Petiole-Lamina Transition Zone: A Functionally Crucial but Often Overlooked Leaf Trait
Abstract
1. Introduction
1.1. Damage-Resistant Transition
1.2. A Bunch of Terms
1.3. Materials Systems with Multiple Gradients
1.4. Motivation
2. Results
2.1. Hosta x tardiana ‘El Niño’
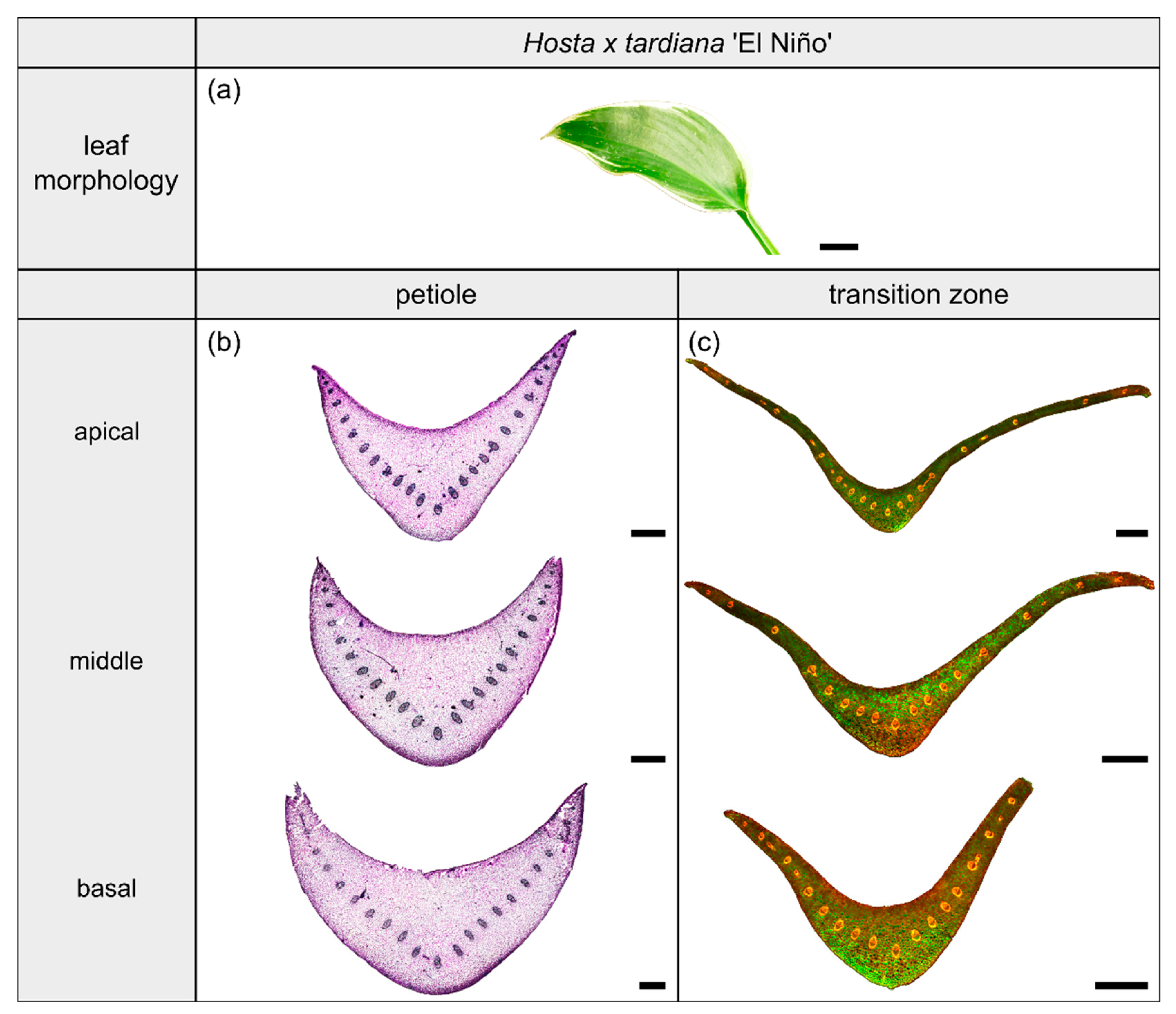
2.1.1. General Description of the Leaf
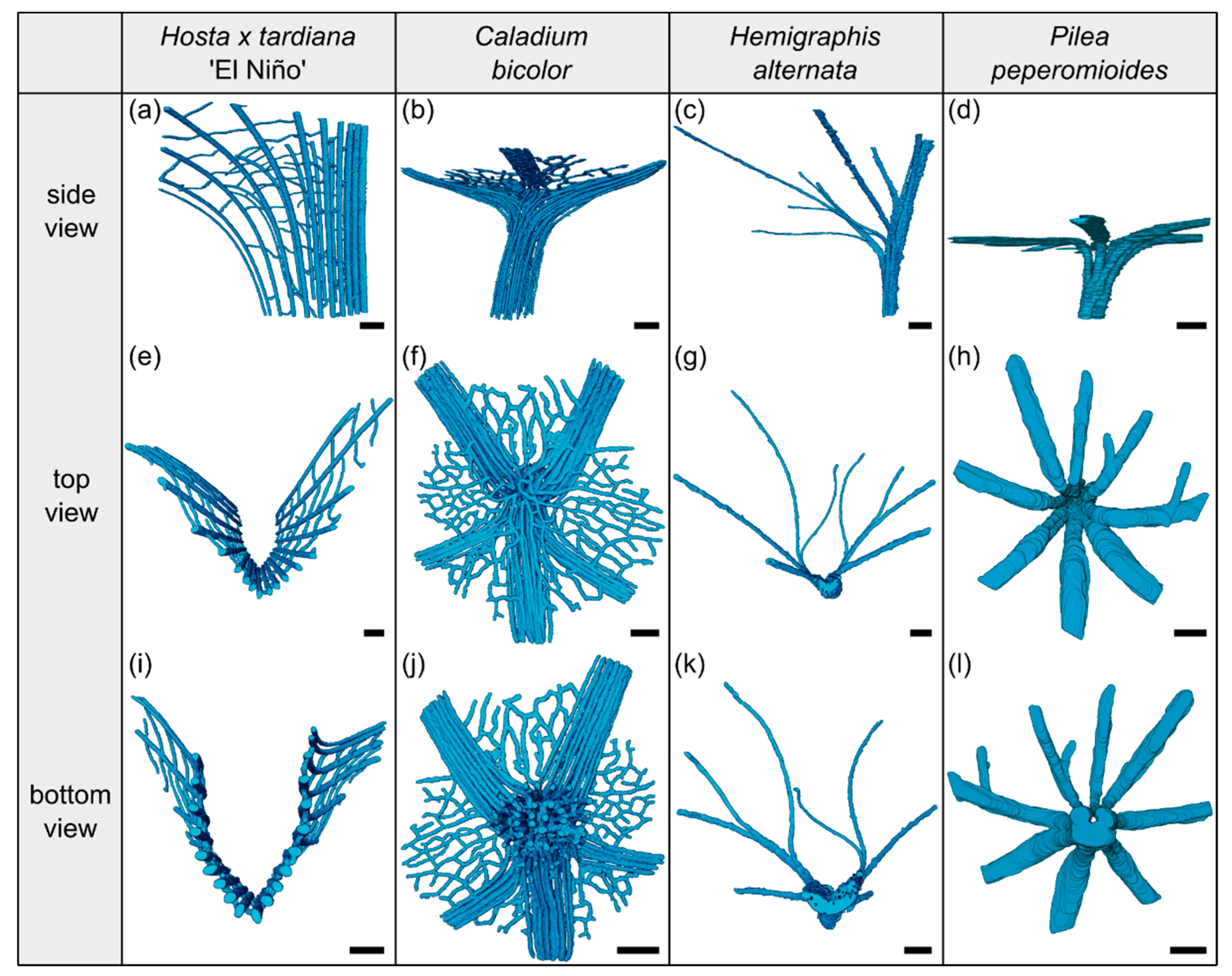
2.1.2. Geometry, Shape and Size
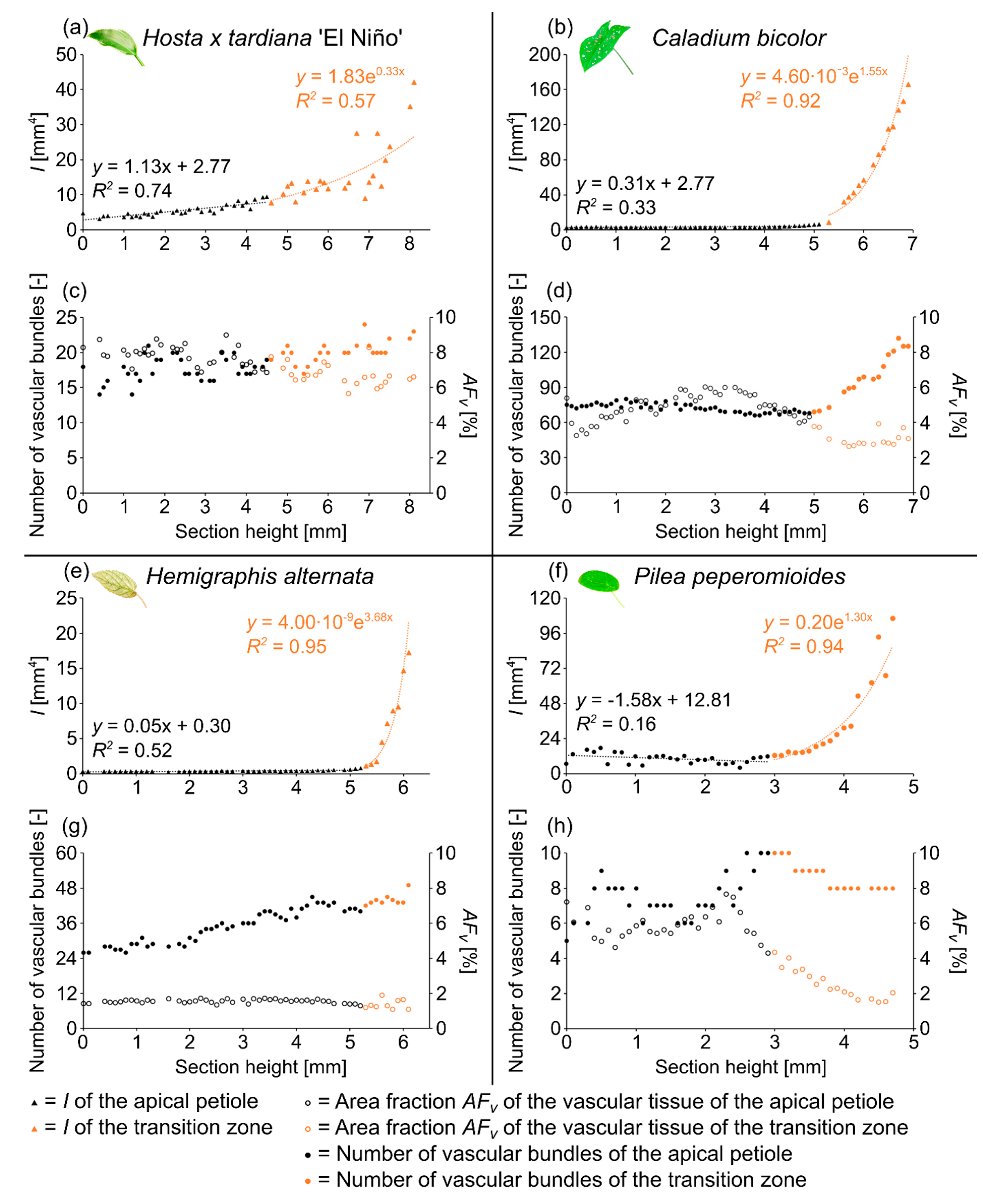
2.1.3. Vascular Tissue
2.2. Caladium bicolor
2.2.1. General Description of the Leaf
2.2.2. Geometry, Shape and Size
2.2.3. Vascular Tissue
2.3. Hemigraphis alternata
2.3.1. General Description of the Leaf
2.3.2. Geometry, Shape and Size
2.3.3. Vascular Tissue
2.4. Pilea peperomioides
2.4.1. General Description of the Leaf
2.4.2. Geometry, Shape and Size
2.4.3. Vascular Tissue
3. Discussion
3.1. Gradual Change of Geometry
3.2. Gradual Change of Shape
3.3. Gradual Change of Size
3.4. Gradual Change of Tissues Arrangement
4. Materials and Methods
4.1. Plant Material
4.2. Geometry, Size and Shape
4.3. µCT Scanning
4.4. Sample Preparation for Histological Studies
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sewell, P. Observations upon the germination and growth of species of Salvia in the garden of Thomas Hanbury, Esq., F.L.S. at La Mortola, Ventimiglia, Italy. Trans. Bot. Soc. Edinburgh 1891, 18, 350–379. [Google Scholar] [CrossRef]
- Yin, H.C. Diaphototropic movement of the leaves of Malva neglecta. Am. J. Bot. 1938, 25, 1–6. [Google Scholar] [CrossRef]
- Foster, A.S.; Arnott, H.J. Morphology and dichotomous vasculature of the leaf of Kingdonia uniflora. Am. J. Bot. 1960, 47, 684–698. [Google Scholar] [CrossRef]
- Isebrands, J.G.; Larson, P.R. Organization and ontogeny of the vascular system in the petiole of eastern cottonwood. Am. J. Bot. 1977, 64, 65–77. [Google Scholar] [CrossRef]
- Roth-Nebelsick, A.; Uhl, D.; Mosbrugger, V.; Kerp, H. Evolution and function of leaf venation architecture: A review. Ann. Bot. 2001, 87, 553–566. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Fleck, S. Petiole mechanics, leaf inclination, morphology, and investment in support in relation to light availability in the canopy of Liriodendron tulipifera. Oecologia 2002, 132, 21–33. [Google Scholar] [CrossRef]
- Poulsen, A.D.; Nordal, I. A phenetic analysis and revision of Guineo-Congolean rain forest taxa of Chlorophytum (Anthericaceae). Bot. J. Linn. Soc. 2005, 148, 1–20. [Google Scholar] [CrossRef]
- Sack, L.; Dietrich, E.M.; Streeter, C.M.; Sánchez-Gómez, D.; Holbrook, N.M. Leaf palmate venation and vascular redundancy confer tolerance of hydraulic disruption. Proc. Natl. Acad. Sci. USA 2008, 105, 1567–1572. [Google Scholar] [CrossRef]
- Jones, A.; Kang, J. Development of leaf lobing and vein pattern architecture in the genus Ipomoea (morning glory). Int. J. Plant Sci. 2015, 176, 820–831. [Google Scholar] [CrossRef]
- Ray, D.M.; Jones, C.S. Scaling relationships and vessel packing in petioles. Am. J. Bot. 2018, 105, 1–10. [Google Scholar] [CrossRef]
- Louf, J.-F.; Nelson, L.; Kang, H.; Song, P.N.; Zehnbauer, T.; Jung, S. How wind drives the correlation between leaf shape and mechanical properties. Sci. Rep. 2018, 8, 16314. [Google Scholar] [CrossRef]
- Langer, M.; Speck, T.; Speck, O. Transition zones between planar and rod-shaped elements-Plant leaves as concept generators for technical applications in architecture. In Bionik: Patente aus der Natur. Innovations- und Nachhaltigkeitspotenziale für Technologieanwendungen: Tagungsbeiträge 9. Bionik-Kongress, Bremen 2018; Kesel, A.B., Zehren, D., Eds.; Bionik-Innovations-Centrum (B-I-C): Bremen, Germany, 2019; pp. 199–204. ISBN 978-3-00-061443-9. [Google Scholar]
- Sacher, M.; Lautenschläger, T.; Kempe, A.; Neinhuis, C. Umbrella leaves—biomechanics of transition zone from lamina to petiole of peltate leaves. Bioinspir. Biomim. 2019, 14, 46011. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Su, X.; Ratkowsky, D.A.; Niklas, K.J.; Gielis, J.; Shi, P. The scaling relationships of leaf biomass vs. leaf surface area of 12 bamboo species. Glob. Ecol. Conserv. 2019, 20, e00793. [Google Scholar] [CrossRef]
- Wilson, H.P.; Paul, O.; Hughes, M. Begonia maguniana (Begoniaceae, Begonia sect. Oligandrae), a new species from New Guinea. Edinb. J. Bot. 2019, 1–7. [Google Scholar] [CrossRef]
- Yeng, W.S.; Boyce, P.C.; Wardi, A.b.; Kian, C.S. Araceae of Mulu National Park. I. Four new species of Schismatoglottis (Araceae). Nord. J. Bot. 2019, 37, e02566. [Google Scholar] [CrossRef]
- Ginebra-Solanellas, R.M.; Holder, C.D.; Lauderbaugh, L.K.; Webb, R. The influence of changes in leaf inclination angle and leaf traits during the rainfall interception process. Agric. For. Meteorol. 2020, 285–286, 107924. [Google Scholar] [CrossRef]
- Wunnenberg, J.; Rjosk, A.; Neinhuis, C.; Lautenschläger, T. Strengthening structures in the petiole-lamina junction of peltate leaves. Biomimetics 2021, 6, 25. [Google Scholar] [CrossRef]
- Niklas, K.J.; Spatz, H.-C. Plant Physics; University of Chicago Press: Chicago, IL, USA; London, UK, 2012; ISBN 9780226586342. [Google Scholar]
- Paolillo, D.J.; Niklas, K.J. Effects of Rht-dosage on the breaking strength of wheat seedling leaves. Am. J. Bot. 1996, 83, 567–572. [Google Scholar] [CrossRef]
- Liu, Z.; Meyers, M.A.; Zhang, Z.; Ritchie, R.O. Functional gradients and heterogeneities in biological materials: Design principles, functions, and bioinspired applications. Prog. Mater. Sci. 2017, 88, 467–498. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef]
- Vogel, S. Twist-to-bend ratios and cross-sectional shapes of petioles and stems. J. Exp. Bot. 1992, 43, 1527–1532. [Google Scholar] [CrossRef]
- Ennos, A.R.; Spatz, H.-C.; Speck, T. The functional morphology of the petioles of the banana, Musa textilis. J. Exp. Bot. 2000, 51, 2085–2093. [Google Scholar] [CrossRef]
- Pasini, D.; Mirjalili, V. The optimized shape of a leaf petiole. In Design and Nature III: Comparing Design in Nature with Science and Engineering; Brebbia, C.A., Ed.; WIT Press: Southampton, UK, 2006; pp. 35–45. [Google Scholar]
- Niklas, K.J. A mechanical perspective on foliage leaf form and function. New Phytol. 1999, 143, 19–31. [Google Scholar] [CrossRef]
- Drost, H.-G.; Janitza, P.; Grosse, I.; Quint, M. Cross-kingdom comparison of the developmental hourglass. Curr. Opin. Genet. Dev. 2017, 45, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S. Graded materials for resistance to contact deformation and damage. Science 2001, 292, 2447–2451. [Google Scholar] [CrossRef]
- Horn, R.; Albrecht, S.; Haase, W.; Langer, M.; Schmeer, D.; Sobek, W.; Speck, O.; Leistner, P. Bio-inspiration as a concept for sustainable constructions illustrated on graded concrete. J. Bionic Eng. 2019, 16, 742–753. [Google Scholar] [CrossRef]
- Speck, T.; Burgert, I. Plant Stems: Functional design and mechanics. Annu. Rev. Mater. Res. 2011, 41, 169–193. [Google Scholar] [CrossRef]
- Speck, T.; Schmitt, M. Mechanische Werte. In Lexikon der Biologie-Biologie im Überblick; Schmitt, M., Ed.; Herder: Freiburg, Germany, 1992; pp. 244–247. [Google Scholar]
- Niklas, K.J. Plant Allometry: The Scaling of Form and Process; University of Chicago Press: Chicago, IL, USA; London, UK, 1994. [Google Scholar]
- Etnier, S.A. Twisting and bending of biological beams: Distribution of biological beams in a stiffness mechanospace. Biol. Bull. 2003, 205, 36–46. [Google Scholar] [CrossRef]
- Wolff-Vorbeck, S.; Langer, M.; Speck, O.; Speck, T.; Dondl, P. Twist-to-bend ratio: An important selective factor for many rod-shaped biological structures. Sci. Rep. 2019, 9, 17182. [Google Scholar] [CrossRef]
- Kull, U.; Herbig, A. Das Blattadersystem der Angiospermen: Form und Evolution. Naturwissenschaften 1995, 82, 441–451. [Google Scholar] [CrossRef]
- Caliaro, M.; Schmich, F.; Speck, T.; Speck, O. Effect of drought stress on bending stiffness in petioles of Caladium bicolor (Araceae). Am. J. Bot. 2013, 100, 2141–2148. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Doube, M.; Kłosowski, M.M.; Arganda-Carreras, I.; Cordelières, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Sakai, W.S. Simple method for differential staining of paraffin embedded plant material using toluidine blue O. Stain Technol. 1973, 48, 247–249. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]



| Description | Plant Species | Scientific Field | Author(s) |
|---|---|---|---|
| various Salvia species | ontogeny and morphology | Sewell, 1891 [1] |
| |||
| Malva neglecta | diaphototropism and anatomy | Yin, 1938 [2] |
| Kingdonia uniflora | morphology and anatomy | Foster and Arnott, 1960 [3] |
| |||
| |||
| Populus deltoides | ontogeny and anatomy | Isebrands and Larson, 1977 [4] |
| Populus balsamifera | functional morphology, anatomy and biomechanics | Roth-Nebelsick et al., 2001 [5] |
| Liriodendron tulipifera | biomechanics, functional morphology and physiology | Niinemets and Fleck, 2002 [6] |
| |||
| various Chlorophytum species | systematics and morphometry | Poulsen and Nordal, 2005 [7] |
| Acer rubrum | physiology and morphology | Sack et al., 2008 [8] |
| Acer saccharum | |||
| Betula alleghaniensis | |||
| Kalmia latifolia | |||
| Quercus rubra | |||
| Viburnum acerifolium | |||
| Viburnum cassinoides | |||
| various Ipomoea species | ontogeny, anatomy and morphometry | Jones and Kang, 2015 [9] |
| various Pelargonium species | anatomy and allometry | Ray and Jones, 2018 [10] |
| Acer saccharum | morphometry and biomechanics | Louf et al., 2018 [11] |
| Liriodendron tulipifera | |||
| Platanus occidentalis | |||
| Quercus rubra | |||
| Caladium bicolor | biomimetics | Langer et al., 2019 [12] |
| Colocasia fallax | functional morphology and biomechanics | Sacher et al., 2019 [13] |
| Tropaeolum majus | |||
| various Phyllostachys and Pleioblastus species | allometry and morphometry | Huang et al., 2019 [14] |
| Begonia maguniana | systematics and morphology | Wilson et al., 2019 [15] |
| various Schismatoglottis species | systematics and morphology | Yeng et al., 2019 [16] |
| Acer saccharinum | functional morphology and biomechanics | Ginebra-Solanellas et al., 2020 [17] |
| Quercus gambelii | |||
| Ulmus pumila | |||
| various species with peltate leaves | functional morphology, systematics | Wunnenberg et al., 2021 [18] |
| Caladium bicolor | functional morphology, biomimetics | this study |
| Hemigraphis alternata | |||
| Hosta x tardiana ‘El Niño’ | |||
| Pilea peperomioides |
| Variable | Description |
|---|---|
| AR | Aspect ratio of the diameters in lateral (dl) and adaxial-abaxial (da) direction of the petiole |
| α | Tapering mode of the petiole |
| A | Cross-sectional area of the transverse section |
| I | Axial second moment of area of the transverse section |
| J | Polar second moment of area of the transverse section |
| I/J | Ratio of axial and polar second moments of area |
| a | Slope of the linear fit calculated for the cross-sectional area (A) or the axial second moment of area (I) or the polar second moment of area (J) |
| b | Growth constant of the exponential fit calculated for the cross-sectional area (A) or the axial second moment of area (I) or the polar second moment of area (J) |
| AFv | Area fraction of vascular tissue in relation to the total cross-sectional area (A) |
| Monocotyledons | Dicotyledons | |||||
|---|---|---|---|---|---|---|
| Configuration | 2D | 3D | 2D | 3D | ||
| Species | Hosta x tardiana | Caladium bicolor | Hemigraphis alternata | Pilea peperomioides | ||
| Variable | Description | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | n |
| Geometry | ||||||
| Geometry change | Change of the cross-sectional geometry from petiole to transition zone | U-profile → V-profile | circular → curvilinear triangle → triangle with lobes | elliptic → circular | circular → elliptic → lobbed | |
| Shape | ||||||
| ARpetiole [-] | Aspect ratio of the petiole | 1.13 (0.13) | 0.95 (0.09) | 1.22 (0.09) | 1.05 (0.08) | 25 |
| αpetiole [-] | Tapering mode of the petiole | 1.47 (0.40) | 0.91 (0.15) | 1.36 (0.57) | 1.18 (0.54) | 25 |
| I / Jpetiole [-] | Ratio of axial and polar second moment of area of the petiole | 0.33 (0.05) | 0.53 (0.05) | 0.40 (0.03) | 0.47 (0.04) | 25 |
| I / Jtransition [-] | Ratio of axial and polar second moment of area of the transition zone | 0.45 (0.14) | 0.60 (0.13) | 0.37 (0.24) | 0.42 (0.08) | 6 |
| Size | ||||||
| aA_petiole [mm2/mm] | Slope of the linear fit for the cross-sectional area of the apical petiole | 0.11 (1.05) | 0.65 (0.52) | −0.02 (0.17) | 0.12 (0.26) | 6 |
| bA_transition [mm−1] | Growth constant of the exponential fit for the cross-sectional area of the transition zone | 0.12 (0.11) | 0.51 (0.10) | 2.73 (1.47) | 1.94 (0.80) | 6 |
| aI_petiole [mm4/mm] | Slope of the linear fit for the axial second moment of area of the apical petiole | 1.60 (4.45) | 1.36 (1.42) | −0.01 (0.07) | 0.08 (0.27) | 6 |
| bI_transition [mm−1] | Growth constant of the exponential fit for the axial second moment of area of the transition zone | 0.47 (0.47) | 1.35 (0.19) | 7.26 (2.11) | 3.87 (1.17) | 6 |
| aJ_petiole [mm4/mm] | Slope of the linear fit for the polar second moment of area of the apical petiole | 4.33 (14.99) | 2.18 (2.17) | −0.01 (0.17) | 0.22 (0.59) | 6 |
| bJ_transition [mm−1] | Growth constant of the exponential fit for the polar second moment of area of the transition zone | 0.47 (0.37) | 1.36 (0.16) | 10.65 (6.27) | 4.18 (1.41) | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langer, M.; Speck, T.; Speck, O. Petiole-Lamina Transition Zone: A Functionally Crucial but Often Overlooked Leaf Trait. Plants 2021, 10, 774. https://doi.org/10.3390/plants10040774
Langer M, Speck T, Speck O. Petiole-Lamina Transition Zone: A Functionally Crucial but Often Overlooked Leaf Trait. Plants. 2021; 10(4):774. https://doi.org/10.3390/plants10040774
Chicago/Turabian StyleLanger, Max, Thomas Speck, and Olga Speck. 2021. "Petiole-Lamina Transition Zone: A Functionally Crucial but Often Overlooked Leaf Trait" Plants 10, no. 4: 774. https://doi.org/10.3390/plants10040774
APA StyleLanger, M., Speck, T., & Speck, O. (2021). Petiole-Lamina Transition Zone: A Functionally Crucial but Often Overlooked Leaf Trait. Plants, 10(4), 774. https://doi.org/10.3390/plants10040774








