Role of Tuber Developmental Processes in Response of Potato to High Temperature and Elevated CO2
Abstract
:1. Introduction
2. Results
2.1. The Effects of High Temperature and Elevated CO2 on Potato Plant Growth
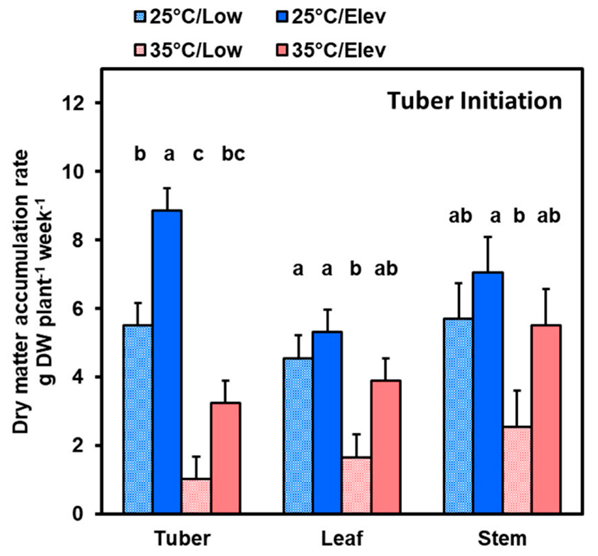
2.1.1. Tuber Initiation Stage
2.1.2. Tuber Bulking Stage
2.2. Tuber Numbers and Size
2.3. The Effects of High Temperature and Elevated CO2 on Potato Tuber Cell Properties
2.4. The Effects of High Temperature and Elevated CO2 on Sugar Levels and Invertases
2.4.1. Sugars
2.4.2. Invertases
3. Discussion
3.1. Elevated Atmospheric CO2 Effect on Whole Plant Biomass Accumulation
3.2. High Temperature Stress Effect on Whole Plant Biomass Accumulation
3.3. Tuber Growth and Development
3.3.1. High Temperature Effects on Components of Tuber Development
3.3.2. Elevated CO2 Effects on Components of Tuber Development
3.3.3. Sugar Signaling in Tuber Development
3.4. Global Climate Change
4. Materials and Methods
4.1. Plant Material
4.2. Temperature and CO2 Control
4.3. Tuber Sampling Method
4.4. Assay Methods
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty. Masson-Delmotte, V., Zhai, P., Pörtner, H.O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; 2018. Available online: https://www.ipcc.ch/site/assets/uploads/sites/2/2019/06/SR15_Full_Report_Low_Res.pdf (accessed on 4 April 2021).
- King, A.W.; Emanuel, W.R.; Post, W.M. Projecting future concentrations of atmospheric carbon dioxide with global carbon cycle models the importance of simulating historical changes. Environ. Manag. 1992, 16, 91–108. [Google Scholar] [CrossRef]
- Jarvis, A.; Ramirez-Villegas, J.; Herrera Campo, B.; Navarro-Racines, C. Is cassava the answer to African climate change adaptation? Trop. Plant Biol. 2012, 5, 9–29. [Google Scholar] [CrossRef]
- Raymundo, R.; Asseng, S.; Prassad, R.; Kleinwechter, U.; Concha, J.; Condori, B.; Bowen, W.; Wolf, J.; Olesen, J.E.; Dong, Q.; et al. Performance of the SUBSTOR-potato model across contrasting growing conditions. Field Crop. Res. 2017, 202, 57–76. [Google Scholar] [CrossRef] [Green Version]
- Fleisher, D.H.; Condori, B.; Quiroz, R.; Alva, A.; Asseng, S.; Barreda, C.; Bindi, M.; Boote, K.J.; Ferrise, R.; Franke, A.C.; et al. A potato model intercomparison across varying climates and productivity levels. Glob. Chang. Biol. 2017, 23, 1258–1281. [Google Scholar] [CrossRef]
- Sun, Q.; Miao, C.; Hanel, M.; Borthwick, A.G.L.; Duan, Q.; Ji, D.; Li, H. Global heat stress on health, wildfires, and agricultural crops under different levels of climate warming. Environ. Int. 2019, 128, 125–136. [Google Scholar] [CrossRef]
- Engineer, C.B.; Hashimoto-Sugimoto, M.; Negi, J.; Israelsson-Nordström, M.; Azoulay-Shemer, T.; Rappel, W.-J.; Iba, K.; Schroeder, J.I. CO2 Sensing and CO2 Regulation of Stomatal Conductance: Advances and Open Questions. Trends Plant Sci. 2016, 21, 16–30. [Google Scholar] [CrossRef] [Green Version]
- Drake, B.G.; Gonzalez-Meler, M.A.; Long, S.P. More efficient plants: A consequence of rising atmospheric CO2? Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 609–639. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Timlin, D.; Rahman, S.M.L.; Baker, J.; Reddy, V.R.; Fleisher, D.; Quebedeaux, B. Whole plant photosynthesis, development, and carbon partitioning in potato as a function of temperature. Agron. J. 2006, 98, 1195–1203. [Google Scholar] [CrossRef] [Green Version]
- Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef]
- Tausz, M.; Tausz-Posch, S.; Norton, R.M.; Fitzgerald, G.J.; Nicolas, M.E.; Seneweera, S. Understanding crop physiology to select breeding targets and improve crop management under increasing atmospheric CO2 concentrations. Environ. Exp. Bot. 2013, 88, 71–80. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Leakey, A.D.B.; Nosberger, J.; Ort, D.R. Food for Thought: Lower-Than-Expected Crop Yield Stimulation with Rising CO2 Concentrations. Science 2006, 312, 1918–1921. [Google Scholar] [CrossRef]
- Miglietta, F.; Magliulo, V.; Bindi, M.; Cerio, L.; Vaccari, F.P.; Loduca, V.; Peressotti, A. Free Air CO2 Enrichment of potato (Solanum tuberosum L.): Development, growth and yield. Glob. Chang. Biol. 1998, 4, 163–172. [Google Scholar] [CrossRef]
- Högy, P.; Fangmeier, A. Atmospheric CO2 enrichment affects potatoes: 1. Aboveground biomass production and tuber yield. Eur. J. Agron. 2009, 30, 78–84. [Google Scholar] [CrossRef]
- Manrique, L.A.; Bartholomew, D.P. Growth and Yield Performance of Potato Grown at Three Elevations in Hawaii: II. Dry Matter Production and Efficiency of Partitioning. Crop Sci. 1991, 31, 367–372. [Google Scholar] [CrossRef]
- Sage, R.F.; Way, D.A.; Kubien, D.S. Rubisco, Rubisco activase, and global climate change. J. Exp. Bot. 2008, 59, 1581–1595. [Google Scholar] [CrossRef] [Green Version]
- Struik, P.C.; Geertsema, J.; Custers, C.-H.M. Effects of shoot; root and stolon temperature on the development of the potato (Solanum tuberosum L.) plant. III. Development of tubers. Potato Res. 1989, 32, 151–158. [Google Scholar] [CrossRef]
- Basu, P.S.; Minhas, J.S. Heat tolerance and assimilate transport in different potato genotypes. J. Exp. Bot. 1991, 42, 861–866. [Google Scholar] [CrossRef]
- Kaminski, K.P.; Korup, K.; Nielsen, K.L.; Liu, F.; Topbjerg, H.B.; Kirk, H.G.; Andersen, M.N. Gas-exchange, water use efficiency and yield responses of elite potato (Solanum tuberosum L.) cultivars to changes in atmospheric carbon dioxide concentration, temperature and relative humidity. Agric. For. Meteorol. 2014, 187, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Kukreja, S.; Goutam, U. Impact of heat stress on potato (Solanum tuberosum L.): Present scenario and future opportunities. J. Hortic. Sci 2020, 95, 407–424. [Google Scholar] [CrossRef]
- Ewing, E.E. Heat stress and the tuberization stimulus. Am. Potato J. 1981, 58, 31–49. [Google Scholar] [CrossRef]
- Mohabir, G.; John, P. Effect of temperature on starch synthesis in potato tuber tissue and in amyloplasts. Plant Physiol. 1989, 88, 1222–1228. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, M.P.; Ewing, E.E. Effects of high air and soil temperature stress on growth and tuberization in Solanum tuberosum. Ann. Bot. 1989, 64, 241–248. [Google Scholar] [CrossRef]
- Hastilestari, B.R.; Lorenz, J.; Reid, S.; Hofmann, J.; Pscheidt, D.; Sonnewald, U.; Sonnewald, S. Deciphering source and sink responses of potato plants (Solanum tuberosum L.) to elevated temperatures. Plant Cell Environ. 2018, 41, 2600–2616. [Google Scholar] [CrossRef]
- Rykaczewska, K. The Effect of High Temperature Occurring in Subsequent Stages of Plant Development on Potato Yield and Tuber Physiological Defects. Am. J. Potato Res. 2015, 92, 339–349. [Google Scholar] [CrossRef] [Green Version]
- Poorter, H. Interspecific variation in the growth response of plants to an elevated ambient CO2 concentration. Vegetatio 1993, 104, 77–97. [Google Scholar] [CrossRef]
- Cure, J.D.; Acock, B. Crop responses to carbon dioxide doubling: A literature survey. Agric. For. Meteorol. 1986, 38, 127–145. [Google Scholar] [CrossRef]
- Bryant, J.; Taylor, G.; Frehner, M. Photosynthetic acclimation to elevated CO2 is modified by source: Sink balance in three component species of chalk grassland swards grown in a free air carbon dioxide enrichment (FACE) experiment. Plant Cell Environ. 1998, 21, 159–168. [Google Scholar] [CrossRef]
- Chen, C.-T.; Setter, T.L. Response of potato dry matter assimilation and partitioning to elevated CO2 at various stages of tuber initiation and growth. Environ. Exp. Bot. 2012, 80, 27–34. [Google Scholar] [CrossRef]
- Midmore, D.J.; Prange, R.K. Growth responses of two Solanum species to contrasting temperatures and irradiance levels relations to photosynthesis dark respiration and chlorophyll fluorescence. Ann. Bot. 1992, 69, 13–20. [Google Scholar] [CrossRef]
- Kim, Y.-U.; Lee, B.-W. Differential Mechanisms of Potato Yield Loss Induced by High Day and Night Temperatures During Tuber Initiation and Bulking: Photosynthesis and Tuber Growth. Front. Plant Sci. 2019, 10, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-H.; Sang, W.-G.; Baek, J.-K.; Kim, J.-H.; Shin, P.; Seo, M.-C.; Cho, J.-I. The effect of concurrent elevation in CO2 and temperature on the growth, photosynthesis, and yield of potato crops. PLoS ONE 2020, 15, e0241081. [Google Scholar] [CrossRef] [PubMed]
- Obiero, C.O.; Milroy, S.P.; Bell, R.W. Importance of whole plant dry matter dynamics for potato (Solanum tuberosum L.) tuber yield response to an episode of high temperature. Environ. Exp. Bot. 2019, 162, 560–571. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Hall, A.E.; Madore, M.A. Interactive effects of high temperature and elevated carbon dioxide concentration on cowpea (Vigna unguiculata L. Walp.). Plant Cell Environ. 1993, 16, 835–842. [Google Scholar] [CrossRef]
- Wayne, P.M.; Reekie, E.G.; Bazzaz, F.A. Elevated CO2 ameliorates birch response to high temperature and frost stress: Implications for modeling climate-induced geographic range shifts. Oecologia 1998, 114, 335–342. [Google Scholar] [CrossRef]
- Xu, X.; Vreugdenhil, D.; Lammeren, A.A.M.V. Cell division and cell enlargement during potato tuber formation. J. Exp. Bot. 1998, 49, 573–582. [Google Scholar] [CrossRef]
- Manrique, L.A.; Bartholomew, D.P.; Ewing, E.E. Growth and yield performance of several potato clones grown at three elevations hawaii usa i. Plant morphology. Crop Sci. 1989, 29, 363–370. [Google Scholar] [CrossRef]
- Nooruddin, A.; Mehta, A.N.; Patel, H.R. Tuber production in relation to weather parameters and agrometeorological indices prevailing during different phenological stages of potato crop. J. Indian Potato Assoc. 1995, 22, 22–117. [Google Scholar]
- Chen, C.-T.; Setter, T.L. Response of potato tuber cell division and growth to shade and elevated CO2. Ann. Bot. 2003, 91, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Dutt, S.; Manjul, A.S.; Raigond, P.; Singh, B.; Siddappa, S.; Bhardwaj, V.; Kawar, P.G.; Patil, V.U.; Kardile, H.B. Key players associated with tuberization in potato: Potential candidates for genetic engineering. Crit. Rev. Biotechnol. 2017, 37, 942–957. [Google Scholar] [CrossRef]
- Jackson, S.D. Multiple signaling pathways control tuber induction in potato. Plant Physiol. 1999, 119, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Van Dam, J.; Kooman, P.L.; Struik, P.C. Effects of temperature and photoperiod on early growth and final number of tubers in potato (Solanum tuberosum L.). Potato Res. 1996, 39, 51–62. [Google Scholar] [CrossRef]
- Wallace, D.; Yan, W. A model of photoperiod x temperature interaction effects on plant development. In Plant Breeding and Whole-System Crop Physiology: Improving Adaptation, Maturity and Yield; CAB International: New York, NY, USA, 1998; pp. 78–98. [Google Scholar]
- Kloosterman, B.; De Koeyer, D.; Griffiths, R.; Flinn, B.; Steuernagel, B.; Scholz, U.; Sonnewald, S.; Sonnewald, U.; Bryan, G.J.; Prat, S.; et al. Genes driving potato tuber initiation and growth: Identification based on transcriptional changes using the POCI array. Funct. Integr. Genom. 2008, 8, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.; Cho, L.-H.; Tun, W.; Jeon, J.-S.; An, G. Sucrose signaling in higher plants. Plant Sci. 2021, 302, 110703. [Google Scholar] [CrossRef]
- Debast, S.; Nunes-Nesi, A.; Hajirezaei, M.R.; Hofmann, J.; Sonnewald, U.; Fernie, A.R.; Börnke, F. Altering Trehalose-6-Phosphate Content in Transgenic Potato Tubers Affects Tuber Growth and Alters Responsiveness to Hormones during Sprouting. Plant Physiol. 2011, 156, 1754–1771. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S.J.; Sonnewald, U. The mode of sucrose degradation in potato tubers determines the fate of assimilate utilisation. Front. Plant Sci. 2012, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Ruan, Y.-L. Regulation of cell division and expansion by sugar and auxin signaling. Front. Plant Sci. 2013, 4, 163. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Sheen, J. Dynamic and diverse sugar signaling. Curr. Opin. Plant Biol. 2016, 33, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.; Wang, L.; Li, J.; Ruan, Y.-L. Cell Wall Invertase Is Essential for Ovule Development through Sugar Signaling Rather Than Provision of Carbon Nutrients. Plant Physiol. 2020, 183, 1126–1144. [Google Scholar] [CrossRef] [Green Version]
- Scholes, D.R.; Paige, K.N. Plasticity in ploidy: A generalized response to stress. Trends Plant Sci. 2015, 20, 165–175. [Google Scholar] [CrossRef]
- Bhargava, S.; Mitra, S. Elevated atmospheric CO2 and the future of crop plants. Plant Breed. 2021, 140, 1–11. [Google Scholar] [CrossRef]
- Cairns, A.J. Colorimetric microtiter plate assay of glucose and fructose by enzyme-linked formazan production: Applicability to the measurement of fructosyl transferase activity in higher plants. Anal. Biochem. 1987, 167, 270–278. [Google Scholar] [CrossRef]
- R_Core_Team. R: A Language and Environment for Statistical Computing. 2017. Available online: https://www.R-project.org/ (accessed on 22 March 2021).






| Stage of Development | |||||
|---|---|---|---|---|---|
| Treatment | When Treatment Was Imposed | ||||
| temperature | atm CO2 | Tuber Initiation | Tuber Bulking | ||
| dry-matter accumulation rate | |||||
| °C | [CO2] | g DW plant−1 wk−1 | |||
| 25 | Elev | 24.3 | c | 37.7 | c |
| 25 | Low | 18.9 | cb | 28.0 | b |
| 35 | Elev | 15.8 | ba | 31.9 | cb |
| 35 | Low | 8.3 | a | 19.0 | a |
| SE | 1.6 | 1.8 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-T.; Setter, T.L. Role of Tuber Developmental Processes in Response of Potato to High Temperature and Elevated CO2. Plants 2021, 10, 871. https://doi.org/10.3390/plants10050871
Chen C-T, Setter TL. Role of Tuber Developmental Processes in Response of Potato to High Temperature and Elevated CO2. Plants. 2021; 10(5):871. https://doi.org/10.3390/plants10050871
Chicago/Turabian StyleChen, Chien-Teh, and Tim L. Setter. 2021. "Role of Tuber Developmental Processes in Response of Potato to High Temperature and Elevated CO2" Plants 10, no. 5: 871. https://doi.org/10.3390/plants10050871







