Over-Expression of Endogenous SUGARWIN Genes Exalted Tolerance against Colletotrichum Infection in Sugarcane
Abstract
:1. Introduction
2. Results
2.1. Callus Induction and Regeneration
2.2. Development of Plant Expression Vectors
2.3. Plant Nuclear Transformation and Recovery of Transgenes
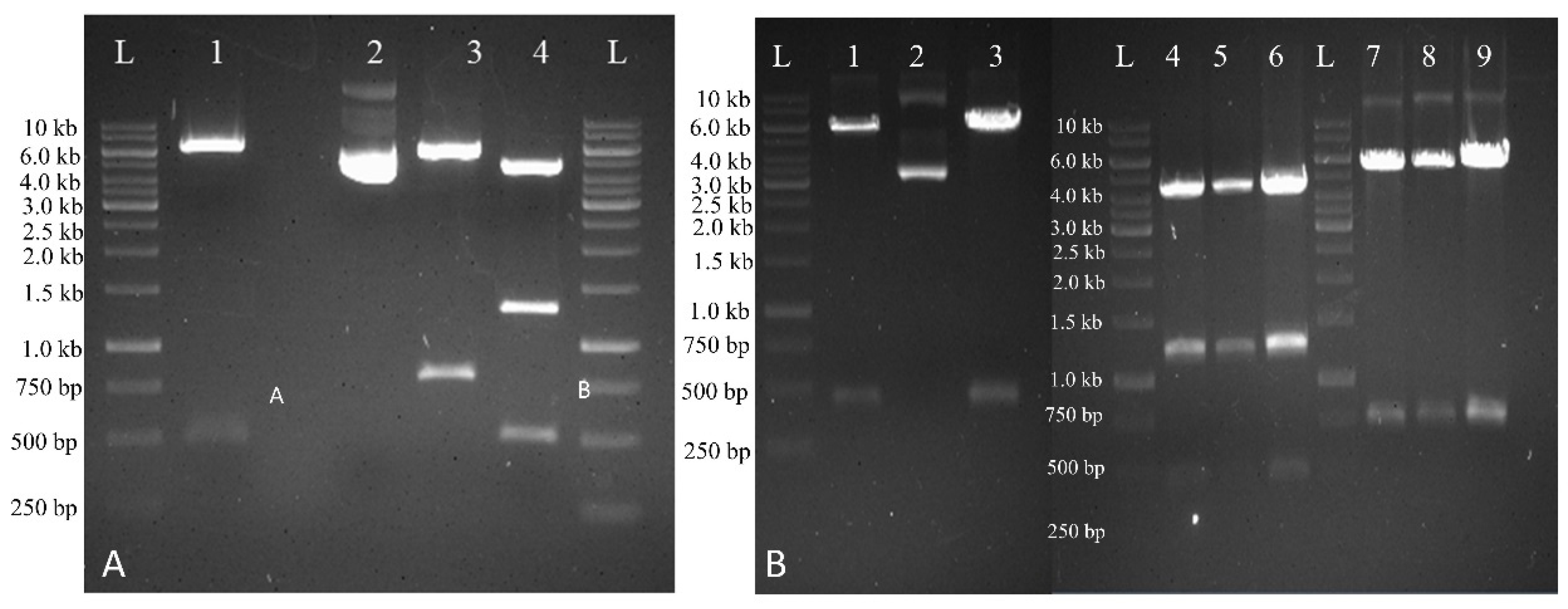
2.4. Tracking Transgene Integration through Ploymerase Chain Reaction (PCR)
2.5. Tracking Transgene Expression by RT-qPCR
2.6. Fungal Bioassay to Assess Anti-Pathogenic Activity of Putative Transgenic Plants
3. Discussion
4. Materials and Methods
4.1. Callus Induction, Regeneration and Rooting
4.2. Development of Plant Expression Vectors
4.3. Genetic Transformation of Sugarcane
4.4. Confirmation of Transgenic Plants by PCR Analysis
4.5. Transcriptomic Quantitative Analysis of Transgenic Sugarcane Plants Using qPCR
4.6. Fungal Bioassays
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tariq, M.; Khan, A.; Tabassum, B.; Toufiq, N.; BHATTI, M.; Riaz, S.; NASIR, I.; Husnain, T. Antifungal activity of chitinase II against Colletotrichum falcatum Went. causing red rot disease in transgenic sugarcane. Turk. J. Biol. 2018, 42, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Awais, M.; Raza, W.; Zia, A. Identification of sugarcane lines with resistance to red rot. Pak. J. Phytopathol. 2011, 23, 98–102. [Google Scholar]
- Hussnain, Z.; Afghan, S. Impact of major cane diseases on sugarcane yield and sugar recovery. Annu. Rep. Shakarganj Sugar Res. Inst. Jhang 2006, 78–80. [Google Scholar]
- Viswanathan, R.; Samiyappan, R. Induced systemic resistance by fluorescent pseudomonads against red rot disease of sugarcane caused by Colletotrichum falcatum. Crop Prot. 2002, 21, 1–10. [Google Scholar] [CrossRef]
- Viswanathan, R. Molecular basis of red rot resistance in sugarcane. Funct. Plant Sci. Biotechnol. 2012, 6, 40–50. [Google Scholar]
- Ruchika, S.; Sushma, T. A review on red rot: The “Cancer” of sugarcane. J. Plant Pathol. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Malathi, P.; Viswanathan, R. Role of microbial chitinase in the biocontrol of sugarcane red rot caused by Colletotrichum falcatum Went. Electron. J. Plant Breed. 2013, 6, 17–23. [Google Scholar]
- Mohanraj, D.; Padmanaban, P.; Karunakaran, M. Effect of phytotoxin of Colletotrichum falcatum Went.(Physalospora tucumanensis) on sugarcane in tissue culture. Acta Phytopathol. Entomol. Hung. 2003, 38, 21–28. [Google Scholar] [CrossRef]
- Sengar, A.; Thind, K.; Kumar, B.; Pallavi, M.; Gosal, S. In vitro selection at cellular level for red rot resistance in sugarcane (Saccharum sp.). Plant Growth Regul. 2009, 58, 201–209. [Google Scholar] [CrossRef]
- Agnihotri, V. Current sugarcane disease scenario and management strategies. Indian Phytopathol. 1996, 49, 109–126. [Google Scholar]
- Enríquez-Obregón, G.A.; Vázquez-Padrón, R.I.; Prieto-Samsonov, D.L.; Gustavo, A.; Selman-Housein, G. Herbicide-resistant sugarcane (Saccharum officinarum L.) plants by Agrobacterium-mediated transformation. Planta 1998, 206, 20–27. [Google Scholar] [CrossRef]
- Jach, G.; Görnhardt, B.; Mundy, J.; Logemann, J.; Pinsdorf, E.; Leah, R.; Schell, J.; Maas, C. Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco. Plant J. 1995, 8, 97–109. [Google Scholar] [CrossRef]
- Medeiros, A.H.; Franco, F.P.; Matos, J.L.; de Castro, P.A.; Santos-Silva, L.K.; Henrique-Silva, F.; Goldman, G.H.; Moura, D.S.; Silva-Filho, M.C. Sugarwin: A sugarcane insect-induced gene with antipathogenic activity. Mol. Plant-Microbe Interact. 2012, 25, 613–624. [Google Scholar] [CrossRef]
- Broekaert, I.; Lee, H.-i.; Kush, A.; Chua, N.-H.; Raikhel, N. Wound-induced accumulation of mRNA containing a hevein sequence in laticifers of rubber tree (Hevea brasiliensis). Proc. Natl. Acad. Sci. USA 1990, 87, 7633–7637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrich, L.; Moyer, M.; Ward, E.; Ryals, J. Pathogenesis-related protein 4 is structurally homologous to the carboxy-terminal domains of hevein, Win-1 and Win-2. Mol. Gen. Genet. Mgg 1991, 230, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Franco, F.P.; Santiago, A.C.; Henrique-Silva, F.; de Castro, P.A.; Goldman, G.H.; Moura, D.S.; Silva-Filho, M.C. The sugarcane defense protein SUGARWIN2 causes cell death in Colletotrichum falcatum but not in non-pathogenic fungi. PLoS ONE 2014, 9, e91159. [Google Scholar] [CrossRef]
- Mustafa, G.; Khan, M.S. Transmission of Engineered Plastids in Sugarcane, a C4 Monocotyledonous Plant, Reveals that Sorting of Preprogrammed Progenitor Cells Produce Heteroplasmy. Plants 2021, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Maliga, P. Fluorescent antibiotic resistance marker for tracking plastid transformation in higher plants. Nat. Biotechnol. 1999, 17, 910–915. [Google Scholar] [CrossRef]
- Bower, R.; Birch, R.G. Transgenic sugarcane plants via microprojectile bombardment. Plant J. 1992, 2, 409–416. [Google Scholar] [CrossRef]
- Mustafa, G.; Khan, M.S. Prospecting the utility of antibiotics as lethal selection agents for chloroplast transformation in sugarcane. Int. J. Agric. Biol. 2012, 14, 307–310. [Google Scholar]
- Mustafa, G.; Khan, M.S. Reproducible in vitro regeneration system for purifying sugarcane clones. Afr. J. Biotechnol. 2012, 11, 9961–9969. [Google Scholar]
- Kim, G.T.; Shoda, K.; Tsuge, T.; Cho, K.H.; Uchimiya, H.; Yokoyama, R.; Nishitani, K.; Tsukaya, H. The ANGUSTIFOLIA gene of Arabidopsis, a plant CtBP gene, regulates leaf-cell expansion, the arrangement of cortical microtubules in leaf cells and expression of a gene involved in cell-wall formation. EMBO J. 2002, 21, 1267–1279. [Google Scholar] [CrossRef] [Green Version]
- Vickers, J.; Grof, C.; Bonnett, G.; Jackson, P.; Morgan, T. Effects of tissue culture, biolistic transformation, and introduction of PPO and SPS gene constructs on performance of sugarcane clones in the field. Aust. J. Agric. Res. 2005, 56, 57–68. [Google Scholar] [CrossRef]
- Nayyar, S.; Sharma, B.K.; Kaur, A.; Kalia, A.; Sanghera, G.S.; Thind, K.S.; Yadav, I.S.; Sandhu, J.S. Red rot resistant transgenic sugarcane developed through expression of β-1, 3-glucanase gene. PLoS ONE 2017, 12, e0179723. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, G.; Joyia, F.A.; Anwar, S.; Parvaiz, A.; Khan, M.S. Biotechnological interventions for the improvement of sugarcane crop and sugar production. Sugarcane-Technol. Res. Intechopen Lond. UK 2018, 113–138. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Estrella, L.R. Genetically modified crops and developing countries. Plant Physiol. 2000, 124, 923–926. [Google Scholar] [CrossRef] [Green Version]
- Bilang, R.; Fütterer, J.; Sautter, C. Transformation of cereals. In Genetic Engineering; Springer: Berlin/Heidelberg, Germany, 1999; pp. 113–157. [Google Scholar]
- McElroy, D.; Brettell, R.I. Foreign gene expression in transgenic cereals. Trends Biotechnol. 1994, 12, 62–68. [Google Scholar] [CrossRef]
- Christensen, A.H.; Sharrock, R.A.; Quail, P.H. Maize polyubiquitin genes: Structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol. Biol. 1992, 18, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wu, J.; Yan, B.; Hao, Q.; Zhang, C.; Lyu, B.; Ni, F.; Caplan, A.; Wu, J.; Fu, D. Remapping of the stripe rust resistance gene Yr10 in common wheat. Theor. Appl. Genet. 2018, 131, 1253–1262. [Google Scholar] [CrossRef]
- Stephenson, E.; Estrada, S.; Meng, X.; Ourada, J.; Muszynski, M.G.; Habben, J.E.; Danilevskaya, O.N. Over-expression of the photoperiod response regulator ZmCCT10 modifies plant architecture, flowering time and inflorescence morphology in maize. PLoS ONE 2019, 14, e0203728. [Google Scholar] [CrossRef] [Green Version]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Phan, T.-T.; Li, Y.-R.; Xing, Y.-X.; Yang, L.-T. Isolation, transformation and overexpression of sugarcane SoP5CS gene for drought tolerance improvement. Sugar Tech 2018, 20, 464–473. [Google Scholar] [CrossRef]
- Liu, G.; Godwin, I.D. Highly efficient sorghum transformation. Plant Cell Rep. 2012, 31, 999–1007. [Google Scholar] [CrossRef] [Green Version]
- Gallo-Meagher, M.; Irvine, J. Herbicide resistant transgenic sugarcane plants containing the bar gene. Crop Sci. 1996, 36, 1367–1374. [Google Scholar] [CrossRef]
- Steib, R.; Chilton, S. Infection of sugar cane stalks by the red rot fungus, physalospora tucumanensis speg. Phytopathology 1951, 41, 522–528. [Google Scholar]
- Srinivasan, K.; Alexander, K. A study of mode of nodal infection and occurrence of dormant infections of red rot. In Proceedings of the All India Conference on Sugarcane Research and Development Workers; Indian Council of Agricultural Research: New Dehli, India, 1966; pp. 676–684. [Google Scholar]
- De Silva, G.; Silva, M.; Carvalho, P. Preliminary studies on invertases of sugarcane stalks inoculated with C. falcatum Went. In Proceedings of the International Society of Sugar Cane Technologists, Sao Paulo, Brazil, 9–25 September 1977; pp. 407–414. [Google Scholar]
- Dickman, M.B.; de Figueiredo, P. Death be not proud—cell death control in plant fungal interactions. PLoS Pathog. 2013, 9, e1003542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant cell wall–degrading enzymes and their secretion in plant-pathogenic fungi. Annu. Rev. Phytopathol. 2014, 52, 427–451. [Google Scholar] [CrossRef]
- Spychalla, J.P.; Bevan, M.W. Agrobacterium-mediated transformation of potato stem and tuber tissue, regeneration and PCR screening for transformation. In Plant Tissue Culture Manual; Springer: Berlin/Heidelberg, Germany, 1993; pp. 379–396. [Google Scholar]
- Begcy, K.; Mariano, E.D.; Gentile, A.; Lembke, C.G.; Zingaretti, S.M.; Souza, G.M.; Menossi, M. A novel stress-induced sugarcane gene confers tolerance to drought, salt and oxidative stress in transgenic tobacco plants. PLoS ONE 2012, 7, e44697. [Google Scholar] [CrossRef] [Green Version]
- Parvaiz, A.; Mustafa, G.; Khan, H.M.W.A.; Joyia, F.A.; Niazi, A.K.; Anwar, S.; Khan, M.S. Field evaluation ratified by transcript and computational analyses unveils myco-protective role of SUGARWIN proteins in sugarcane. 3 Biotech 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Parvaiz, A.; Mustafa, G.; Khan, M.S.; Ali, M.A. Characterization and Expression Analysis of Resistance Gene Analogues in Elite Sugarcane Genotypes. Protein Pept. Lett. 2021. [Google Scholar] [CrossRef] [PubMed]



 p = 0.01.
p = 0.01.
 p = 0.01.
p = 0.01.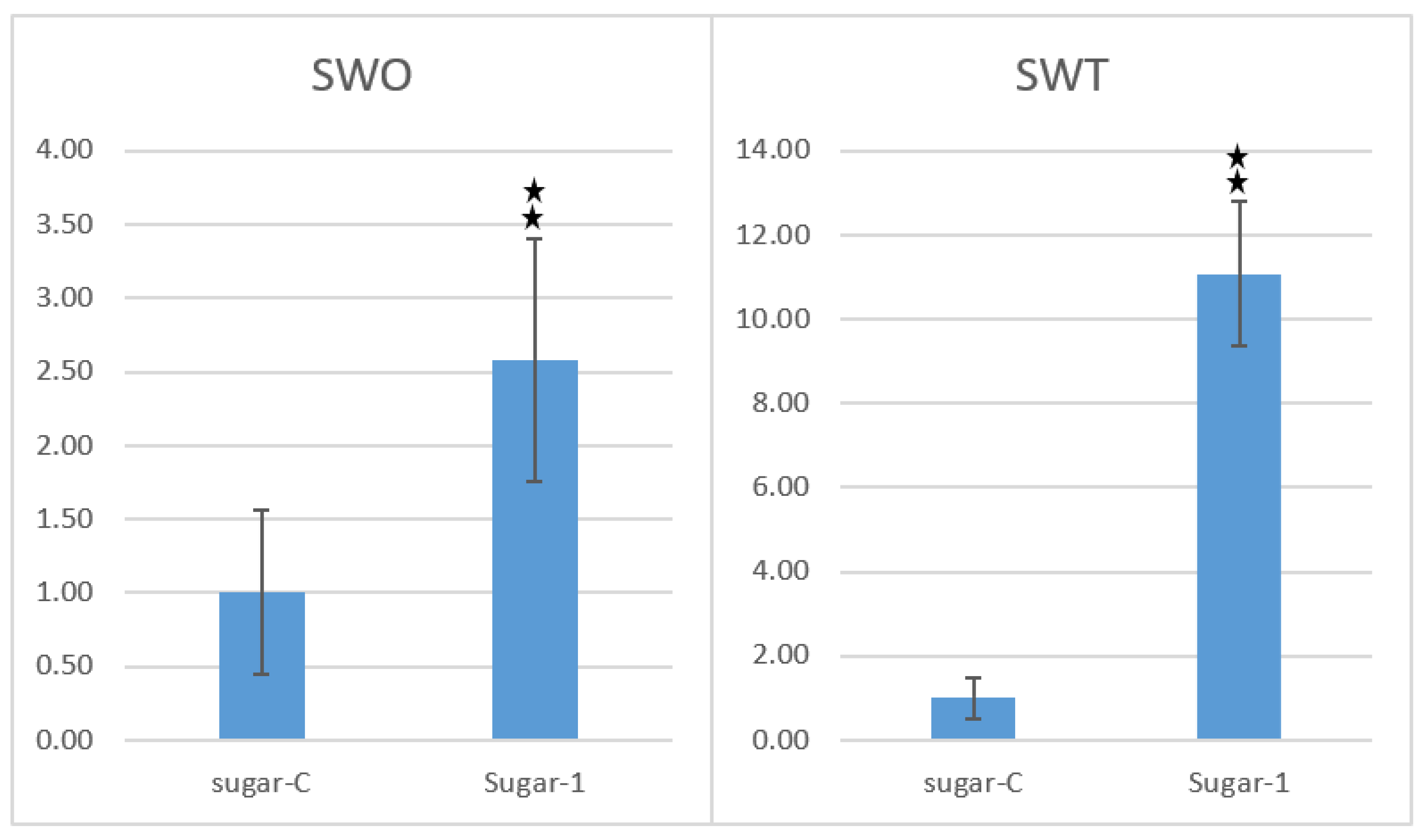

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvaiz, A.; Mustafa, G.; Khan, M.S.; Ali, M.A. Over-Expression of Endogenous SUGARWIN Genes Exalted Tolerance against Colletotrichum Infection in Sugarcane. Plants 2021, 10, 869. https://doi.org/10.3390/plants10050869
Parvaiz A, Mustafa G, Khan MS, Ali MA. Over-Expression of Endogenous SUGARWIN Genes Exalted Tolerance against Colletotrichum Infection in Sugarcane. Plants. 2021; 10(5):869. https://doi.org/10.3390/plants10050869
Chicago/Turabian StyleParvaiz, Aqsa, Ghulam Mustafa, Muhammad Sarwar Khan, and Muhammad Amjad Ali. 2021. "Over-Expression of Endogenous SUGARWIN Genes Exalted Tolerance against Colletotrichum Infection in Sugarcane" Plants 10, no. 5: 869. https://doi.org/10.3390/plants10050869







