Plant Species of Sub-Family Valerianaceae—A Review on Its Effect on the Central Nervous System
Abstract
:1. Introduction
2. Habitat, Distribution, and Traditional Uses of Valerianaceae Sub-Family
3. Effect on the Central Nervous System
4. Molecular Mechanism of Action of Pharmacological Potential
5. Other Pharmacological Potential of Valerianaceae
5.1. Antibacterial Effect
5.2. Anti-Cancer Effect
5.3. Anti-Inflammatory Effect
5.4. Antioxidant Effect
5.5. Cardiovascular Effect
6. Phytochemical Configuration of Valerianaceae
7. Extraction and Isolation Procedure of Major Compounds from Valerianaceae
8. Preclinical and Clinical Effectiveness in Humans and Patents
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Backlund, A.; Moritz, T. Phylogenetic implications of an expanded valepotriate distribution in the Valerianaceae. Biochem. Syst. Ecol. 1998, 26, 309–335. [Google Scholar] [CrossRef]
- Donoghue, M.J.; Bell, C.D.; Winkworth, R.C. The evolution of reproductive characters in Dipsacales. Int. J. Plant Sci. 2003, 164, S453–S464. [Google Scholar] [CrossRef]
- Lin, S.; Chen, T.; Liu, X.-H.; Shen, Y.-H.; Li, H.-L.; Shan, L.; Liu, R.-H.; Xu, X.-K.; Zhang, W.-D.; Wang, H. Iridoids and lignans from Valeriana jatamansi. J. Nat. Prod. 2010, 73, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, X.; Yu, B. Iridoids from the Rhizomes and Roots of Valeriana jatamansi. J. Nat. Prod. 2002, 65, 1949–1952. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xiao, D.; Bian, Y.-H.; Zhang, X.-Y.; Li, B.-J.; Ding, L.-S.; Peng, S.-L. Minor iridoids from the roots of Valeriana wallichii. J. Nat. Prod. 2008, 71, 1254–1257. [Google Scholar] [CrossRef]
- Bell, C.D.; Edwards, E.J.; Kim, S.-T.; Donoghue, M.J. Dipsacales phylogeny based on chloroplast DNA sequences. Harv. Pap. Bot. 2001, 6, 481–499. [Google Scholar]
- Bell, C.D. Preliminary phylogeny of Valerianaceae (Dipsacales) inferred from nuclear and chloroplast DNA sequence data. Mol. Phylogenetics Evol. 2004, 31, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.D.; Donoghue, M.J. Phylogeny and biogeography of Valerianaceae (Dipsacales) with special reference to the South American valerians. Org. Divers. Evol. 2005, 5, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Kutschker, A.; Morrone, J.J. Distributional patterns of the species of Valeriana (Valerianaceae) in southern South America. Plant Syst. Evol. 2012, 298, 535–547. [Google Scholar] [CrossRef]
- Olsen, C. Trade and conservation of Himalayan medicinal plants: Nardostachys grandiflora DC. and Neopicrorhiza scrophulariiflora (Pennell) Hong. Biol. Conserv. 2005, 125, 505–514. [Google Scholar] [CrossRef]
- Estrada-Soto, S.; Rivera-Leyva, J.; Ramírez-Espinosa, J.J.; Castillo-España, P.; Aguirre-Crespo, F.; Hernández-Abreu, O. Vasorelaxant effect of Valeriana edulis ssp. procera (Valerianaceae) and its mode of action as calcium channel blocker. J. Pharm. Pharmacol. 2010, 62, 1167–1174. [Google Scholar] [CrossRef]
- Houghton, P.J. The scientific basis for the reputed activity of Valerian. J. Pharm. Pharmacol. 1999, 51, 505–512. [Google Scholar] [CrossRef]
- Oshima, Y.; Matsuoka, S.; Ohizumi, Y. Antidepressant principles of Valeriana fauriei roots. Chem. Pharm. Bull. 1995, 43, 169–170. [Google Scholar] [CrossRef] [Green Version]
- Morazzoni, P.; Bombardelli, E. Valeriana officinalis: Traditional used and recent evaluation of activity. Fitoterapia 1995, 66, 99–112. [Google Scholar]
- Hendriks, H.; Bos, R.; Allersma, D.; Malingré, T.M.; Koster, A.S. Pharmacological screening of valerenal and some other components of essential oil of Valeriana officinalis. Planta Med. 1981, 42, 62–68. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Luan, F.; Zhao, Z.; Ning, N.; Li, M.; Jin, L.; Chang, Y.; Zhang, Q.; Wu, N.; Huang, L. The genus Patrinia: A review of traditional uses, phytochemical and pharmacological studies. Am. J. Chin. Med. 2017, 45, 637–666. [Google Scholar] [CrossRef]
- Cornara, L.; La Rocca, A.; Marsili, S.; Mariotti, M. Traditional uses of plants in the Eastern Riviera (Liguria, Italy). J. Ethnopharmacol. 2009, 125, 16–30. [Google Scholar] [CrossRef]
- Rehman, T.; Ahmad, S. Nardostachys chinensis Batalin: A review of traditional uses, phytochemistry, and pharmacology. Phytother. Res. 2019, 33, 2622–2648. [Google Scholar] [CrossRef]
- Lentini, F.; Venza, F. Wild food plants of popular use in Sicily. J. Ethnobiol. Ethnomed. 2007, 3, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- (HMPC/EMEA); Committee on Herbal Medicinal Product from European Medicines Agency. Community Monograph on European Medicine Agency on Valerian Root, Valeriana officinalis L. Radix. European Medicines Agency. 2016. Available online: https://www.ema.europa.eu/en/documents/herbal-summary/valerian-root-summary-public_en.pdf (accessed on 28 November 2020).
- Hattesohl, M.; Feistel, B.; Sievers, H.; Lehnfeld, R.; Hegger, M.; Winterhoff, H. Extracts of Valeriana officinalis L. sl show anxiolytic and antidepressant effects but neither sedative nor myorelaxant properties. Phytomedicine 2008, 15, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Yoo, D.Y.; Kim, W.; Nam, S.M.; Kim, J.W.; Choi, J.H.; Kwak, Y.-G.; Yoon, Y.S.; Hwang, I.K. Valeriana officinalis root extract suppresses physical stress by electric shock and psychological stress by nociceptive stimulation-evoked responses by decreasing the ratio of monoamine neurotransmitters to their metabolites. BMC Complement. Altern. Med. 2014, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.; Kubin, Z.; Shepherd, J.; Ettinger, R. Valeriana officinalis root extracts have potent anxiolytic effects in laboratory rats. Phytomedicine 2010, 17, 674–678. [Google Scholar] [CrossRef]
- Marder, M.; Viola, H.; Wasowski, C.; Fernández, S.; Medina, J.H.; Paladini, A.C. 6-Methylapigenin and hesperidin: New valeriana flavonoids with activity on the CNS. Pharmacol. Biochem. Behav. 2003, 75, 537–545. [Google Scholar] [CrossRef]
- Fernández, S.; Wasowski, C.; Paladini, A.C.; Marder, M. Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis. Pharmacol. Biochem. Behav. 2004, 77, 399–404. [Google Scholar] [CrossRef]
- Granger, R.E.; Campbell, E.L.; Johnston, G.A. (+)-And (−)-borneol: Efficacious positive modulators of GABA action at human recombinant α1β2γ2L GABAA receptors. Biochem. Pharmacol. 2005, 69, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Oliva, I.; González-Trujano, M.E.; Arrieta, J.; Enciso-Rodríguez, R.; Navarrete, A. Neuropharmacological profile of hydroalcohol extract of Valeriana edulis ssp. procera roots in mice. Phytother. Res. 2004, 18, 290–296. [Google Scholar] [CrossRef]
- Takemoto, H.; Omameuda, Y.; Ito, M.; Fukuda, T.; Kaneko, S.; Akaike, A.; Kobayashi, Y. Inhalation administration of valerena-4, 7 (11)-diene from Nardostachys chinensis roots ameliorates restraint stress-induced changes in murine behavior and stress-related factors. Biol. Pharm. Bull. 2014, 37, 1050–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.-Q.; Yu, Y.-F.; Zhao, Y.; Yu, C.-X.; Zhi, D.-J.; Qi, F.-M.; Fei, D.-Q.; Zhang, Z.-X. Four novel sesquiterpenoids with their anti-Alzheimer’s disease activity from Nardostachys chinensis. Org. Biomol. Chem. 2018, 16, 9038–9045. [Google Scholar] [CrossRef]
- Lyle, N.; Chakrabarti, S.; Sur, T.; Gomes, A.; Bhattacharyya, D. Nardostachys jatamansi protects against cold restraint stress induced central monoaminergic and oxidative changes in rats. Neurochem. Res. 2012, 37, 2748–2757. [Google Scholar] [CrossRef] [PubMed]
- Bae, G.-S.; Heo, K.-H.; Choi, S.B.; Jo, I.-J.; Kim, D.-G.; Shin, J.-Y.; Seo, S.-H.; Park, K.-C.; Lee, D.-S.; Oh, H. Beneficial effects of fractions of Nardostachys jatamansi on lipopolysaccharide-induced inflammatory response. Evid. Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Razack, S.; Kandikattu, H.K.; Venuprasad, M.; Amruta, N.; Khanum, F.; Chuttani, K.; Mishra, A.K. Anxiolytic actions of Nardostachys jatamansi via GABA benzodiazepine channel complex mechanism and its biodistribution studies. Metab. Brain Dis. 2018, 33, 1533–1549. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.-S.; Kim, D.-C.; Park, J.-S.; Kim, K.-W.; Kim, Y.-C.; Oh, H. Isolation of Novel Sesquiterpeniods and Anti-neuroinflammatory Metabolites from Nardostachys jatamansi. Molecules 2018, 23, 2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, C.-S.; Kim, K.-W.; Lee, S.-C.; Kim, Y.-C.; Oh, H. Anti-neuroinflammatory effects of sesquiterpenoids isolated from Nardostachys jatamansi. Bioorg. Med. Chem. Lett. 2018, 28, 140–144. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.; Shu, Z.; Chan, K.; Huang, S.; Li, Y.; Xiao, Y.; Wu, L.; Kuang, H.; Sun, X. Valeriana amurensis improves Amyloid-beta 1-42 induced cognitive deficit by enhancing cerebral cholinergic function and protecting the brain neurons from apoptosis in mice. J. Ethnopharmacol. 2014, 153, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guo, J.; Du, X.; Mcgeer, P.L. Anti-inflammatory and neuroprotective effects of kissoone B and extracts of Valeriana amurensis. Rev. Bras. Farmacogn. 2020, 30, 474–481. [Google Scholar] [CrossRef]
- Liu, X.G.; Gao, P.Y.; Wang, G.S.; Song, S.J.; Li, L.Z.; Li, X.; Yao, X.S.; Zhang, Z.X. In vivo antidepressant activity of sesquiterpenes from the roots of Valeriana fauriei Briq. Fitoterapia 2012, 83, 599–603. [Google Scholar] [CrossRef]
- Lee, H.; Won, H.; Im, J.; Kim, Y.O.; Lee, S.; Cho, I.-H.; Kim, H.-K.; Kwon, J.-T.; Kim, H.-J. Effect of Valeriana fauriei extract on the offspring of adult rats exposed to prenatal stress. Int. J. Mol. Med. 2016, 38, 251–258. [Google Scholar] [CrossRef]
- Lee, H.; Im, J.; Won, H.; Kim, J.Y.; Kim, H.K.; Kwon, J.T.; Kim, Y.O.; Lee, S.; Cho, I.H.; Lee, S.W. Antinociceptive effect of Valeriana fauriei regulates BDNF signaling in an animal model of fibromyalgia. Int. J. Mol. Med. 2018, 41, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, L.G.; Salles, L.A.; Stein, A.C.; Betti, A.H.; Sakamoto, S.; Cassel, E.; Vargas, R.F.; von Poser, G.L.; Rates, S.M. Antidepressant-like effect of Valeriana glechomifolia Meyer (Valerianaceae) in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 36, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, L.G.; Borsoi, M.; Stolz, E.D.; Herzfeldt, V.; Viana, A.F.; Ravazzolo, A.P.; Rates, S.M.K. Diene valepotriates from Valeriana glechomifolia prevent lipopolysaccharide-induced sickness and depressive-like behavior in mice. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- de Almeida, T.M.; Danielli, L.J.; Apel, M.A.; Cassel, E.; Vargas, R.M.; Von Poser, G.L.; Müller, L.G.; Rates, S.M. A valepotriate-enriched fraction from Valeriana glechomifolia Meyer inhibits leukocytes migration and nociception in formalin test in rodents. Rev. Bras. Farmacogn. 2019, 29, 477–482. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, P.; Guo, Y.; Xie, C.; Jin, D.-Q.; Ma, Y.; Hou, W.; Zhang, T. Iridoids from the roots of Valeriana jatamansi and their neuroprotective effects. Fitoterapia 2011, 82, 1133–1136. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Guo, Y.; Guo, P.; Yamakuni, T.; Ohizumi, Y. Isolation, structural elucidation, and neuroprotective effects of iridoids from Valeriana jatamansi. Biosci. Biotechnol. Biochem. 2012, 76, 1401–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, J.-S.; Peng, M.; Shi, J.-L.; Zheng, H.-Z.; Liu, Y.; Zhao, B.-S.; Guo, J.-Y. Evaluation of anxiolytic activity of compound Valeriana jatamansi Jones in mice. BMC Complement. Altern. Med. 2012, 12, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.-N.; Ding, Y.-S.; Ma, X.-J.; Zhao, C.-B.; Lin, M.-X.; Luo, J.; Jiang, Y.-N.; He, S.; Guo, J.-Y.; Shi, J.-L. Identification of bioactive chemical markers in Zhi zhu xiang improving anxiety in rat by fingerprint-efficacy study. Molecules 2018, 23, 2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wu, L.; Chen, C.; Wang, L.; Guo, C.; Zhao, X.; Zhao, T.; Wang, X.; Liu, A.; Yan, Z. Serum Metabolic Profiling Reveals the Antidepressive Effects of the Total Iridoids of Valeriana jatamansi Jones on Chronic Unpredictable Mild Stress Mice. Front. Pharmacol. 2020, 11, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nouri, M.H.K.; Abad, A.N.A. Gabaergic system role in aqueous extract of Valeriana officinalis L. root on PTZ-induced clonic seizure threshold in mice. Afr. J. Pharm. Pharmacol. 2011, 5, 1212–1217. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.M.; Choi, J.H.; Yoo, D.Y.; Kim, W.; Jung, H.Y.; Kim, J.W.; Kang, S.-Y.; Park, J.; Kim, D.-W.; Kim, W.J. Valeriana officinalis extract and its main component, valerenic acid, ameliorate D-galactose-induced reductions in memory, cell proliferation, and neuroblast differentiation by reducing corticosterone levels and lipid peroxidation. Exp. Gerontol. 2013, 48, 1369–1377. [Google Scholar] [CrossRef]
- Shahidi, S.; Bathaei, A.; Pahlevani, P. Antinociceptive effects of Valeriana extract in mice: Involvement of the dopaminergic and serotonergic systems. Neurophysiology 2013, 45, 448–452. [Google Scholar] [CrossRef] [Green Version]
- Torres-Hernández, B.A.; Del Valle-Mojica, L.M.; Ortíz, J.G. Valerenic acid and Valeriana officinalis extracts delay onset of Pentylenetetrazole (PTZ)-Induced seizures in adult Danio rerio (Zebrafish). BMC Complement. Altern. Med. 2015, 15, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yoo, D.Y.; Jung, H.Y.; Nam, S.M.; Kim, J.W.; Choi, J.H.; Kwak, Y.-G.; Yoo, M.; Lee, S.; Yoon, Y.S.; Hwang, I.K. Valeriana officinalis extracts ameliorate neuronal damage by suppressing lipid peroxidation in the gerbil hippocampus following transient cerebral ischemia. J. Med. Food 2015, 18, 642–647. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-W.; He, X.-H.; Yuan, R.; Wei, B.-J.; Chen, Z.; Dong, J.-X.; Wang, J. Sesquiterpenes and a monoterpenoid with acetylcholinesterase (AchE) inhibitory activity from Valeriana officinalis var. latiofolia in vitro and in vivo. Fitoterapia 2016, 110, 142–149. [Google Scholar] [CrossRef]
- Santos, G.; Giraldez-Alvarez, L.D.; Ávila-Rodriguez, M.; Capani, F.; Galembeck, E.; Neto, A.G.; Barreto, G.E.; Andrade, B. SUR1 receptor interaction with hesperidin and linarin predicts possible mechanisms of action of Valeriana officinalis in Parkinson. Front. Aging Neurosci. 2016, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Lee, Y.; Lee, H.J.; Kwon, Y.-S.; Chun, W. In silico screening of GABA aminotransferase inhibitors from the constituents of Valeriana officinalis by molecular docking and molecular dynamics simulation study. J. Mol. Model. 2020, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Amaral de Brito, A.P.; Galvão de Melo, I.M.d.S.; El-Bachá, R.S.; Guedes, R.C.A. Valeriana officinalis Counteracts Rotenone Effects on Spreading Depression in the Rat Brain in vivo and Protects Against Rotenone Cytotoxicity Toward Rat Glioma C6 Cells in vitro. Front. Neurosci. 2020, 14, 759. [Google Scholar] [CrossRef] [PubMed]
- de Ávila, J.M.; Pereira, A.O.; Zachow, L.L.; Gehm, A.Z.; Santos, M.Z.; Mostardeiro, M.A.; Back, D.; Morel, A.F.; Dalcol, I.I. Chemical constituents from Valeriana polystachya Smith and evaluation of their effects on the acetylcholinesterase and prolyl oligopeptidase activities. Fitoterapia 2018, 131, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, I.; Cechinel Filho, V.; Mora, T.C.; Cáceres, A.; Martínez, J.V.; Cruz, S.M.; de Souza, M.M. Evaluation of behavioral and pharmacological effects of hydroalcoholic extract of Valeriana prionophylla Standl. from Guatemala. Evid. Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sah, S.P.; Mathela, C.S.; Chopra, K. Antidepressant effect of Valeriana wallichii patchouli alcohol chemotype in mice: Behavioural and biochemical evidence. J. Ethnopharmacol. 2011, 135, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.P.; Mathela, C.S.; Chopra, K. Involvement of nitric oxide (NO) signalling pathway in the antidepressant activity of essential oil of Valeriana wallichii Patchouli alcohol chemotype. Phytomedicine 2011, 18, 1269–1275. [Google Scholar] [CrossRef]
- Sahu, S.; Ray, K.; Kumar, M.Y.; Gupta, S.; Kauser, H.; Kumar, S.; Mishra, K.; Panjwani, U. Valeriana wallichii root extract improves sleep quality and modulates brain monoamine level in rats. Phytomedicine 2012, 19, 924–929. [Google Scholar] [CrossRef]
- Sridharan, S.; Mohankumar, K.; Jeepipalli, S.P.K.; Sankaramourthy, D.; Ronsard, L.; Subramanian, K.; Thamilarasan, M.; Raja, K.; Chandra, V.K.; Sadras, S.R. Neuroprotective effect of Valeriana wallichii rhizome extract against the neurotoxin MPTP in C57BL/6 mice. Neurotoxicology 2015, 51, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, M.E.; Roohbakhsh, A.; Allahtavakoli, M.; Shamsizadeh, A. Anticonvulsant effect of aqueous extract of Valeriana officinalis in amygdala-kindled rats: Possible involvement of adenosine. J. Ethnopharmacol. 2010, 127, 313–318. [Google Scholar] [CrossRef]
- Björkholm, C.; Monteggia, L.M. BDNF–a key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonulalan, E.-M.; Bayazeid, O.; Yalcin, F.-N.; Demirezer, L.-O. The roles of valerenic acid on BDNF expression in the SH-SY5Y cell. Saudi Pharm. J. 2018, 26, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Sudati, J.H.; Vieira, F.A.; Pavin, S.S.; Dias, G.R.M.; Seeger, R.L.; Golombieski, R.; Athayde, M.L.; Soares, F.A.; Rocha, J.B.T.; Barbosa, N.V. Valeriana officinalis attenuates the rotenone-induced toxicity in Drosophila melanogaster. Neurotoxicology 2013, 37, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Jugran, A.K.; Rawat, S.; Bhatt, I.D.; Rawal, R.S. Valeriana jatamansi: An herbaceous plant with multiple medicinal uses. Phytother. Res. 2019, 33, 482–503. [Google Scholar] [CrossRef]
- Shalam, M.; Shantakumar, S.; Narasu, M.L. Pharmacological and biochemical evidence for the antidepressant effect of the herbal preparation Trans-01. Indian J. Pharmacol. 2007, 39, 231. [Google Scholar] [CrossRef] [Green Version]
- Subhan, F.; Karim, N.; Gilani, A.H.; Sewell, R.D. Terpenoid content of Valeriana wallichii extracts and antidepressant-like response profiles. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2010, 24, 686–691. [Google Scholar]
- Sah, S.P.; Mathela, C.S.; Chopra, K. Elucidation of possible mechanism of analgesic action of Valeriana wallichii DC chemotype (patchouli alcohol) in experimental animal models. Indian J. Exp. Biol. 2010, 48, 289–293. [Google Scholar]
- Kim, B.; Ma, S.-S.; Jo, C.; Lee, S.; Choi, H.; Lee, K.; Ham, I.; Choi, H.-Y. Vasorelaxant effect of the ethanol extract from Valeriana fauriei briquet root and rhizome on rat thoracic aorta. Pharmacogn. Mag. 2019, 15, 59. [Google Scholar]
- Kim, J.S.; Ahn, J.D.; Cho, S.-I. Effects of Valerianae Radix et Rhizoma extract on psychological stress in mice. Pharmacogn. Mag. 2015, 11, 381. [Google Scholar]
- Chen, J.; Wu, J.; Liu, L.; Zhang, Y.; Wang, F.; Du, X. Studies on improving sleep function and relative mechanism of mice by petroleum extract of Valeriana amurensis. Chin. J. Exp. Tradit. Med Formulae 2013, 19, 245–249. [Google Scholar]
- Lyle, N.; Gomes, A.; Sur, T.; Munshi, S.; Paul, S.; Chatterjee, S.; Bhattacharyya, D. The role of antioxidant properties of Nardostachys jatamansi in alleviation of the symptoms of the chronic fatigue syndrome. Behav. Brain Res. 2009, 202, 285–290. [Google Scholar] [CrossRef]
- Takemoto, H.; Ito, M.; Shiraki, T.; Yagura, T.; Honda, G. Sedative effects of vapor inhalation of agarwood oil and spikenard extract and identification of their active components. J. Nat. Med. 2008, 62, 41–46. [Google Scholar] [CrossRef]
- Takemoto, H.; Ito, M.; Asada, Y.; Kobayashi, Y. Inhalation administration of the sesquiterpenoid aristolen-1 (10)-en-9-ol from Nardostachys chinensis has a sedative effect via the GABAergic system. Planta Med. 2015, 81, 343–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Song, X.; Gao, Z.; Zhao, H.; Wang, X.; Liu, M.; Jia, L. Anti-hyperlipidemic, antioxidant and organic protection effects of acidic-extractable polysaccharides from Dictyophora indusiata. Int. J. Biol. Macromol. 2019, 129, 281–292. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Ying, S.-S.; Zheng, H.-H.; Liu, Y.-T.; Wang, Z.-P.; Zhang, H.; Deng, X.; Wu, Y.-J.; Gao, X.-M.; Li, T.-X. Novel serotonin transporter regulators: Natural aristolane-and nardosinane-types of sesquiterpenoids from Nardostachys chinensis Batal. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Wu, Y.-J.; Chen, Y.-P.; Zheng, H.-H.; Wang, Z.-P.; Zhu, Y.; Gao, X.-M.; Xu, Y.-T.; Wu, H.-H. Nardonaphthalenones A and B from the roots and rhizomes of Nardostachys chinensis Batal. Bioorg. Med. Chem. Lett. 2017, 27, 875–879. [Google Scholar] [CrossRef]
- Rao, V.S.; Rao, A.; Karanth, K.S. Anticonvulsant and neurotoxicity profile of Nardostachys jatamansi in rats. J. Ethnopharmacol. 2005, 102, 351–356. [Google Scholar] [CrossRef]
- Purushotham, K.; Pl, B. Anticonvulsant profile of Nardostachys jatamansi roots in albino rats. Int. J. Basic Clin. Pharmacol. 2016, 758–762. [Google Scholar]
- Liu, Q.F.; Jeon, Y.; Sung, Y.-W.; Lee, J.H.; Jeong, H.; Kim, Y.-M.; Yun, H.S.; Chin, Y.-W.; Jeon, S.; Cho, K.S. Nardostachys jatamansi ethanol extract ameliorates Aβ42 cytotoxicity. Biol. Pharm. Bull. 2018, 41, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Ko, W.; Park, J.-S.; Kim, K.-W.; Kim, J.; Kim, Y.-C.; Oh, H. Nardosinone-type sesquiterpenes from the hexane fraction of Nardostachys jatamansi attenuate NF-κB and MAPK signaling pathways in lipopolysaccharide-stimulated BV2 microglial cells. Inflammation 2018, 41, 1215–1228. [Google Scholar] [CrossRef]
- Kim, K.-W.; Yoon, C.-S.; Kim, Y.-C.; Oh, H. Desoxo-narchinol a and narchinol b isolated from Nardostachys jatamansi exert anti-neuroinflammatory effects by up-regulating of nuclear transcription factor erythroid-2-related factor 2/heme oxygenase-1 signaling. Neurotox. Res. 2019, 35, 230–243. [Google Scholar] [CrossRef]
- Sieghart, W. Structure and pharmacology of γ-amino butyric acid_A receptor subtypes. Pharmacol. Rev. 1995, 47, 181–234. [Google Scholar]
- Dietz, B.M.; Mahady, G.B.; Pauli, G.F.; Farnsworth, N.R. Valerian extract and valerenic acid are partial agonists of the 5-HT5a receptor in vitro. Mol. Brain Res. 2005, 138, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Awad, R.; Levac, D.; Cybulska, P.; Merali, Z.; Trudeau, V.; Arnason, J. Effects of traditionally used anxiolytic botanicals on enzymes of the γ-aminobutyric acid (GABA) system. Can. J. Physiol. Pharmacol. 2007, 85, 933–942. [Google Scholar] [CrossRef]
- Felgentreff, F.; Becker, A.; Meier, B.; Brattström, A. Valerian extract characterized by high valerenic acid and low acetoxy valerenic acid contents demonstrates anxiolytic activity. Phytomedicine 2012, 19, 1216–1222. [Google Scholar] [CrossRef]
- Valle-Mojica, D.; Lisa, M.; Ayala-Marín, Y.M.; Ortiz-Sanchez, C.M.; Torres-Hernández, B.A.; Abdalla-Mukhaimer, S.; Ortiz, J.G. Selective interactions of Valeriana officinalis extracts and valerenic acid with [3H] glutamate binding to rat synaptic membranes. Evid. Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Becker, A.; Felgentreff, F.; Schröder, H.; Meier, B.; Brattström, A. The anxiolytic effects of a Valerian extract is based on valerenic acid. BMC Complement. Altern. Med. 2014, 14, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Khom, S.; Hintersteiner, J.; Luger, D.; Haider, M.; Pototschnig, G.; Mihovilovic, M.D.; Schwarzer, C.; Hering, S. Analysis of β-subunit-dependent GABAA receptor modulation and behavioral effects of valerenic acid derivatives. J. Pharmacol. Exp. Ther. 2016, 357, 580–590. [Google Scholar] [CrossRef] [Green Version]
- Sichardt, K.; Vissiennon, Z.; Koetter, U.; Brattström, A.; Nieber, K. Modulation of postsynaptic potentials in rat cortical neurons by valerian extracts macerated with different alcohols: Involvement of adenosine A1-and GABAA-receptors. Phytother. Res. 2007, 21, 932–937. [Google Scholar] [CrossRef]
- Valle-Mojica, D.; Lisa, M.; Cordero-Hernández, J.M.; González-Medina, G.; Ramos-Vélez, I.; Berríos-Cartagena, N.; Torres-Hernández, B.A.; Ortíz, J.G. Aqueous and ethanolic Valeriana officinalis extracts change the binding of ligands to glutamate receptors. Evid. Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Sun, Y.; Zhao, T.; Li, Y.; Zhao, X.; Zhang, L.; Wu, L.; Zhang, L.; Zhang, T.; Wei, G. Antidepressant effects and mechanisms of the total iridoids of Valeriana jatamansi on the brain-gut Axis. Planta Med. 2020, 86, 172–179. [Google Scholar] [CrossRef]
- Nencini, C.; Cavallo, F.; Capasso, A.; De Feo, V.; De Martino, L.; Bruni, G.; Giorgi, G.; Micheli, L. Binding studies for serotoninergic, dopaminergic and noradrenergic receptors of Valeriana adscendens Trel. extracts. J. Ethnopharmacol. 2006, 108, 185–187. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, M.J.; Chang, Y.; Lee, S.; Kim, H.-J.; Lee, S.W.; Kim, Y.O.; Cho, I.-H. Valeriana fauriei exerts antidepressant-like effects through anti-inflammatory and antioxidant activities by inhibiting brain-derived neurotrophic factor associated with chronic restraint stress. Rejuvenation Res. 2020, 23, 245–255. [Google Scholar] [CrossRef]
- Jiang, H.-H.; Dong, F.-W.; Zhou, J.; Hu, J.-M.; Yang, J.; Nian, Y. Ca v 2.2 and Ca v 3.1 calcium channel inhibitors from Valeriana jatamansi Jones. RSC Adv. 2017, 7, 45878–45884. [Google Scholar] [CrossRef] [Green Version]
- Dong, F.-W.; Jiang, H.-H.; Yang, L.; Gong, Y.; Zi, C.-T.; Yang, D.; Ye, C.-J.; Li, H.; Yang, J.; Nian, Y. Valepotriates from the roots and rhizomes of Valeriana jatamansi Jones as novel N-type calcium channel antagonists. Front. Pharmacol. 2018, 9, 885. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.; Zuo, Y.; Wang, Z.; Yang, B.; Kuang, H. Compounds from the roots and rhizomes of Valeriana amurensis protect against neurotoxicity in PC12 cells. Molecules 2012, 17, 15013–15021. [Google Scholar] [CrossRef] [Green Version]
- Mineo, L.; Concerto, C.; Patel, D.; Mayorga, T.; Paula, M.; Chusid, E.; Aguglia, E.; Battaglia, F. Valeriana officinalis root extract modulates cortical excitatory circuits in humans. Neuropsychobiology 2017, 75, 46–51. [Google Scholar] [CrossRef]
- Khuda, F.; Iqbal, Z.; Khan, A.; Nasir, F. Antimicrobial and anti-inflammatory activities of leaf extract of Valeriana wallichii DC. Pak J. Pharm. Sci. 2012, 25, 715–719. [Google Scholar]
- Liu, Y.-H.; Wu, P.-Q.; Hu, Q.-L.; Pei, Y.-J.; Qi, F.-M.; Zhang, Z.-X.; Fei, D.-Q. Cytotoxic and antibacterial activities of iridoids and sesquiterpenoids from Valeriana jatamansi. Fitoterapia 2017, 123, 73–78. [Google Scholar] [CrossRef]
- Li, X.; Chen, T.; Lin, S.; Zhao, J.; Chen, P.; Ba, Q.; Guo, H.; Liu, Y.; Li, J.; Chu, R. Valeriana jatamansi constituent IVHD-valtrate as a novel therapeutic agent to human ovarian cancer: In vitro and in vivo activities and mechanisms. Curr. Cancer Drug Targets 2013, 13, 472–483. [Google Scholar] [CrossRef]
- Lin, S.; Fu, P.; Chen, T.; Ye, J.; Su, Y.-Q.; Yang, X.-W.; Zhang, Z.-X.; Zhang, W.-D. Minor valepotriates from Valeriana jatamansi and their cytotoxicity against metastatic prostate cancer cells. Planta Med. 2015, 81, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.-J.; Chen, H.-M.; Yang, F.; Deng, Y.; Hui, A.; Xie, X.-F.; Li, H.-X.; Zhang, H.; Cao, Z.-X.; Zhu, L.-X. Iridoids from the roots of Valeriana jatamansi Jones. Phytochemistry 2017, 141, 156–161. [Google Scholar] [CrossRef]
- Zhu, Z.; Shen, W.; Tian, S.; Yang, B.; Zhao, H. F3, a novel active fraction of Valeriana jatamansi Jones induces cell death via DNA damage in human breast cancer cells. Phytomedicine 2019, 57, 245–254. [Google Scholar] [CrossRef]
- Han, R.; Nusbaum, O.; Chen, X.; Zhu, Y. Valeric acid suppresses liver cancer development by acting as a novel HDAC inhibitor. Mol. Ther. Oncolytics 2020, 19, 8–18. [Google Scholar] [CrossRef]
- Honma, T.; Shiratani, N.; Banno, Y.; Kataoka, T.; Kimura, R.; Sato, I.; Endo, Y.; Kita, K.; Suzuki, T.; Takayanagi, T. Seeds of Centranthus ruber and Valeriana officinalis Contain Conjugated Linolenic Acids with Reported Antitumor Effects. J. Oleo Sci. 2019, 68, 481–491. [Google Scholar] [CrossRef] [Green Version]
- Cravotto, G.; Boffa, L.; Genzini, L.; Garella, D. Phytotherapeutics: An evaluation of the potential of 1000 plants. J. Clin. Pharm. Ther. 2010, 35, 11–48. [Google Scholar] [CrossRef]
- Khuda, F.; Iqbal, Z.; Khan, A.; Nasir, F.; Shah, Y. Anti-inflammatory activity of the topical preparation of Valeriana wallichii and Achyranthes aspera leaves. Pak. J. Pharm. Sci. 2013, 26, 451–454. [Google Scholar]
- Sudati, J.H.; Fachinetto, R.; Pereira, R.P.; Boligon, A.A.; Athayde, M.L.; Soares, F.A.; de Vargas Barbosa, N.B.; Rocha, J.B.T. In vitro antioxidant activity of Valeriana officinalis against different neurotoxic agents. Neurochem. Res. 2009, 34, 1372–1379. [Google Scholar] [CrossRef]
- Dugaheh, M.A.; Meisami, F.; Torabian, Z.; Sharififar, F. Antioxidant effect and study of bioactive components of Valeriana sisymbriifolia and Nardostachys jatamansii in comparison to Valeriana officinalis. Pak. J. Pharm. Sci. 2013, 26, 53–58. [Google Scholar]
- Jugran, A.K.; Rawat, S.; Bhatt, I.D.; Rawal, R.S. Essential oil composition, phenolics and antioxidant activities of Valeriana jatamansi at different phenological stages. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2020, 1–8. [Google Scholar] [CrossRef]
- Gan, L.; Wei, S.; Xiang, B.; Wang, W.; Xue, C. Effect of Valerian Ligusticum Pill on angiogenesis after injury of cerebral ischemia reperfusion in rats. Yao Xue Xue Bao Acta Pharm. Sin. 2016, 51, 1423–1428. [Google Scholar]
- Wu, P.-Q.; Li, B.; Yu, Y.-F.; Zhao, Y.; Yu, C.-X.; Zhi, D.-J.; Qi, F.-M.; Su, P.-J.; Wang, R.-Y.; Fei, D.-Q. (±)-Neonardochinone A, a pair of enantiomeric neoligans from Nardostachys chinensis with their anti-Alzheimer’s disease activities. Phytochem. Lett. 2020, 39, 39–42. [Google Scholar] [CrossRef]
- Takemoto, H.; Yagura, T.; Ito, M. Evaluation of volatile components from spikenard: Valerena-4, 7 (11)-diene is a highly active sedative compound. J. Nat. Med. 2009, 63, 380–385. [Google Scholar] [CrossRef]
- Rekha, K.; Rao, R.R.; Pandey, R.; Prasad, K.R.; Babu, K.S.; Vangala, J.R.; Kalivendi, S.V.; Rao, J.M. Two new sesquiterpenoids from the rhizomes of Nardostachys jatamansi. J. Asian Nat. Prod. Res. 2013, 15, 111–116. [Google Scholar] [CrossRef]
- Lin, J.; Cai, Q.-Y.; Xu, W.; Lin, J.-M.; Peng, J. Chemical composition, anticancer, anti-neuroinflammatory, and antioxidant activities of the essential oil of Patrinia scabiosaefolia. Chin. J. Integr. Med. 2018, 24, 207–212. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, Y.; Yang, B.; Wang, Z.; Wu, L.; Su, X.; Brantner, A.; Kuang, H.; Wang, Q. Isolation and screened neuroprotective active constituents from the roots and rhizomes of Valeriana amurensis. Fitoterapia 2014, 96, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Bettero, G.M.; Salles, L.; Rosário Figueira, R.M.; Poser, G.; Rates, S.M.; Noël, F.; Quintas, L.E. In vitro effect of valepotriates isolated from Valeriana glechomifolia on rat P-type ATPases. Planta Med. 2011, 77, 1702–1706. [Google Scholar] [CrossRef] [Green Version]
- Qi, S.G.; Quan, L.Q.; Cui, X.Y.; Li, H.M.; Zhao, X.D.; Li, R.T. A natural compound obtained from Valeriana jatamansi selectively inhibits glioma stem cells. Oncol. Lett. 2020, 19, 1384–1392. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.; Ye, X.; Huang, Q.; Dai, W.-M.; Zhang, J.-M. Anti-epileptic effects of valepotriate isolated from Valeriana jatamansi jones and its possible mechanisms. Pharmacogn. Mag. 2017, 13, 512. [Google Scholar]
- Tan, Y.-Z.; Yong, Y.; Dong, Y.-H.; Wang, R.-J.; Li, H.-X.; Zhang, H.; Guo, D.-L.; Zhang, S.-J.; Dong, X.-P.; Xie, X.-F. A new secoiridoid glycoside and a new sesquiterpenoid glycoside from Valeriana jatamansi with neuroprotective activity. Phytochem. Lett. 2016, 17, 177–180. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.-N.; Shi, J.-L.; Liu, Y.; Wang, Y.-L.; Wang, C.-G.; Hou, W.-H.; Guo, J.-Y. The anxiolytic effects of valtrate in rats involves changes of corticosterone levels. Evid. Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, Y.; Xie, C.; Jin, D.Q.; Gao, J.; Gui, L. Isolation and neuroprotective activities of acylated iridoids from Valeriana jatamansi. Chem. Biodivers. 2012, 9, 1382–1388. [Google Scholar] [CrossRef]
- Wasowski, C.; Marder, M.; Viola, H.; Medina, J.H.; Paladini, A.C. Isolation and identification of 6-methylapigenin, a competitive ligand for the brain GABAA receptors, from Valeriana wallichii. Planta Med. 2002, 68, 934–936. [Google Scholar] [CrossRef]
- Xu, J.; Yang, B.; Guo, Y.; Jin, D.-Q.; Guo, P.; Liu, C.; Hou, W.; Zhang, T.; Gui, L.; Sun, Z. Neuroprotective bakkenolides from the roots of Valeriana jatamansi. Fitoterapia 2011, 82, 849–853. [Google Scholar] [CrossRef]
- Choi, H.-S.; Hong, K.-B.; Han, S.H.; Suh, H.J. Valerian/Cascade mixture promotes sleep by increasing non-rapid eye movement (NREM) in rodent model. Biomed. Pharmacother. 2018, 99, 913–920. [Google Scholar] [CrossRef]
- Moradi-Afrapoli, F.; Ebrahimi, S.N.; Smiesko, M.; Hamburger, M. HPLC-based activity profiling for GABAA receptor modulators in extracts: Validation of an approach utilizing a larval zebrafish locomotor assay. J. Nat. Prod. 2017, 80, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.-Z.; Zu, X.-P.; Wang, J.-X.; Li, H.-L.; Chen, B.-Y.; Liu, Q.-X.; Hu, X.-Q.; Yan, Z.-H.; Zhang, W.-D. Neomerane-type sesquiterpenoids from Valeriana officinalis var. latifolia. Tetrahedron 2014, 70, 962–966. [Google Scholar] [CrossRef]
- Wang, P.-C.; Ran, X.-H.; Chen, R.; Luo, H.-R.; Liu, Y.-Q.; Zhou, J.; Zhao, Y.-X. Germacrane-type sesquiterpenoids from the roots of Valeriana officinalis var. latifolia. J. Nat. Prod. 2010, 73, 1563–1567. [Google Scholar] [CrossRef]
- Giraldo, S.E.; Rincon, J.; Guerrero, M.F.; Lopez, I.; Jimenez, I.A.; Marder, M.; Wasowski, C.; Vergel, N.E. Valepotriate Hydrines Isolated from an Anticonvulsant Fraction of Valeriana pavonii Poepp. & Endl. Lat. Am. J. Pharm. 2013, 32, 1224–1230. [Google Scholar]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases. Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Kempinski, C.; Chappell, J. Extraction and analysis of terpenes/terpenoids. Curr. Protoc. Plant Biol. 2016, 1, 345–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perveen, S.; Al-Taweel, A. Introductory chapter: Terpenes and terpenoids. Terpenes Terpenoids 2018, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tantengco, O.A.G.; Condes, M.L.C.; Estadilla, H.H.T.; Ragragio, E.M. Ethnobotanical survey of medicinal plants used by ayta communities in Dinalupihan, Bataan, Philippines. Pharmacogn. J. 2018, 10, 859–870. [Google Scholar] [CrossRef] [Green Version]
- Dhiman, B.; Sharma, P.; Pal, P.K. Biology, chemical diversity, agronomy, conservation and industrial importance of Valeriana jatamansi: A natural sedative. J. Appl. Res. Med. Aromat. Plants 2020, 16, 100243. [Google Scholar] [CrossRef]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Cai, L. Thin layer chromatography. Curr. Protoc. Essent. Lab. Tech. 2014, 8, 6.3.1–6.3.18. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Mtewa, A.G.; Deyno, S.; Kasali, F.M.; Annu, A.; Sesaazi, D.C. General extraction, isolation and characterization techniques in drug discovery: A review. Int. J. Sci. Basic Appl. Res. 2018, 38, 10–24. [Google Scholar]
- Rasul, M.G. Extraction, isolation and characterization of natural products from medicinal plants. Int. J. Basic Sci. Appl. Comput. 2018, 2, 1–6. [Google Scholar]
- Ho, C.S.; Lam, C.; Chan, M.; Cheung, R.; Law, L.; Lit, L.; Ng, K.; Suen, M.; Tai, H. Electrospray ionisation mass spectrometry: Principles and clinical applications. Clin. Biochem. Rev. 2003, 24, 3. [Google Scholar]
- Ranjbar, B.; Gill, P. Circular dichroism techniques: Biomolecular and nanostructural analyses—A review. Chem. Biol. Drug Des. 2009, 74, 101–120. [Google Scholar] [CrossRef]
- Gutierrez, S.; Ang-Lee, M.K.; Walker, D.J.; Zacny, J.P. Assessing subjective and psychomotor effects of the herbal medication valerian in healthy volunteers. Pharmacol. Biochem. Behav. 2004, 78, 57–64. [Google Scholar] [CrossRef]
- Yu, L.; Ke-Ke, X.; Chao-Yong, C.; Rui-Tong, Z.; Ming, L.; Shao-Hua, L.; Ling-Zhen, P.; Tian-E, Z.; Zhi-Yong, Y. A study of the substance dependence effect of the ethanolic extract and iridoid-rich fraction from Valeriana jatamansi Jones in mice. Pharmacogn. Mag. 2015, 11, 745. [Google Scholar]
- Gooneratne, N.S. Complementary and alternative medicine for sleep disturbances in older adults. Clin. Geriatr. Med. 2008, 24, 121–138. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, B.; Scholle, S.; Hölzl, J.; Khudeir, N.; Hess, S.; Müller, C.E. Lignans isolated from valerian: Identification and characterization of a new olivil derivative with partial agonistic activity at A1 adenosine receptors. J. Nat. Prod. 2002, 65, 1479–1485. [Google Scholar] [CrossRef]
- Maurmann, N.; Reolon, G.K.; Rech, S.B.; Fett-Neto, A.G.; Roesler, R. A valepotriate fraction of Valeriana glechomifolia shows sedative and anxiolytic properties and impairs recognition but not aversive memory in mice. Evid. Based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Morais, L.; Barbosa-Filho, J.; Almeida, R. Central depressant effects of reticuline extracted from Ocotea duckei in rats and mice. J. Ethnopharmacol. 1998, 62, 57–61. [Google Scholar] [CrossRef]
- Khom, S.; Baburin, I.; Timin, E.; Hohaus, A.; Trauner, G.; Kopp, B.; Hering, S. Valerenic acid potentiates and inhibits GABAA receptors: Molecular mechanism and subunit specificity. Neuropharmacology 2007, 53, 178–187. [Google Scholar] [CrossRef]
- Benke, D.; Barberis, A.; Kopp, S.; Altmann, K.-H.; Schubiger, M.; Vogt, K.E.; Rudolph, U.; Möhler, H. GABAA receptors as in vivo substrate for the anxiolytic action of valerenic acid, a major constituent of valerian root extracts. Neuropharmacology 2009, 56, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Rahman, H.; Shaik, H.A.; Madhavi, P.; Eswaraiah, M.C. A review: Pharmacognostics and pharmacological profiles of Nardastachys jatamansi DC. Elixir Pharm. 2011, 39, 5017–5020. [Google Scholar]
- Torres-Hernández, B.A.; Colón, L.R.; Rosa-Falero, C.; Torrado, A.; Miscalichi, N.; Ortíz, J.G.; González-Sepúlveda, L.; Pérez-Ríos, N.; Suárez-Pérez, E.; Bradsher, J.N.; et al. Reversal of pentylenetetrazole-altered swimming and neural activity-regulated gene expression in zebrafish larvae by valproic acid and valerian extract. Psychopharmacology 2016, 233, 2533–2547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulyawan, E.; Ahmad, M.R.; Islam, A.A.; Massi, M.N.; Hatta, M.; Arif, S.K. Analysis of GABRB3 Protein Level After Administration of Valerian Extract (Valeriana officinalis) in BALB/c mice. Pharmacogn. J. 2020, 12, 821–827. [Google Scholar] [CrossRef]
- González-Trujano, M.E.; Contreras-Murillo, G.; López-Najera, C.A.; Hidalgo-Flores, F.J.; Navarrete-Castro, A.; Sánchez, C.G.; Magdaleno-Madrigal, V.M. Anticonvulsant activity of Valeriana edulis roots and valepotriates on the pentylenetetrazole-induced seizures in rats. J. Ethnopharmacol. 2021, 265, 113299. [Google Scholar] [CrossRef]
- Bent, S.; Padula, A.; Moore, D.; Patterson, M.; Mehling, W. Valerian for sleep: A systematic review and meta-analysis. Am. J. Med. 2006, 119, 1005–1012. [Google Scholar] [CrossRef] [Green Version]
- De Feo, V.; Faro, C. Pharmacological effects of extracts from Valeriana adscendens Trel. II. Effects on GABA uptake and amino acids. Phytother. Res. 2003, 17, 661–664. [Google Scholar] [CrossRef]
- Vissiennon, Z.; Sichardt, K.; Koetter, U.; Brattström, A.; Nieber, K. Valerian extract Ze 911 inhibits postsynaptic potentials by activation of adenosine A1 receptors in rat cortical neurons. Planta Med. 2006, 72, 579–583. [Google Scholar] [CrossRef]
- Lacher, S.K.; Mayer, R.; Sichardt, K.; Nieber, K.; Müller, C.E. Interaction of valerian extracts of different polarity with adenosine receptors: Identification of isovaltrate as an inverse agonist at A1 receptors. Biochem. Pharmacol. 2007, 73, 248–258. [Google Scholar] [CrossRef]
- Bloodgood, B.L.; Sharma, N.; Browne, H.A.; Trepman, A.Z.; Greenberg, M.E. The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition. Nature 2013, 503, 121–125. [Google Scholar] [CrossRef]
- Lin, Y.; Bloodgood, B.L.; Hauser, J.L.; Lapan, A.D.; Koon, A.C.; Kim, T.-K.; Hu, L.S.; Malik, A.N.; Greenberg, M.E. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature 2008, 455, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, I.; Mardinly, A.R.; Gabel, H.W.; Bazinet, J.E.; Couch, C.H.; Tzeng, C.P.; Harmin, D.A.; Greenberg, M.E. Npas4 regulates excitatory-inhibitory balance within neural circuits through cell-type-specific gene programs. Cell 2014, 157, 1216–1229. [Google Scholar] [CrossRef] [Green Version]
- Phiel, C.J.; Zhang, F.; Huang, E.Y.; Guenther, M.G.; Lazar, M.A.; Klein, P.S. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 2001, 276, 36734–36741. [Google Scholar] [CrossRef] [Green Version]
- Jacobo-Herrera, N.J.; Vartiainen, N.; Bremner, P.; Gibbons, S.; Koistinaho, J.; Heinrich, M. F-κB modulators from Valeriana officinalis. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 917–919. [Google Scholar]
- Cornara, L.; Ambu, G.; Trombetta, D.; Denaro, M.; Alloisio, S.; Frigerio, J.; Labra, M.; Ghimire, G.; Valussi, M.; Smeriglio, A. Comparative and functional screening of three species traditionally used as antidepressants: Valeriana officinalis L., Valeriana jatamansi Jones ex Roxb. and Nardostachys jatamansi (D. Don) DC. Plants 2020, 9, 994. [Google Scholar] [CrossRef]
- NIH Office of Dietary Supplements. Available online: https://ods.od.nih.gov/factsheets/Valerian-HealthProfessional/#en13 (accessed on 30 November 2020).
- Leathwood, P.D.; Chauffard, F.; Heck, E.; Munoz-Box, R. Aqueous extract of valerian root (Valeriana officinalis L.) improves sleep quality in man. Pharmacol. Biochem. Behav. 1982, 17, 65–71. [Google Scholar] [CrossRef]
- Leathwood, P.; Chauffard, F. Aqueous extract of valerian reduces latency to fall asleep in man. Planta Med. 1985, 51, 144–148. [Google Scholar] [CrossRef]
- Vorbach, E.; Gortelmeyer, R.; Bruning, J. Treatment of insomnia: Effectiveness and tolerance of a valerian extract. Psychopharmakotherapie 1996, 3, 109–115. [Google Scholar]
- Dorn, M. Baldrian versus Oxazepam: Efficacy and Tolerability in Non-Organic and Non-Psychiatric Insomniacs. A Randomised, Double-Blind, Clinical, Comparative Study. Forsch. Komplement. Und Klass. Nat. 2000, 7, 79–84. [Google Scholar]
- Donath, F.; Quispe, S.; Diefenbach, K.; Maurer, A.; Fietze, I.; Roots, I. Critical evaluation of the effect of valerian extract on sleep structure and sleep quality. Pharmacopsychiatry 2000, 33, 47–53. [Google Scholar] [CrossRef]
- Shinjyo, N.; Waddell, G.; Green, J. Valerian Root in Treating Sleep Problems and Associated Disorders—A Systematic Review and Meta-Analysis. J. Evid. Based Integr. Med. 2020, 25, 2515690X20967323. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.L.P.; Alcântara, C.E.P.; de Moraes, M.; de Andrade, E.D. Valeriana officinalis L. for conscious sedation of patients submitted to impacted lower third molar surgery: A randomized, double-blind, placebo-controlled split-mouth study. J. Pharm. Bioallied Sci. 2014, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Narkevich, I.A.; Flisyuk, E.V.; Luzhanin, V.G.; Pozharitskaya, O.N. Medicinal plants from the 14th edition of the Russian Pharmacopoeia, recent updates. J. Ethnopharmacol. 2021, 268, 113685. [Google Scholar] [CrossRef]

| Plant Species | Extract/Compound | Effect/Main Findings | Model/Assays | Dose | Administration | Reference |
|---|---|---|---|---|---|---|
| Nardostachys chinensis Batalin (synonym of Nardostachys jatamansi (D.Don) DC.) | Valerena-4,7(11)-diene from roots | Anti-stress, inhibited stress-induced excitatory behaviors and reduced stress-induced blood corticosterone, cerebral serotonin, and dopamine levels | Mice, a model of acute stress (restraint stress for 15 min) | 300 µg/cage | Inhalation | [28] |
| Nardostachys chinensis Batalin (synonym of Nardostachys jatamansi (D.Don) DC.) | Sesquiterpenoids isolated from underground parts | Alleviate the Alzheimer’s disease-like symptom of paralysis in worms | Caenorhabditis elegans Alzheimer’s disease pathological model | 50 μM | Added to the culture medium | [29] |
| Nardostachys jatamansi (D. Don) DC. | Rhizomes 70% ethanol extract | Anti-stress effect, inhibited cold restraint stress-induced oxidative stress | Rats, the cold restraint stress model | 200 and 500 mg/kg | Orally | [30] |
| Nardostachys jatamansi (D. Don) DC. | Root fractions | Anti-inflammatory effects reduced lipopolysaccharide-induced inflammatory response | Lipopolysaccharide-induced inflammation in murine peritoneal macrophages and mice model of lipopolysaccharide-induced endotoxin shock | 10–100 μg/mL | Intraperitoneally | [31] |
| Nardostachys jatamansi (D. Don) DC. | Root 70% ethanol extract | Anxiolytic effects | Mice, models of anxiety (elevated plus maze, open field test, light-dark box test, and Vogel’s conflict test) | 250 mg/kg | Orally | [32] |
| Nardostachys jatamansi (D. Don) DC. | Sesquiterpeniods | Anti-neuroinflammatory effects | BV2 microglial cells | Several (10–80 μM) | In vitro | [33,34] |
| Valeriana amurensis P. Smir. ex Kim. | Isolated compounds from roots and rhizomes | Ameliorate amyloid-beta-induced cognitive dysfunction | Amyloid-beta1-42 induced Alzheimer’s disease mice model | 0.2–0.8 g/kg | Intrahippocampal injection | [35] |
| Valeriana amurensis P. Smir. ex Kim. | Petroleum ether, ethyl acetate, n-butanol, and aqueous extract, and kissoone B from roots and rhizomes | Anti-inflammatory and neuroprotective effects | Cell (THP-1 cells as surrogates for microglia, SH-SY5Ycells as surrogates for neurons, and U373 cells as surrogates for astrocytes) and mice models | 400 μM kissoone B and 100 μg/mL extracts | Intragastric | [36] |
| Valeriana fauriei Briq. | Sesquiterpenes from the roots | Antidepressant | Mice, Forced swim test | 20 mg/kg, during seven consecutive days | Orally | [37] |
| Valeriana fauriei Briq. | Commercial root extract | Reduction the incidence of prenatal stress related-psychiatric disorders | Rats, prenatal stress model, evaluation of behavioral patterns and changes in protein levels in the prefrontal cortex | 100 mg/kg/day, administered on postnatal days 35–56 | Orally | [38] |
| Valeriana fauriei Briq. | Aqueous extract | Antinociceptive effect | Mice, a model of fibromyalgia (induced by intermittent cold stress) | 100 mg/kg/day for 24 days | Orally | [39] |
| Valeriana glechomifolia Meyer | Diene valepotriates fraction from underground parts | Antidepressant, interaction with dopaminergic and noradrenergic neurotransmission | Mice, tail suspension test (TST), and forced swimming test (FST) | 0.25–20 mg/kg | Orally | [40] |
| Valeriana glechomifolia Meyer | Valepotriate-enriched extract from aerial and underground parts | Antidepressant potential, prevent lipopolysaccharide-induced sickness and depressive behavior | Mice submitted to a forced swimming session as a stressful stimulus (experimental model of depression associated with inflammation) | 10 mg/kg | Orally | [41] |
| Valeriana glechomifolia Meyer | Valepotriate-enriched fraction from aerial and underground parts | Anti-inflammatory activity, inhibition of leukocytes migration | Formalin test in CF1 mice and Wistar rat’s leukocytes migration assay | 1, 10 and 30 mg/kg; 0.1–1 g/mL | Orally | [42] |
| Valeriana jatamansi Jones | Bakkenolides from rosts | Neuroprotective effects | 1-Methyl-4-phenylpyridinium-induced neuronal cell death in human dopaminergic neuroblastoma SH-SY5Y cells | 1.5, 5 and 15 μM | In vitro | [43] |
| Valeriana jatamansi Jones | Iridoids from roots | Neuroprotective effects | 1-Methyl-4-phenylpyridinium-induced neuronal cell death in human dopaminergic neuroblastoma SH-SY5Y cells | 3, 10 and 30 μM | In vitro | [44] |
| Valeriana jatamansi Jones | Root ethanol extract | Anxiolytic action | Mice, elevated plus maze, light/dark box test, and spontaneous activity | 1.2, 2.4 and 4.8 g/kg, for 10 days | Orally | [45] |
| Valeriana jatamansi Jones | Root and rhizome (Zhi zhu xiang) 35% ethanol extract | Anti-anxiety activity | Empty bottle stimulated rats, open field test, and the elevated plus-maze test | 1.2 g/kg, for 7 days | Orally | [46] |
| Valeriana jatamansi Jones | Iridoid-rich fraction from roots and rhizomes | Antidepressive | Unpredictable mild stress mouse model | 5.73, 11.47 and 22.94 mg/kg | Orally | [47] |
| Valeriana officinalis L. | Extracts from roots | Anxiolytic and antidepressant effect | Mice elevated plus maze test and forced swimming test | 100–1000 mg/kg | Orally and intraperitoneally | [21] |
| Valeriana officinalis L. | Root ethanol extract and valerenic acid | Anxiolytic effects, reduction in anxious behavior | Rats, elevated plus maze | 3 mL/kg extract and 3 mg/kg valerenic acid | Intraperitoneally | [23] |
| Valeriana officinalis L. | Roots aqueous extract | Anticonvulsant effects | Mice, pentylenetetrazole-induced clonic seizure | 0.25, 0.5 and 1 g/kg | Intraperitoneally | [48] |
| Valeriana officinalis L. | Root extract and valerenic acid | Memory function, cell proliferation, neuroblast differentiation, serum corticosterone, and lipid peroxidation | Mice, D-galactose-induced aging model | 100 mg/kg extracts and 340 μg/kg valerenic acid | Orally | [49] |
| Valeriana officinalis L. | Root ethanol extract | Antinociceptive effects, pain modulation | Mice, Tail-Flick Test, Acetic Acid Writhing Test | 50, 200 and 800 mg/kg | Intraperitoneally | [50] |
| Valeriana officinalis L. | Root extract | Anti-stress effects | Mice, exposure to physical stress psychological in a communication box | 100 mg/kg/0.5 mL | Orally | [22] |
| Valeriana officinalis L. | Root aqueous and ethanol extracts | Anticonvulsant effects | Zebrafish (Danio rerio), an animal model used to study clonic-like behaviors | 1 mg/mL; 5 mg/mL | Dissolved in aquarium water | [51] |
| Valeriana officinalis L. | Root ethanol extract | Protective effects against ischemic injury in the hippocampal pyramidal neurons | Gerbils subjected to ischemia/reperfusion injury | 100 mg/kg | Orally | [52] |
| Valeriana officinalis var. latiofolia | Sesquiterpenes and a monoterpenoid from roots | Inhibition of acetylcholinesterase | In vitro and in vivo in mice | 0.65, 1.30 and 2.6 mg/kg/day, for 90 days | Intragastric | [53] |
| Valeriana officinalis L. | Root aqueous extract | Elucidation of mechanisms of neuroprotective action against rotenone-induced cellular damage | Theoretical analysis (microarray data) | - | - | [54] |
| Valeriana officinalis L. | Eighteen root compounds | Inhibition of GABA aminotransferase | Molecular docking and molecular dynamics simulations | - | - | [55] |
| Valeriana officinalis L. | Root aqueous extract | Protective action against rotenone effects (counteract Cortical spreading depression propagation velocity and C6 glioma cytotoxicity) | Cortical spreading depression (in vivo) and C6 glioma cell culture (in vitro) models | 250 mg/kg/day, for 15 days | Orally | [56] |
| Valeriana polystachya Smith | Extract from roots and rhizome, and isolated compounds from roots and rhizomes | Inhibition of acetylcholinesterase and prolyl oligopeptidase activities | In vitro | 200 μg/mL extract and 150 μM of isolated compounds | In vitro | [57] |
| Valeriana prionophylla Standl. | Roots and rhizomes 50% ethanol extract | Anxiolytic, antidepressant, and hypno-sedative effects | Swiss mice and male Wistar rats, open field, rota rod, elevated plus-maze, forced swimming, strychnine- and pentobarbital-induced sleeping time, pentylenetetrazole-induced seizures, and the inhibitory avoidance tests | 50, 100 and 150 mg/kg | Orally and intraperitoneally | [58] |
| Valeriana wallichii DC (synonym of Valeriana jatamansi Jones) | Roots and rhizomes dichloromethane extract | Antidepressant effect | Mice, acute toxicity, studies forced swim test, locomotor activity and measurement of biogenic amines | 10, 20 and 40 mg/kg | Orally | [59] |
| Valeriana wallichii DC (synonym of Valeriana jatamansi Jones) | Roots and rhizomes essential oil | Antidepressant effect | Mice, acute toxicity, studies forced swim test, locomotor activity, measurement of biogenic amines and effect of nitric oxide modulators | 10, 20 and 40 mg/kg | Orally | [60] |
| Valeriana wallichii DC (synonym of Valeriana jatamansi Jones) | Root aqueous extract | Sleep quality improvement | Rats, estimation of the sleep-wake profile, electroencephalogram delta activity, and estimation of regional brain monoamines. | 200 and 300 mg/kg | Orally | [61] |
| Valeriana wallichii DC (synonym of Valeriana jatamansi Jones) | Rhizome methanol extract | Neuroprotective effect | Mice, 1-methyl-4-phenyl-1,2,3,6-tet-rahydropyridine-induced Parkinson’s disease model | 50, 100 and 200 mg/kg | Orally | [62] |
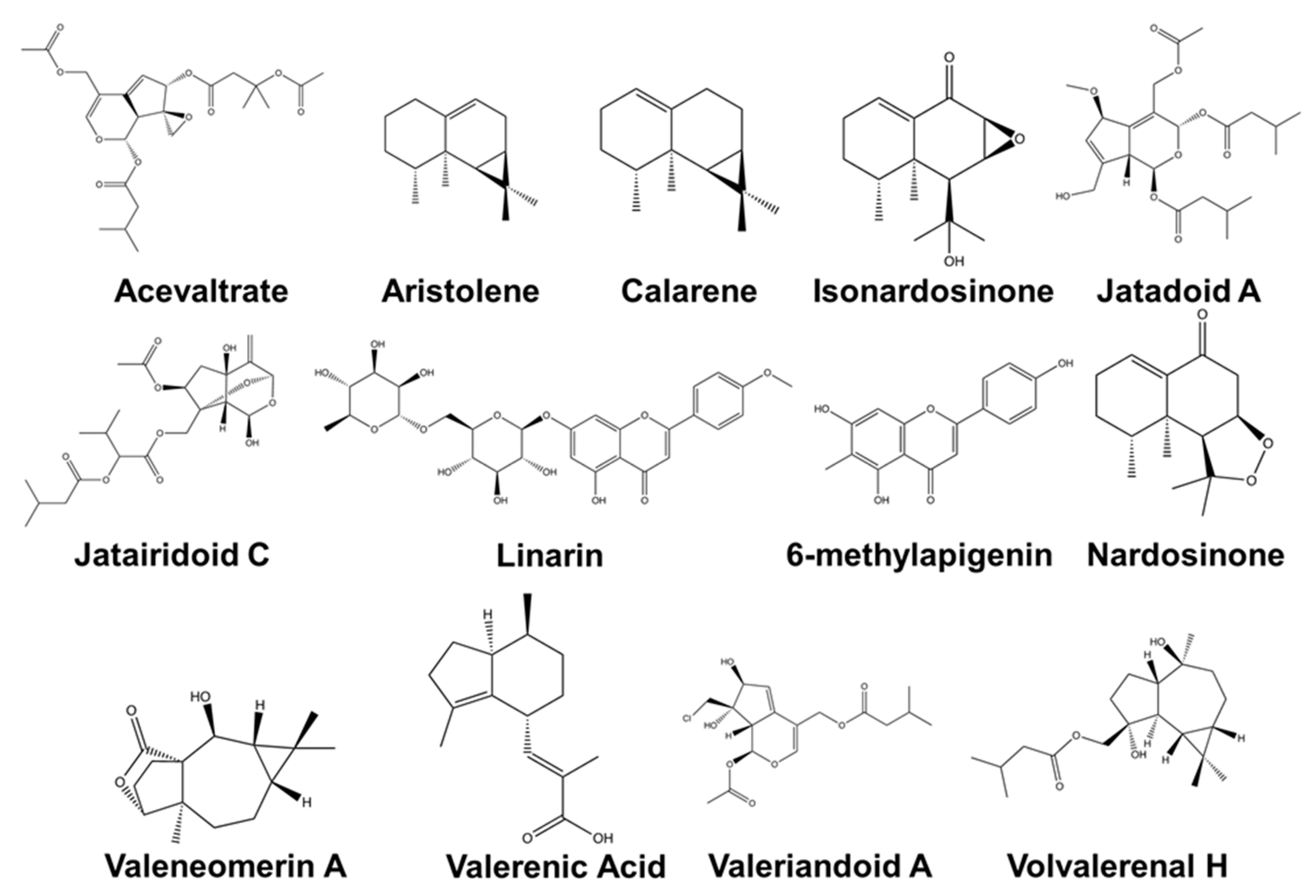
| Major Compound | Known Biological Activity | Isolation Techniques Used * | Detection Methods ** | First Author and Year |
|---|---|---|---|---|
| Nardostachys jatamansi (D.Don) DC. | ||||
| (–)-(8R)-neonardochinoneA and (+)-(8S)neonardochinoneA | Anti-Alzheimer’s disease (AD) activity | Silica gel CC, MCI gel CC, and Sephadex LH-20 CC | HRESIMS NMR XRC | [115] |
| Nardochinins A-D | Silica gel CC, MCI gel CC, and Sephadex LH-20 CC | HRESIMS NMR XRC | [29] | |
| Kanshone C—inhibits SERT and Desoxo-nachinol A—enhances SERT | Natural serotonin regulator using SERT activity assay | Silica gel CC and preparative HPLC | HRESIMS NMR XRC | [78] |
| Aristolen-1(10)-en-9-ol | Sedative effect via GABAergic system | Silica gel CC and preparative HPLC | GC-FID GC-MS | [76] |
| Valerena-4,7(11)-diene | The stress-reducing effect in mice | Silica gel CC and preparative HPLC | GC-MS | [28] |
| Valerena-4,7(11)-diene and b-maaliene | Sedative effects in mice | Silica gel CC, gel permeation chromatography, and HPLC | GC NMR | [116] |
| Aristolene, calarene, and valerena-4,7(11)-diene | Sedative effects in mice | Silica gel CC, gel permeation chromatography, and HPLC | GC/GC-MS NMR | [75] |
| Kanshone L, Kanshone M | Anti-inflammatory effects in BV2 and primary microglial cells | Silica gel CC and preparative HPLC | NMR HRESIMS | [34] |
| Sesquiterpenoids: Kanshone J and Kanshone K | Anti-inflammatory effects in BV2 and primary microglial cells | Solvent partition, CC, and HPLC | NMR HRESIMS | [34] |
| Nardosinone, Isonardosinone, Kanshone E, Kanshone B | Anti-inflammatory effects in BV2 and microglial cells | Silica gel CC | NMR MS | [83] |
| Compounds 5 and 6 | Cytotoxic activity against a neuroblastoma cell line | Silica gel CC | FT-IR MS NMR | [117] |
| Patrinia scabiosifolia Link | ||||
| Caryophyllene oxide | Anti-inflammatory activity in BV-2 cells | Distillation | GC-MS | [118] |
| Valeriana amurensis P. Smirn. ex Kom. | ||||
| Kissoone B | Anti-inflammatory and neuroprotective effects | Percolation, Sephadex LH-20 CC, and paper chromatography | EIMS NMR | [36] |
| Xiecaoside E and Lignin 11-17 | Neuroprotective effects in PC12 cells | Silica gel CC and preparative HPLC | FT-IR NMR | [119] |
| Lignans (e.g., (þ) pinoresinol-4, 4′-di-O-β-D-glucopyranoside, (þ) 8-hydroxypinoresinol-4′-Oβ-D-glucopyranoside); Iridoids (e.g., patrinoside and kanokoside A) | Activity on cerebral cholinergic function and neuroprotective effect from an αβ-induced cognitive deficit in mice | Silica gel CC, octadecyl silica gel CC, and preparative HPLC | NMR EIMS | [35] |
| Heishuixiecaoline A, B, and C, volvalerenal C, (+) pinoresinol-4,4′-di-O-β-D-glucopyranoside, (+) pinoresinol-8-O-β-D-glucopyranoside, and 8-hydroxypinoresinol-4,4′-di-O-β-D-glucopyranoside | Neuroprotective effects in PC12 cells | AB-8 macroporous resin CC and silica gel CC | HRESIMS NMR FT-IR | [99] |
| Valeriana fauriei Briq. | ||||
| 8α-acetoxyl-3α,4α,10-trihydroxyl-guaia-1(2)-ene-12,6α-olide and 2-Ethylhexyl-4-hydroxybenzoate | An antidepressant activity using forced swim test in a mouse model | Silica gel CC and Sephadex LH-20 CC | FT-IR MS NMR | [37] |
| Valeriana glechomifolia F.G. Mey. | ||||
| Valtrate, Acevaltrate, 1-β-acevaltrate, 1-β-aceacevaltrate and isovaltrate | Activity on depressive-like behavior in mice | Supercritical CO2 (SCCO2) extraction and HPLC | HPLC | [41] |
| Valtrate, acevaltrate, and 1-β-acevaltrate | Inhibition of Na+/K+-ATPase activity in the brain hemispheres of rat | Ultrasonic bath and preparative TLC | NMR | [120] |
| Valeriana jatamansi Jones | ||||
| Rupesin E | Anticancer and pro-apoptotic activity against glioma stem cells. | Silica gel CC, Sephadex LH-20 CC, and semi-preparative HPLC | NMR | [121] |
| (4β,8β)-8-methoxy-3-methoxy-10-methylene-2,9-dioxatricyclo[4.3.1.0]decan-4-ol and (1S,3R,5R,7S,8R,9S)-3,8-epoxy-1- O-ethyl-5-hydroxyvalechlorine | Neuroprotective effects in PC12 cells | Silica gel CC, semipreparative HPLC, Sephadex LH-20 CC, preparative TLC | NMR ECD FT-IR UV Vis HRESIMS | [105] |
| Valepotriate | The anti-epileptic effect in mice | Silica gel CC | NMR HPLC | [122] |
| Isopatrinioside and Valeriananoid F | Neuroprotective effects in PC12 cells | Silica gel CC, Sephadex LH-20 CC, preparative TLC, and preparative HPLC | NMR HRESIMS FT-IR MCP UV-Vis | [123] |
| Valtrate | The anxiolytic effect in rats | Chromatography in AB-8 macroporous adsorption resin, silica gel CC, and TLC | NMR EIMS FT-IR UV Vis | [124] |
| Iridoids: Jatadoids A and B | Neuroprotective effects in SH-SY5Y cells | Silica gel CC, TLC, ODS CC, and preparative HPLC | NMR HRESIMS | [44] |
| Jatairidoids A–C | Neuroprotective effects in SH-SY5Y cells | Silica gel CC, ODS CC, and preparative HPLC | NMR HRESIMS EIMS FT-IR | [125] |
| Valeriandoids A–C and chlorovaltrate | Neuroprotective effects in SH-SY5Y cells | Silica gel CC, ODS CC, and preparative HPLC | NMR HRESIMS EIMS FT-IR | [44] |
| 2S(−)-hesperidin | Sedative and sleep-enhancing properties in mice | Silica gel CC | UV-Vis NMR EIMS | [24] |
| 6-methylapigenin | Anxiolytic and sleep-enhancing properties in mice | Silica gel CC and C18 column chromatography | UV-Vis NMR EIMS | [126] |
| Valerilactones A and B, and bakkenolide-H | Neuroprotective effects in human dopaminergic neuroblastoma SH-SY5Y cells | Silica gel CC, ODS CC, and preparative HPLC | NMR HRESIMS ESIMS FT-IR | [127] |
| Valeriana officinalis L. | ||||
| Acetoxyvalerenic acid and valerinic acid | Sleep promoting properties in mice model | Soxhlet extraction, rotary vacuum evaporation, and C18 CC | HPLC UV-Vis | [128] |
| Valerenic acid | GABAA receptor modulator using a larval zebrafish seizure model | ASE® 200 solvent extraction system | HPLC | [129] |
| Volvalerenal H, Volvalerenal I, Volvalerenal J, Volvalerenal acid K, and Densispicnins C | Acetylcholinesterase inhibitory activity | Silica gel CC, Sephadex LH-20 CC, preparative TLC, and preparative HPLC | NMR HRESIMS FT-IR | [53] |
| Valeneomerin A, Valeneomerin B, Valeneomerin D | Neuroprotective effects against H2O2 induced oxidative stress in SH-SY5Y cells | Silica gel CC, RP-MPLC, and Sephadex LH-20 (MeOH) CC, and preparative TLC | NMR HRESIMS FT-IR XRC | [130] |
| Linarin | Sedative and sleep-enhancing property | Silica gel CC | NMR EIMS UV-Vis | [25] |
| Volvalerenals A-E and volvalerenic acids A-C | Weak acetylcholinesterase inhibitory activities | Silica gel CC, and Sephadex LH-20 CC | NMR HRESIMS EIMS XRC | [131] |
| Valeriana laurifolia Kunth | ||||
| Valtrate acetoxyhydrine, valtrate, isovaleroyloxyhydrine, and valtrate chlorohydrine | Anticonvulsant property in mice | Silica gel CC, and preparative TLC | NMR HRESIMS EIMS FT-IR | [132] |
| Compound/Species | Animal Model | Dosage | Outcomes | Ref. |
|---|---|---|---|---|
| Valerenic acid derivatives | Male mice (c57Bl/6N) | 3 mg/kg | Anxiolytic effect | [151] |
| Valerenic acid derivatives | Mutant mice (GABAA receptor b3 subunit mutation) | From 1 to 30 mg/kg | Anxiolitic effects | [152] |
| V. glechomifolia | Swiss male CF1 mice | 1, 3, and 10 mg/kg | Sedative effects | [149] |
| V. prionophylla | Swiss female mice; Wistar male rats | 50, 100, and 150 mg/kg | Anxiolytic; antidepressant; hypno-sedative effects | [58] |
| N. chinensis | Different animal model | Different dosages | Antidepressant; anticonvulsant; neuroprotective and antiparkinson activities; cognition and memory improvement | [153] |
| V. jatamansi | Kunming mice | Ethanolic extract | Anxiolytic effects; No drug dependence | [146] |
| V. officinalis | Zebrafish larvae | 0.3 g/kg, 0.9 g/kg | Regulation of neural-activity genes | [154] |
| V. officinalis | BALB/c mice | 1, 2.5, 5, and 7 mg/mL | Modulate GABAA subunit β3 receptors; sedative effects | [155] |
| V. edulis | Wistar male rats | 2.5 mg/10 g; 5 mg/10 g | Anticonvulsant properties | [156] |
| Species | Systematic Review | Indication | Conclusions | Source |
|---|---|---|---|---|
| V. officinalis | 5 clinical trials | Sleep disorders | Not sufficient for determining the effectiveness | [167] |
| V. officinalis | 60 studies and meta-analysis | Sleep disorders | Sufficient for determining the effectiveness but standardization and quality control is needed | [173] |
| N. grandiflora | Preliminary clinical studies | Aggressiveness, restlessness, stubbornness, sleep disorders | Further studies are needed | [153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, G.; Shin, H.-S.; Tundis, R.; Gonçalves, S.; Tantengco, O.A.G.; Campos, M.G.; Acquaviva, R.; Malfa, G.A.; Romano, A.; Robles, J.A.H.; et al. Plant Species of Sub-Family Valerianaceae—A Review on Its Effect on the Central Nervous System. Plants 2021, 10, 846. https://doi.org/10.3390/plants10050846
Das G, Shin H-S, Tundis R, Gonçalves S, Tantengco OAG, Campos MG, Acquaviva R, Malfa GA, Romano A, Robles JAH, et al. Plant Species of Sub-Family Valerianaceae—A Review on Its Effect on the Central Nervous System. Plants. 2021; 10(5):846. https://doi.org/10.3390/plants10050846
Chicago/Turabian StyleDas, Gitishree, Han-Seung Shin, Rosa Tundis, Sandra Gonçalves, Ourlad Alzeus G. Tantengco, Maria G. Campos, Rosaria Acquaviva, Giuseppe Antonio Malfa, Anabela Romano, Joyce Ann H. Robles, and et al. 2021. "Plant Species of Sub-Family Valerianaceae—A Review on Its Effect on the Central Nervous System" Plants 10, no. 5: 846. https://doi.org/10.3390/plants10050846
APA StyleDas, G., Shin, H.-S., Tundis, R., Gonçalves, S., Tantengco, O. A. G., Campos, M. G., Acquaviva, R., Malfa, G. A., Romano, A., Robles, J. A. H., Clores, M. Q., & Patra, J.-K. (2021). Plant Species of Sub-Family Valerianaceae—A Review on Its Effect on the Central Nervous System. Plants, 10(5), 846. https://doi.org/10.3390/plants10050846















