Effects of Maternal Environment on Seed Germination and Seedling Vigor of Petunia × hybrida under Different Abiotic Stresses
Abstract
1. Introduction
2. Results
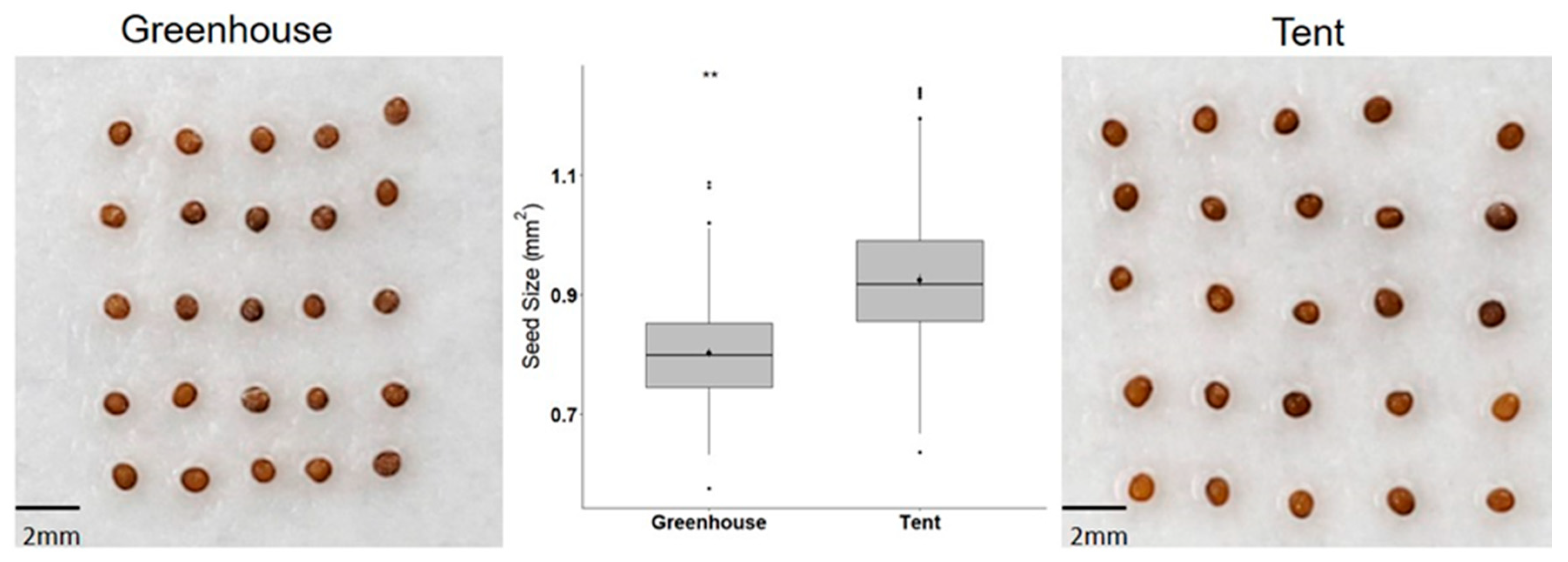
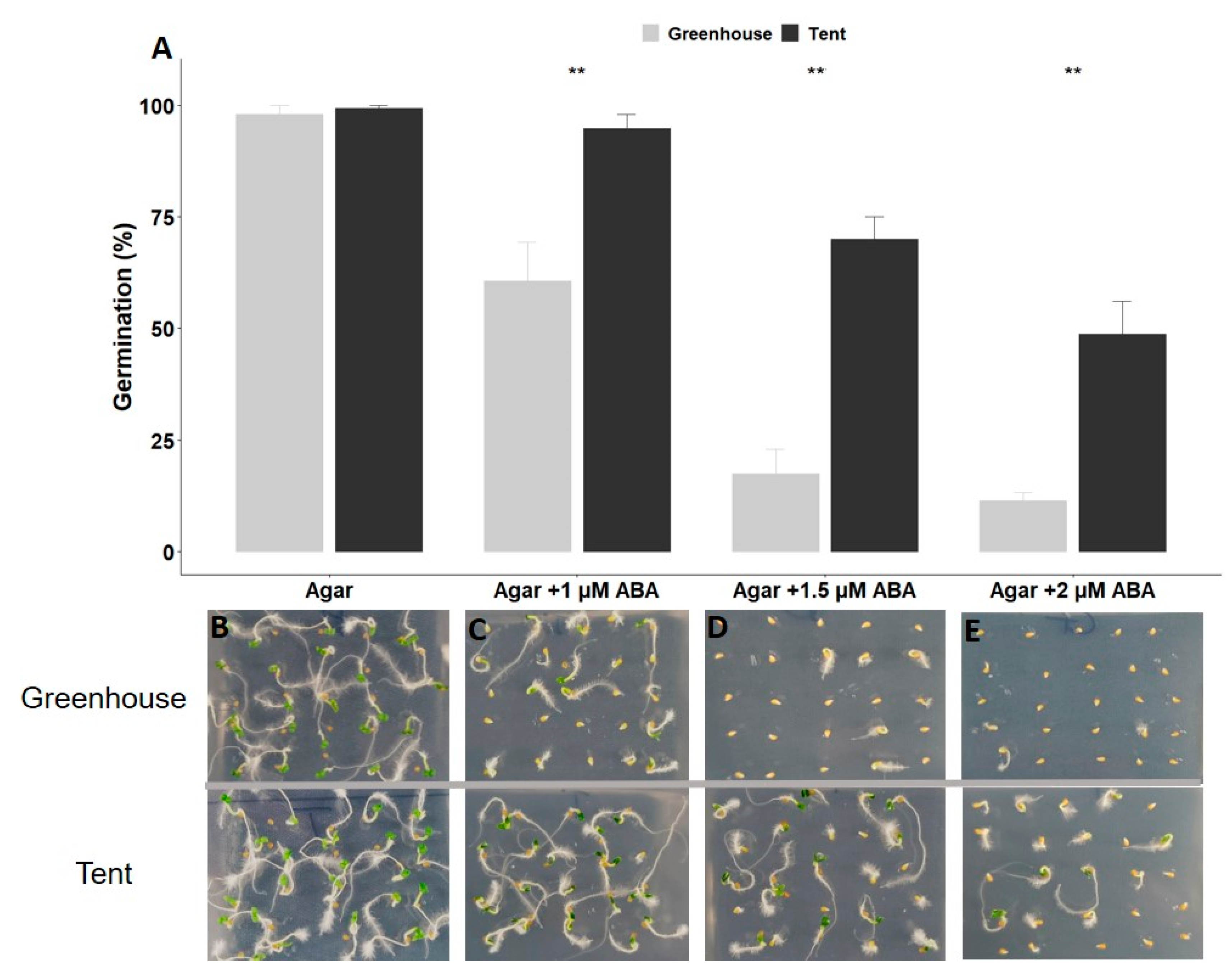
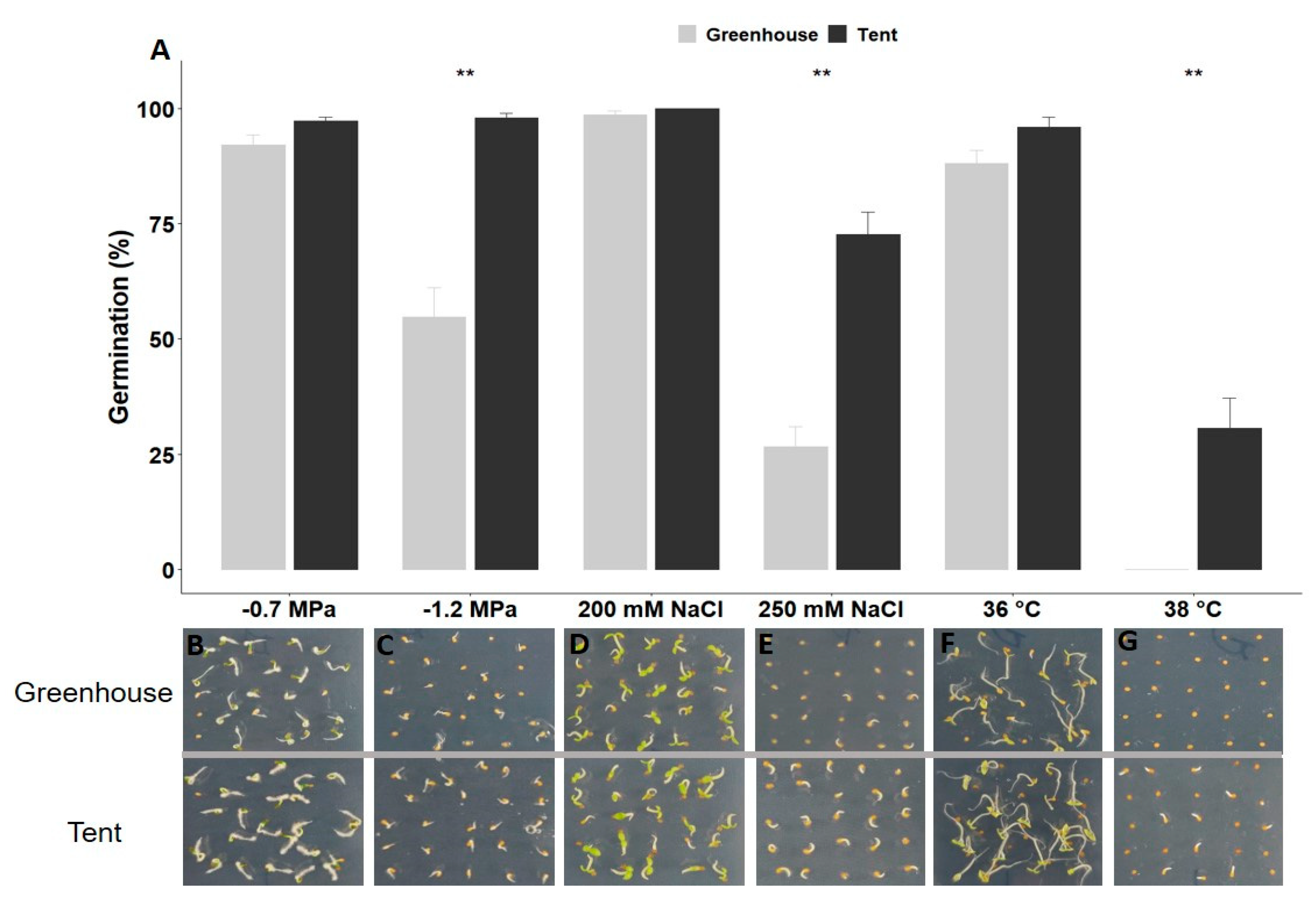
2.1. Petunia Seed Size and Seed Germination under Different Types of Abiotic Stress
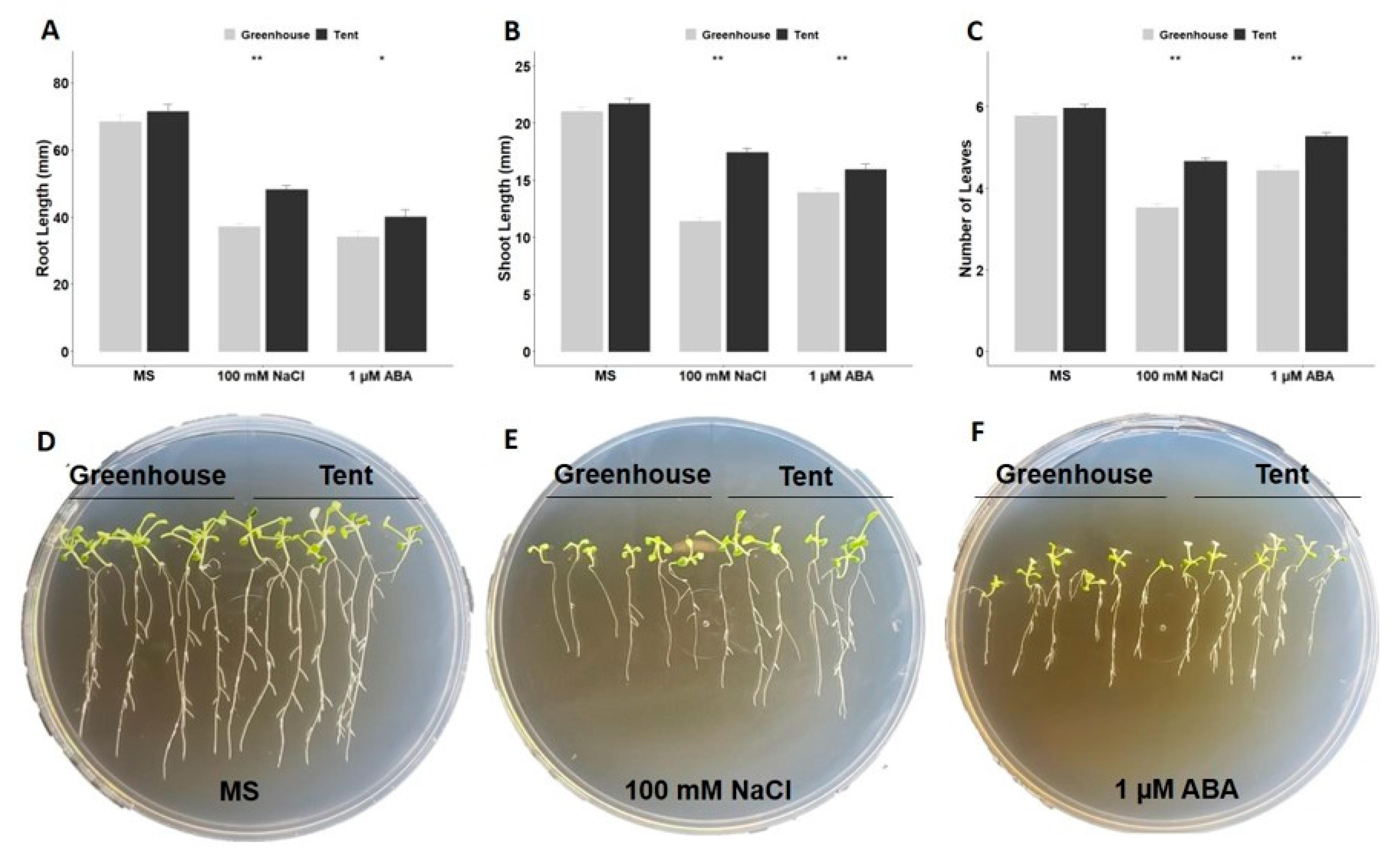
2.2. Post-Germination Growth under ABA or NaCl Stress
2.3. Alterations in the Reactive Oxygen Species (ROS) and Electrolyte Leakage of Petunia Seedlings under Different Types of Abiotic Stress
3. Discussion
3.1. Petunia Seed Germination and Seedling Vigor under Different Types of Abiotic Stress
3.2. Petunia Seedling Reactive Oxygen Species (ROS) Accumulation and Electrolyte Leakage in Response to Different Types of Abiotic Stress
4. Materials and Methods
4.1. Plant Materials and Maternal Environments
4.2. Seed Size Measurement
4.3. Seed Germination under Different Types of Abiotic Stress
4.4. Seedling Vigor Assay
4.5. Seedling Stress Test: Electrolyte Leakage Measurement and Reactive Oxygen Species (ROS) Staining
4.6. Statistical Analysis
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donohue, K. Completing the cycle: Maternal effects as the missing link in plant life histories. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Geshnizjani, N.; Khorami, S.S.; Willems, L.A.J.; Snoek, B.L.; Hilhorst, H.W.M.; Ligterink, W. The interaction between genotype and maternal nutritional environments affects tomato seed and seedling quality. J. Exp. Bot. 2019, 70, 2905–2918. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Michelangeli, J.A.C.; Gezan, S.A.; Lee, H.; Vallejos, C.E. Maternal Effects on Seed and Seedling Phenotypes in Reciprocal F1 Hybrids of the Common Bean (Phaseolus vulgaris L.). Front. Plant Sci. 2017, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Finch-savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed]
- Hampton, J.G.; Boelt, B.; Rolston, M.P.; Chastain, T.G. Effects of elevated CO2 and temperature on seed quality. J. Agric. Sci. 2012, 151, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Egli, D.B.; Wardlaw, I.F. Temperature Response of Seed Growth Characteristics of Soybeans 1. Agron. J. 1980, 72, 560–564. [Google Scholar] [CrossRef]
- Shinohara, T.; Hampton, J.G.; Hill, M.J. Location of deterioration within garden pea(Pisum sativum)cotyledons is associated with the timing of exposure to high temperature. N. Z. J. Crop. Hortic. Sci. 2006, 34, 299–309. [Google Scholar] [CrossRef]
- Pagamas, P.; Nawata, E. Sensitive stages of fruit and seed development of chili pepper (Capsicum annuum L. var. Shishito) exposed to high-temperature stress. Sci. Hortic. 2008, 117, 21–25. [Google Scholar] [CrossRef]
- Van Der Weele, C.M.; Spollen, W.G.; Sharp, R.E.; Baskin, T.I. Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J. Exp. Bot. 2000, 51, 1555–1562. [Google Scholar] [CrossRef]
- Donohue, K.; De Casas, R.R.; Burghardt, L.T.; Kovach, K.; Willis, C.G. Germination, Postgermination Adaptation, and Species Ecological Ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Penfield, S.; MacGregor, D.R. Effects of environmental variation during seed production on seed dormancy and germination. J. Exp. Bot. 2016, 68, 436. [Google Scholar] [CrossRef]
- Li, R.; Chen, L.; Wu, Y.; Zhang, R.; Baskin, C.C.; Baskin, J.M.; Hu, X. Effects of Cultivar and Maternal Environment on Seed Quality in Vicia sativa. Front. Plant Sci. 2017, 8, 8. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Floriculture Crops 2018 Summary; United States Department of Agriculture: Washington, DC, USA, 2018.
- Arshad, M.S.; Farooq, M.; Asch, F.; Krishna, J.S.; Prasad, P.V.; Siddique, K.H. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiol. Biochem. 2017, 115, 57–72. [Google Scholar] [CrossRef]
- Adams, S.R.; Cockshull, K.E.; Cave, C.R.J. Effect of Temperature on the Growth and Development of Tomato Fruits. Ann. Bot. 2001, 88, 869–877. [Google Scholar] [CrossRef]
- Garner, W.W.; Allard, H.A. Effect of the relative length of day and night and other factors of the environment on growth and reproduction in plants1. Mon. Weather. Rev. 1920, 48, 415. [Google Scholar] [CrossRef]
- Lin, C. Photoreceptors and Regulation of Flowering Time. Plant Physiol. 2000, 123, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Barrero, J.M.; Downie, A.B.; Xu, Q.; Gubler, F. A Role for Barley CRYPTOCHROME1 in Light Regulation of Grain Dormancy and Germination. Plant Cell 2014, 26, 1094–1104. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, W.; Yin, H.; Luo, X.; Chen, W.; Liu, X.; Wang, X.; Meng, Y.; Feng, L.; Qin, Y.; et al. Shading of the mother plant during seed development promotes subsequent seed germination in soybean. J. Exp. Bot. 2020, 71, 2072–2084. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, M.J.; Finch-Savage, W.E.; Grappin, P.; Job, D. Post-genomics dissection of seed dormancy and germination. Trends Plant Sci. 2008, 13, 7–13. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gacio, M.D.C.; Matilla-Vázquez, M.A.; Matilla, A.J. Seed dormancy and ABA signaling. Plant Signal. Behav. 2009, 4, 1035–1048. [Google Scholar] [CrossRef]
- Kermode, A.R. Role of Abscisic Acid in Seed Dormancy. J. Plant Growth Regul. 2005, 24, 319–344. [Google Scholar] [CrossRef]
- Huo, H.; Dahal, P.; Kunusoth, K.; McCallum, C.M.; Bradford, K.J. Expression of 9-cis-EPOXYCAROTENOID DIOXYGENASE4 Is Essential for Thermoinhibition of Lettuce Seed Germination but Not for Seed Development or Stress Tolerance. Plant Cell 2013, 25, 884–900. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.; Henry, I.M.; Coppoolse, E.R.; Verhoef-Post, M.; Schut, J.W.; De Rooij, H.; Vogelaar, A.; Joosen, R.V.; Woudenberg, L.; Comai, L.; et al. Rapid identification of lettuce seed germination mutants by bulked segregant analysis and whole genome sequencing. Plant J. 2016, 88, 345–360. [Google Scholar] [CrossRef]
- Vamerali, T.; Saccomani, M.; Bona, S.; Mosca, G.; Guarise, M.; Ganis, A. A comparison of root characteristics in relation to nutrient and water stress in two maize hybrids. Plant Soil 2003, 255, 157–167. [Google Scholar] [CrossRef]
- Rosental, L.; Perelman, A.; Nevo, N.; Toubiana, D.; Samani, T.; Batushansky, A.; Sikron, N.; Saranga, Y.; Fait, A. Environmental and genetic effects on tomato seed metabolic balance and its association with germination vigor. BMC Genom. 2016, 17, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Pannico, A.; Cirillo, C.; Fascella, G.; El-Nakhel, C.; De Pascale, S.; Rouphael, Y. Influence of priming methods on seed germinability and transplants performance in six vegetable species. Acta Hort. 2020, 1296, 297–304. [Google Scholar] [CrossRef]
- Massimi, M. Impact of seed size on seeds viability, vigor and storability of Hordeum vulgare (L.). Agric. Sci. Dig. A Res. J. 2018, 62–64. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, R.; Chen, J.; Cao, F.; Jiang, L.; Zou, Y. Morphological and physiological traits of seeds and seedlings in two rice cultivars with contrasting early vigor. Plant Prod. Sci. 2016, 20, 95–101. [Google Scholar] [CrossRef][Green Version]
- Lu, X.-L.; Niu, A.-L.; Cai, H.-Y.; Zhao, Y.; Liu, J.-W.; Zhu, Y.-G.; Zhang, Z.-H. Genetic dissection of seedling and early vigor in a recombinant inbred line population of rice. Plant Sci. 2007, 172, 212–220. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef]
- Mohammad, H.; Fujita, M. Hydrogen Peroxide Priming Stimulates Drought Tolerance in Mustard (Brassica juncea L.). Seedlings. Plant Gene Trait 2013, 4, 109–123. [Google Scholar] [CrossRef]
- Gong, M.; Chen, B.; Li, Z.-G.; Guo, L.-H. Heat-shock-induced cross adaptation to heat, chilling, drought and salt stress in maize seedlings and involvement of H2O2. J. Plant Physiol. 2001, 158, 1125–1130. [Google Scholar] [CrossRef]
- Guzel, S.; Terzi, R. Exogenous hydrogen peroxide increases dry matter production, mineral content and level of osmotic solutes in young maize leaves and alleviates deleterious effects of copper stress. Bot. Stud. 2013, 54, 26. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Zhu, X.; Liu, S.; Song, F.; Liu, F.; Wang, Y.; Qi, X.; Wang, F.; Zuo, Z.; et al. Salt acclimation induced salt tolerance is enhanced by abscisic acid priming in wheat. Plant Soil Environ. 2017, 63, 307–314. [Google Scholar]
- He, F.; Wang, H.-L.; Li, H.-G.; Su, Y.; Li, S.; Yang, Y.; Feng, C.-H.; Yin, W.; Xia, X. PeCHYR1, a ubiquitin E3 ligase from Populus euphratica, enhances drought tolerance via ABA-induced stomatal closure by ROS production in Populus. Plant Biotechnol. J. 2018, 16, 1514–1528. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ge, Y.; Zhang, W.; Zhao, Y.; Yang, G. The walnut JrVHAG1 gene is involved in cadmium stress response through ABA-signal pathway and MYB transcription regulation. BMC Plant Biol. 2018, 18, 19. [Google Scholar] [CrossRef]
- Walter, J.; Jentsch, A.; Beierkuhnlein, C.; Kreyling, J. Ecological stress memory and cross stress tolerance in plants in the face of climate extremes. Environ. Exp. Bot. 2013, 94, 3–8. [Google Scholar] [CrossRef]
- Crisp, P.A.; Ganguly, D.; Eichten, S.R.; Borevitz, J.O.; Pogson, B.J. Reconsidering plant memory: Intersections between stress recovery, RNA turnover, and epigenetics. Sci. Adv. 2016, 2, e1501340. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Hyvärinen, L.; Piskurewicz, U.; Lopez-Molina, L. Non-canonical RNA-directed DNA methylation participates in maternal and environmental control of seed dormancy. eLife 2019, 8, 8. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image Processing with Image. J. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Cao, W.-H.; Liu, J.; He, X.-J.; Mu, R.-L.; Zhou, H.-L.; Chen, S.-Y.; Zhang, J.-S. Modulation of Ethylene Responses Affects Plant Salt-Stress Responses. Plant Physiol. 2007, 143, 707–719. [Google Scholar] [CrossRef]
- Ilík, P.; Špundová, M.; Šicner, M.; Melkovičová, H.; Kučerová, Z.; Krchnák, P.; Fürst, T.; Večeřová, K.; Panzarová, K.; Benediktyová, Z.; et al. Estimating heat tolerance of plants by ion leakage: A new method based on gradual heating. New Phytol. 2018, 218, 1278–1287. [Google Scholar] [CrossRef]
- Team, R. RStudio: Integrated Development Environment for R. 2016. Available online: http://www.rstudio.com (accessed on 28 May 2019).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, C.D.; Chen, J.; Clark, D.; Perez, H.; Huo, H. Effects of Maternal Environment on Seed Germination and Seedling Vigor of Petunia × hybrida under Different Abiotic Stresses. Plants 2021, 10, 581. https://doi.org/10.3390/plants10030581
Nguyen CD, Chen J, Clark D, Perez H, Huo H. Effects of Maternal Environment on Seed Germination and Seedling Vigor of Petunia × hybrida under Different Abiotic Stresses. Plants. 2021; 10(3):581. https://doi.org/10.3390/plants10030581
Chicago/Turabian StyleNguyen, Chi D., Jianjun Chen, David Clark, Hector Perez, and Heqiang (Alfred) Huo. 2021. "Effects of Maternal Environment on Seed Germination and Seedling Vigor of Petunia × hybrida under Different Abiotic Stresses" Plants 10, no. 3: 581. https://doi.org/10.3390/plants10030581
APA StyleNguyen, C. D., Chen, J., Clark, D., Perez, H., & Huo, H. (2021). Effects of Maternal Environment on Seed Germination and Seedling Vigor of Petunia × hybrida under Different Abiotic Stresses. Plants, 10(3), 581. https://doi.org/10.3390/plants10030581







