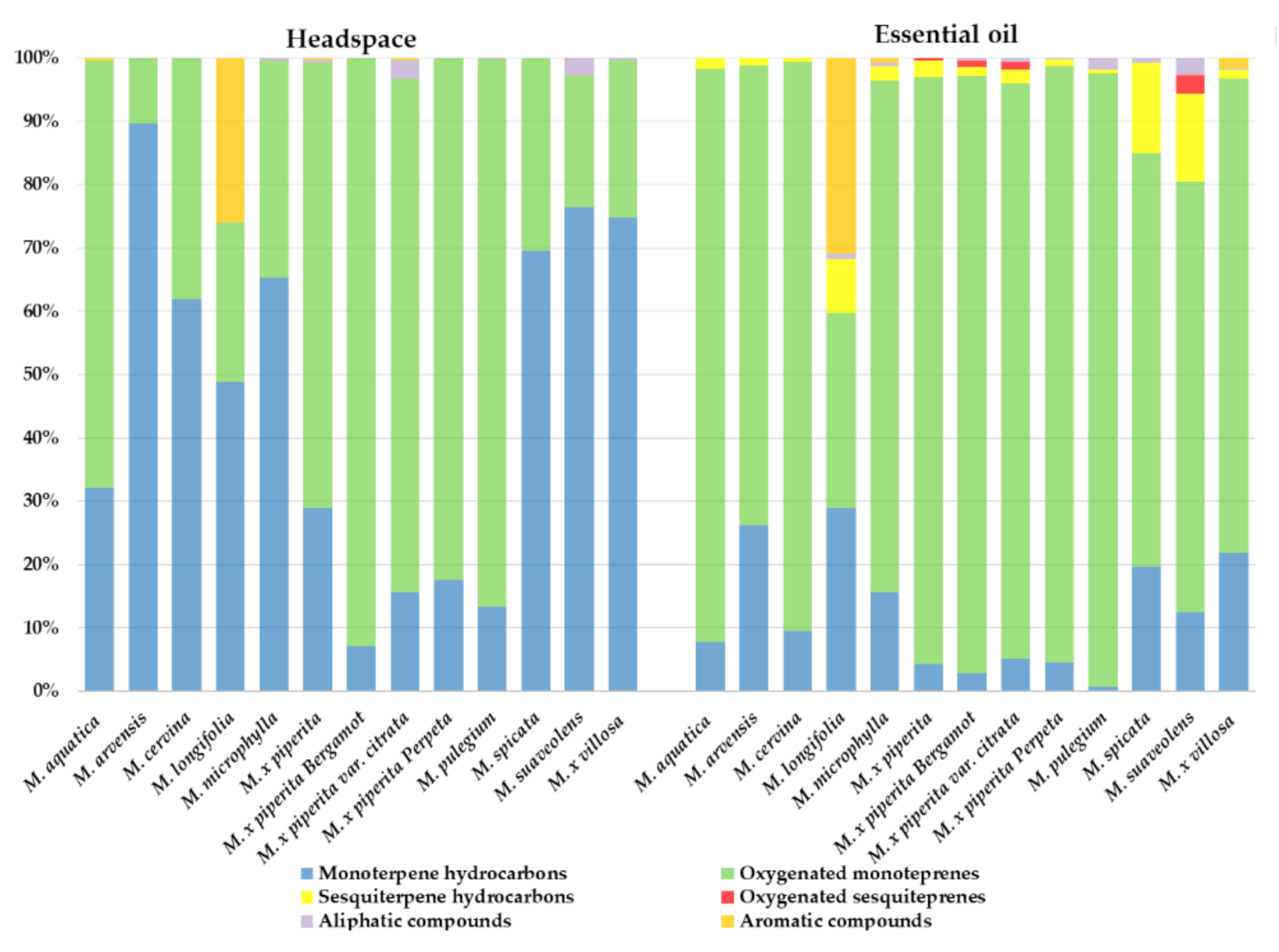
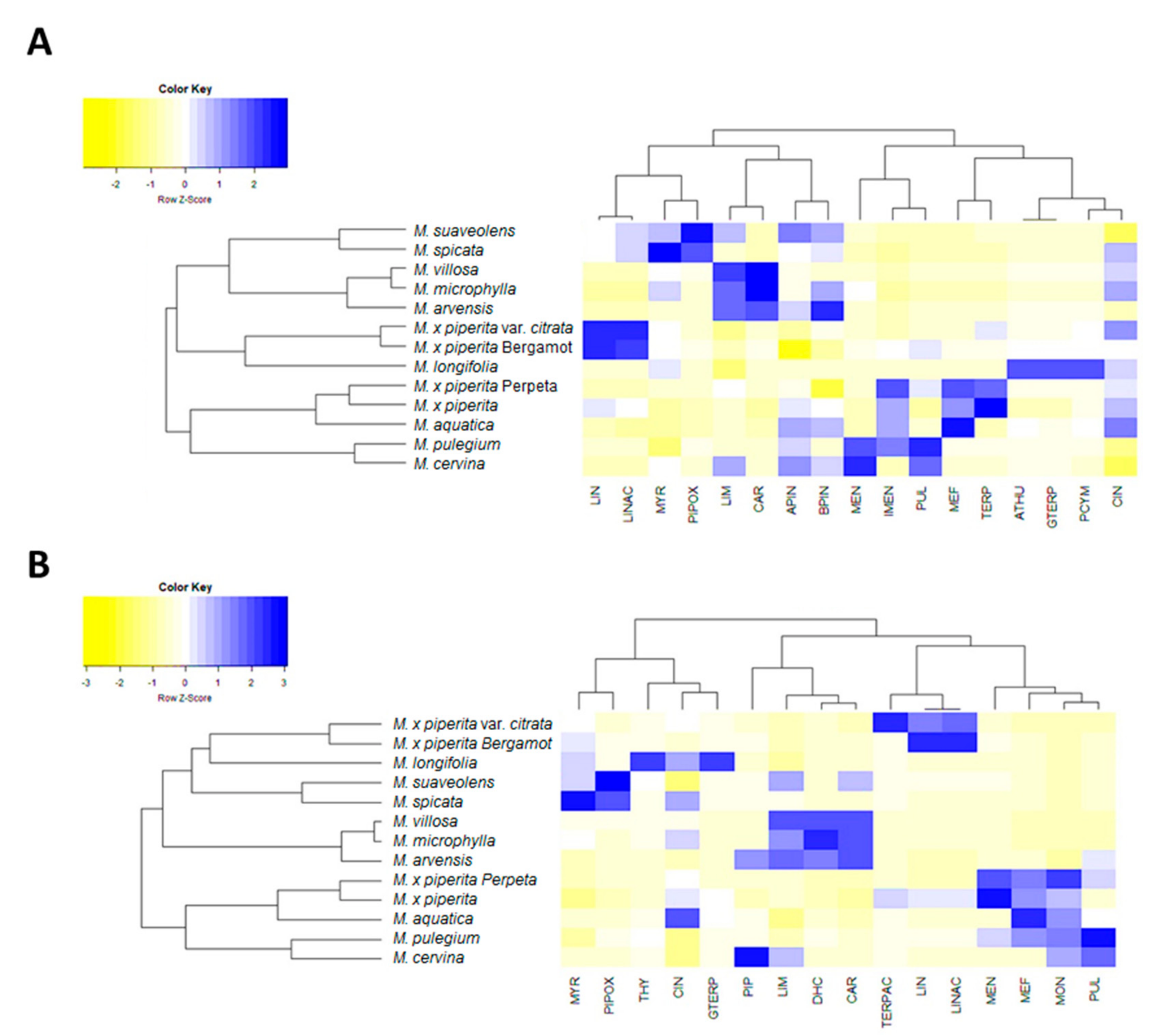
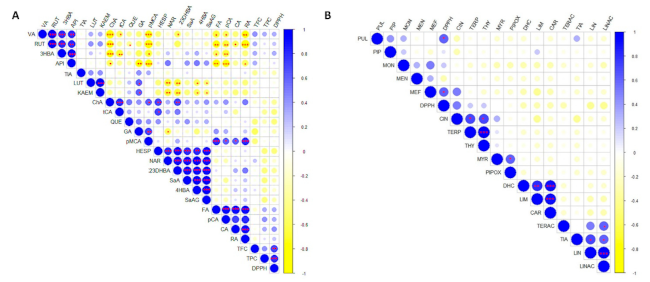

| Species | Origin | TPC 1 (mg/g) | TFC 2 (mg/g) | Major Phenolics | DPPH 3 (IC50 μg/mL) | Ref. |
|---|---|---|---|---|---|---|
| Mentha aquatica | Finland | 152.5 4 | rosmarinic acid, luteolin glucoside | 275 | [12] | |
| Greece | 172–185 4 | 29 ± 1.5 | [25] | |||
| Italy | 337 ± 2.15 5 | 15.75 ± 0.25 6 | [26] | |||
| Portugal | rosmarinic acid, luteolin glucoside | 8.1 ± 1.3 | [27] | |||
| Romania | 212.9 ± 0.00 4 | 0.27 ± 0.00 6 | rosmarinic acid, caffeic acid | [28] | ||
| Mentha arvensis | Finland | rosmarinic acid | [29] | |||
| Mentha cervina | Italy | rosmarinic acid | 0.21 ± 0.01 | [30] | ||
| Portugal | 0.15 ± 0.00 4,7 | chlorogenic acid, caffeic acid | [31] | |||
| Mentha longifolia | Croatia | rosmarinic acid, chlorogenic acid | 8.43 ± 0.28 | [32] | ||
| Greece | 115–216 4 | 36 ± 0.6 7 | [25] | |||
| Hungary | 19.35–47.52 3 | rosmarinic acid, rutin, apigenin | [33] | |||
| Poland | rosmarinic acid | [34] | ||||
| Romania | 48 4 | 18 7 | [35] | |||
| Slovakia | rutin, kaempferol | [36] | ||||
| lithospermic acid, rosmarinic acid | 25.39 | [37] | ||||
| Mentha microphylla | Greece | 217–233 3 | 29 ± 1.2 | [25] | ||
| Mentha pulegium | Greece | 138–188 4 | 28 ± 1.0 | [25] | ||
| 82.9 ± 0.1 4 | 13.2 ± 1.8 8 | caffeic acid, thymol, carvacrol | 179.5 ± 4.9 9 | [38] | ||
| Portugal | rosmarinic acid | 23 | [39] | |||
| 81.2 ± 7.6 | 8.9 ± 0.2 | [40] | ||||
| Serbia | 25 4 | 0.05% | 19.27 | [41] | ||
| Mentha x piperita | Bulgaria | 45.22 ± 0.10 4 | [42] | |||
| 35.1 ± 1.2 4 | 12.5 ± 0.4 6 | 250 9 | [43] | |||
| Croatia | rosmarinic acid | 8.88 ± 0.13 | [32] | |||
| Czechia | 63.0 4 | 147.5 9 | [44] | |||
| Finland | 1142.4 ± 10.7 4 | 709.3 ± 24.0 6 | eriocitrin, rosmarinic acid | [45] | ||
| Germany | eriodyctiol, luteolin | [46] | ||||
| Poland | eriodyctiol, luteolin, rosmarinic acid | [34,47] | ||||
| 5.58–36.04 4 | [48] | |||||
| Portugal | eriodyctiol, luteolin, rosmarinic acid | [49] | ||||
| Serbia | 50.05 ± 0.31 4 | 25.95 ± 1.16 6 | 98.43 ± 2.39 10 | [50] | ||
| Slovakia | rutin, gallic acid, epicatechin | [36] | ||||
| rosmarinic acid, lithospermic acid | 28.45 | [37] | ||||
| Spain | eriodyctiol, hesperidin, rosmarinic acid | [51] | ||||
| Mentha spicata | Cyprus | 18.91 ± 0.20 4 | 90 8 | [52] | ||
| Finland | 214 4 | eriocitrin, luteolin, rosmarinic acid | 210 | [12] | ||
| Italy | 9.6 ± 0.4 6 | catechin, caffeic acid, rutin | [53] | |||
| Portugal | 81.2 ± 7.6 4 | 65.2 ± 0.1 | [40] | |||
| rosmarinic acid | 546 ± 17 | [54] | ||||
| 87.06 ± 4.56 4 | rosmarinic acid, hesperetin | 8.93 ± 0.27 | [55] | |||
| Mentha suaveolens | Slovakia | 2.25 ± 0.297 4 | 3.9 ± 0.001 6 | cinnamic acid, chlorogenic acid | [56] | |
| Mentha x villosa | Slovakia | rosmarinic acid, luteolin glucoside | 7.1 ± 0.09 | [57] | ||
| rosmarinic acid, lithospermic acid | 39.48 | [37] |
| Species | Antioxidant Activity (DPPH) | Tyrosinase Inhibitory Activity (TIA) | ||
|---|---|---|---|---|
| Methanolic extract (mg TE 1/g) | Essential Oil (mg TE 1/mL) | Methanolic Extract (μg KAE 2/g) | Essential Oil (mg KAE 2/mL) | |
| M. aquatica | 67.70 ± 7.65 | 43.86 ± 0.33 | 38.45 ± 1.75 | 1.42 ± 0.19 |
| M. arvensis | 54.65 ± 5.30 | 7.83 ± 0.21 | 41.60 ± 2.61 | 54.36 ± 12.09 |
| M. cervina | 22.79 ± 1.85 | 20.96 ± 0.19 | 91.77 ± 6.11 | 56.61 ± 7.21 |
| M. longifolia | 91.01 ± 8.95 | 22.10 ± 0.56 | 50.36 ± 6.70 | 1.73 ± 0.15 |
| M. microphylla | 57.71 ± 6.42 | 7.28 ± 0.10 | 40.97 ± 3.45 | 164.06 ± 22.28 |
| M. x piperita | 85.90 ± 8.53 | 2.85 ± 0.38 | 102.82 ± 15.16 | 42.01 ± 6.55 |
| M. x piperita Bergamot | 90.60 ± 1.48 | 3.15 ± 0.68 | 36.11 ± 6.47 | 636.97 ± 32.58 |
| M. x piperita var. citrata | 84.34 ± 6.54 | 2.71 ± 0.34 | 43.51 ± 0.89 | 120.58 ± 19.40 |
| M. x piperita Perpeta | 52.63 ± 5.48 | 7.09 ± 0.89 | 75.74 ± 1.06 | 26.87 ± 7.54 |
| M. pulegium | 68.24 ± 3.34 | 40.01 ± 0.86 | 123.89 ± 5.64 | 403.19 ± 55.93 |
| M. spicata | 88.96 ± 10.38 | 14.70 ± 0.56 | 56.29 ± 7.21 | 1.33 ± 0.21 |
| M. suaveolens | 91.65 ± 2.92 | 7.11 ± 0.15 | 43.01 ± 1.10 | 40.31 ± 3.41 |
| M. x villosa | 106.04 ± 3.26 | 8.01 ± 0.74 | 49.83 ± 6.12 | 75.63 ± 9.95 |
| Species | ECN | Collection code | Origin | Plant Height | Leaves | Corolla Color | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Petiole/Sessile | Shape | Margin | Surface | Hairiness | ||||||
| Mentha aquatica | - | 4348 | Switzerland | 30–40 | shortly petiolate | ovate | serrate | smooth | glabrous | lilac |
| Mentha arvensis | 09A6400048 | 3422 | Slovakia | 15–20 | shortly petiolate | lanceolate-ovate | serrate | smooth | hairy | lilac |
| Mentha cervina | 09A6400017 | 2112 | Germany | 20–25 | sessile | linear-oblanceolate | obscurely toothed | smooth | glabrous | white |
| Mentha longifolia | - | 4641 | Slovakia | 60–70 | sessile | oblong-elliptical | serrate | smooth | hairy | lilac |
| Mentha microphylla | 09A6400054 | 4344 | Switzerland | 50–60 | sessile | lanceolate | serrate | slightly rugose | tomentose | lilac |
| Mentha x piperita | - | 4371 | Czechia | 30–35 | long petiolate | lanceolate | serrate | smooth | glabrous | lilac-pink |
| Mentha x piperita Bergamot | - | 4338 | Switzerland | 15–20 | long petiolate | subcordate | serrate | smooth | glabrous | lilac |
| Mentha x piperita var. citrata | - | 4354 | Switzerland | 15–20 | long petiolate | subcordate | serrate | smooth | glabrous | lilac |
| Mentha x piperita Perpeta | 09A6400045 | 3433 | Czechia | 35–40 | long petiolate | lanceolate | serrate | smooth | glabrous | lilac-pink |
| Mentha pulegium | - | 3522 | Slovenia | 20–29 | shortly petiolate | narrowly-elliptical | entire | smooth | subglabrous | lilac |
| Mentha spicata | 09A6400015 | 2081 | Germany | 50–60 | petiolate | lanceolate-ovate | serrate | smooth | glabrous | lilac |
| Mentha suaveolens | 09A6400052 | 4340 | Switzerland | 50–60 | sessile | ovate-oblong | serrate | rugose | hairy | pinkish |
| Mentha x villosa | - | 4347 | Switzerland | 80–100 | shortly petiolate | broadly ovate | irregular serrate | slightly rugose | hairy | pink |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ćavar Zeljković, S.; Šišková, J.; Komzáková, K.; De Diego, N.; Kaffková, K.; Tarkowski, P. Phenolic Compounds and Biological Activity of Selected Mentha Species. Plants 2021, 10, 550. https://doi.org/10.3390/plants10030550
Ćavar Zeljković S, Šišková J, Komzáková K, De Diego N, Kaffková K, Tarkowski P. Phenolic Compounds and Biological Activity of Selected Mentha Species. Plants. 2021; 10(3):550. https://doi.org/10.3390/plants10030550
Chicago/Turabian StyleĆavar Zeljković, Sanja, Jana Šišková, Karolína Komzáková, Nuria De Diego, Katarína Kaffková, and Petr Tarkowski. 2021. "Phenolic Compounds and Biological Activity of Selected Mentha Species" Plants 10, no. 3: 550. https://doi.org/10.3390/plants10030550
APA StyleĆavar Zeljković, S., Šišková, J., Komzáková, K., De Diego, N., Kaffková, K., & Tarkowski, P. (2021). Phenolic Compounds and Biological Activity of Selected Mentha Species. Plants, 10(3), 550. https://doi.org/10.3390/plants10030550







