Genome-Wide Identification and Expression Analyses of AnSnRK2 Gene Family under Osmotic Stress in Ammopiptanthus nanus
Abstract
:1. Introduction
2. Results
2.1. The SnRK2 Genes in A. nanus
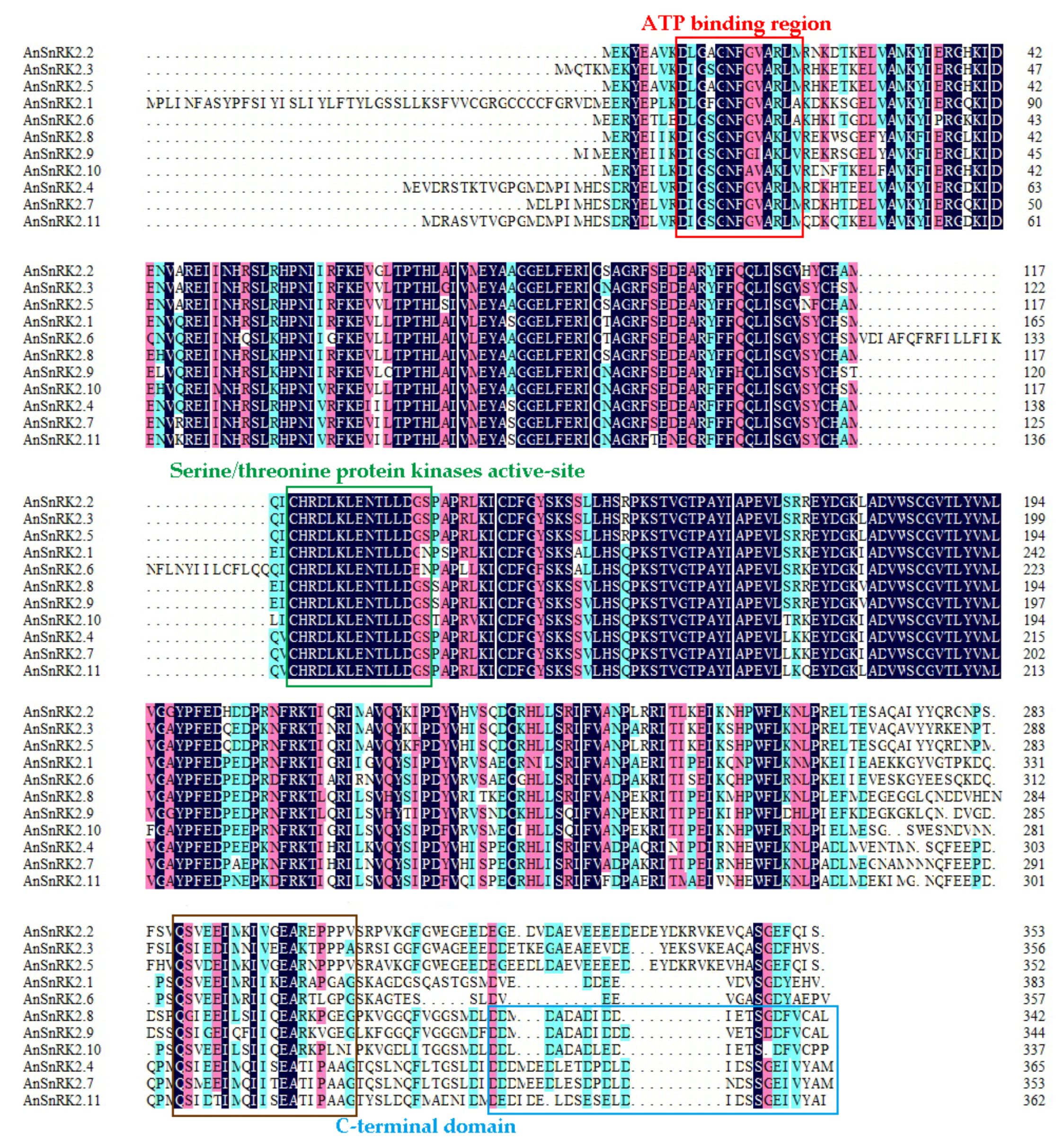
2.2. Multiple Alignment and Phylogenetic Analysis of AnSnRK2s
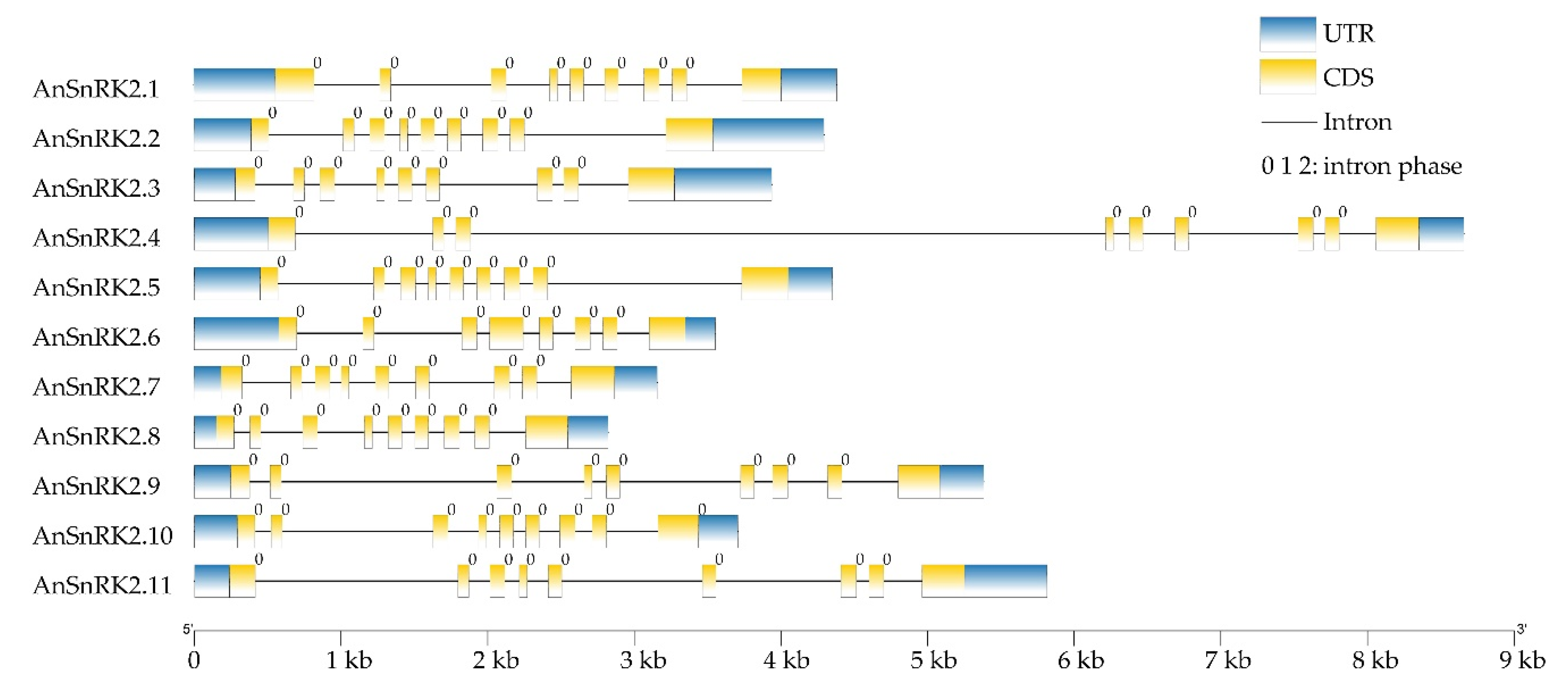
2.3. Gene Structure and Motifs of AnSnRK2s
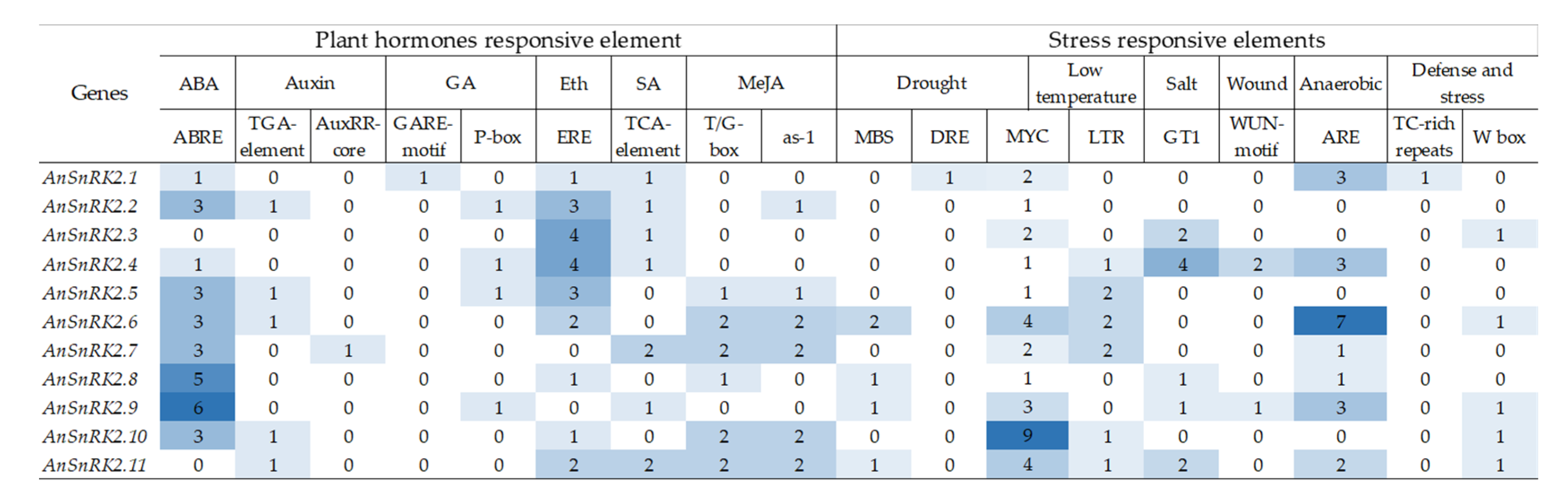
2.4. The cis-Elements in the Promoter of AnSnRK2s
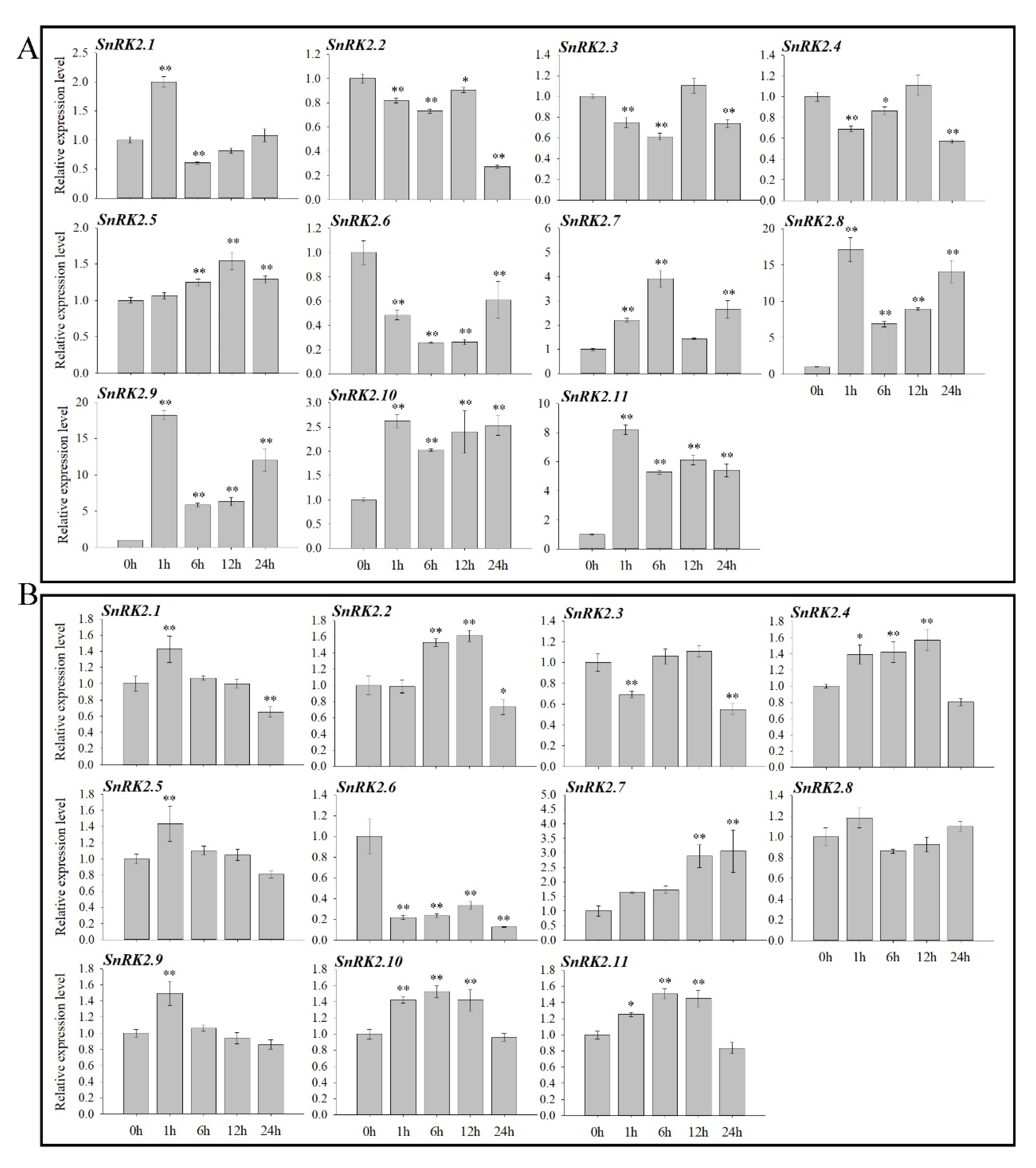
2.5. Expression of AnSnRK2 Genes under Drought and Salinity Stress
3. Discussion
4. Materials and Methods
4.1. Identification of AnSnRK2 Genes in A. nanus
4.2. Multiple Alignment and Phylogenetic Analysis
4.3. Gene Structure and Analysis of cis-Acting Elements
4.4. Plant Materials and Treatments
4.5. qRT-PCR Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, L.; Hu, X.; Song, J.; Zong, X.; Li, D.; Li, D. Over-expression of a Zea mays L. protein phosphatase 2c gene (ZmPP2C) in arabidopsis thaliana decreases tolerance to salt and drought. J. Plant Physiol. 2009, 166, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Tougane, K.; Komatsu, K.; Bhyan, S.B.; Sakata, Y.; Ishizaki, K.; Yamato, K.T.; Kohchi, T.; Takezawa, D. Evolutionarily conserved regulatory mechanisms of abscisic acid signaling in land plants: Characterization of abscisic acid insensitive1-like type 2C protein phosphatase in the liverwort marchantia polymorpha. Plant Physiol. 2010, 152, 1529–1543. [Google Scholar] [CrossRef] [Green Version]
- Nie, L.L.; Feng, J.J.; Fan, P.X.; Chen, X.Y.; Guo, J.; Lv, S.L.; Bao, H.; Jia, W.T.; Tai, F.; Jiang, P.; et al. Comparative proteomics of root plasma membrane proteins reveals the involvement of calcium signalling in nacl-facilitated nitrate uptake in salicornia europaea. J. Exp. Bot. 2015, 66, 4497–4510. [Google Scholar] [CrossRef] [Green Version]
- Umezawa, T.; Sugiyama, N.; Takahashi, F.; Anderson, J.C.; Ishihama, Y.; Peck, S.C.; Shinozaki, K. Genetics and phosphoproteomics reveal a protein phosphorylation network in the abscisic acid signaling pathway in arabidopsis thaliana. Sci. Signal. 2013, 6, rs8. [Google Scholar] [CrossRef] [PubMed]
- Teige, M.; Scheikl, E.; Eulgem, T.; Dóczi, R.; Ichimura, K.; Shinozaki, K.; Dangl, J.L.; Hirt, H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol. Cell 2004, 15, 141–152. [Google Scholar] [CrossRef]
- Kong, X.; Pan, J.; Zhang, D.; Jiang, S.; Cai, G.; Wang, L.; Li, D. Identification of mitogen-activated protein kinase kinase gene family and MKK–MAPK interaction network in maize. Biochem. Bioph. Res. Co. 2013, 441, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Hadiarto, T.; Nanmori, T.; Matsuoka, D.; Iwasaki, T.; Sato, K.; Fukami, Y.; Azuma, T.; Yasuda, T. Activation of Arabidopsis MAPK kinase kinase (AtMEKK1) and induction of AtMEKK1–AtMEK1 pathway by wounding. Planta 2006, 223, 708–713. [Google Scholar] [CrossRef]
- Boudsocq, M.; Sheen, J. CDPKs in immune and stress signaling. Trends Plant Sci. 2013, 18, 30–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.; Li, Y.; Fan, R.C.; Shang, Y.; Du, S.Y.; Wang, X.F.; Wu, F.Q.; Xu, Y.H.; Zhang, X.Y.; Zhang, D.P. The Arabidopsis Ca2+-dependent protein kinase CPK12 negatively regulates abscisic acid signaling in seed germination and post-germination growth. New Phytol. 2011, 192, 61–73. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Yu, X.C.; Wang, X.J.; Rui, Z.; Zhang, D.P. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell 2007, 19, 3019–3036. [Google Scholar] [CrossRef] [Green Version]
- Boudsocq, M.; Laurière, C. Osmotic signaling in plants: Multiple pathways mediated by emerging kinase families. Plant Physiol. 2005, 138, 1185–1194. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Zhang, H.; Tian, S.; Chang, X.; Jing, R. TaSnRK2.4, an SNF1-type serine/threonine protein kinase of wheat (Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis. J. Exp. Bot. 2010, 61, 683–696. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, K.; Fujita, Y.; Kanamori, N.; Katagiri, T.; Umezawa, T.; Kidokoro, S.; Maruyama, K.; Yoshida, T.; Ishiyama, K.; Kobayashi, M.; et al. Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2. 2, SRK2E/SnRK2. 6/OST1 and SRK2I/SnRK2. 3, involved in ABA signaling are essential for the control of seed development and dormancy. Plant. Cell Physiol. 2009, 50, 1345–1363. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Zhang, X.; Nocker, S.V.; Gong, X.Q.; Ma, F.W. Overexpression of a protein kinase gene MpSnRK2. 10 from Malus prunifolia confers tolerance to drought stress in transgenic Arabidopsis thaliana and apple. Gene 2019, 692, 26–34. [Google Scholar] [CrossRef]
- Wang, P.; Xue, L.; Batelli, G.; Lee, S.; Hou, Y.J.; Van Oosten, M.J.; Zhang, H.; Tao, W.A.; Zhu, J.K. Quantitative phosphoproteomics identifies SnRK2 protein kinase substrates and reveals the effectors of abscisic acid action. Proc. Natl. Acad. Sci. USA 2013, 110, 11205–11210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrabak, E.M.; Chan, C.W.M.; Gribskov, M.; Harper, J.F.; Choi, J.H.; Halford, N.; Kudla, J.; Luan, S.; Nimmo, H.G.; Sussman, M.R.; et al. The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 2003, 132, 666–680. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, Y.; Yamamoto, S.; Minami, H.Y.; Hattori, T. Differential activation of the rice sucrose nonfermenting1–related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant. Cell 2004, 16, 1163–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jossier, M.; Bouly, J.P.; Meimoun, P.; Arjmand, A.; Lessard, P.; Hawley, S.; Hardie, D.G.; Thomas, M. SnRK1 (SNF1-related kinase 1) has a central role in sugar and ABA signalling in Arabidopsis thaliana. Plant J. 2009, 59, 316–328. [Google Scholar] [CrossRef]
- Halford, N.G.; Hey, S.; Jhurreea, D.; Laurie, S.; McKibbin, R.S.; Paul, M.; Zhang, Y.H. Metabolic signalling and carbon partitioning: Role of Snf1-related (SnRK1) protein kinase. J. Exp. Bot. 2003, 382, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Halford, N.G.; Hey, S. Snf1-related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signalling in plants. Biochem. J. 2009, 419, 247–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coello, P.; Hey, S.J.; Halford, N.G. The sucrose non-fermenting-1-related (SnRK) family of protein kinases: Potential for manipulation to improve stress tolerance and increase yield. J. Exp. Bot. 2011, 62, 883–893. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant. Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef]
- Fujita, Y.; Yoshida, T.; Yamaguchi-Shinozaki, K. Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants. Physiol. Plant. 2013, 147, 15–27. [Google Scholar] [CrossRef]
- Anderberg, R.J.; Walker-Simmons, M.K. Isolation of a wheat cDNA clone for an abscisic acid-inducible transcript with homology to protein kinases. Proc. Natl. Acad. Sci. USA 1992, 82, 10183–10187. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Cadenas, A.; Verhey, S.D.; Holappa, L.D.; Shen, Q.; Ho, T.H.; Walker-Simmons, M.K. An abscisic acid-induced protein kinase, PKABA1, mediates abscisic acid-suppressed gene expression in barley aleurone layers. Proc. Natl. Acad. Sci. USA 1999, 96, 1767–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, R.R. The Abscisic Acid-Responsive Kinase PKABA1 Interacts with a Seed-Specific Abscisic Acid Response Element-Binding Factor, TaABF, and Phosphorylates TaABF Peptide Sequences. Plant Physiol. 2002, 130, 837–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudsocq, M.; Barbier-Brygoo, H.; Lauriere, C. Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana. J. Biol. Chem. 2004, 279, 41758–41766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, Y.; Nakashima, K.; Yoshida, T.; Katagiri, T.; Kidokoro, S.; Kanamori, N.; Umezawa, T.; Fujita, M.; Maruyama, K.; Ishiyama, K.; et al. Three SnRK2 protein kinases are the main positive regulators of abscisic acid signaling in response to water stress in Arabidopsis. Plant Cell Physiol. 2009, 50, 2123–2132. [Google Scholar] [CrossRef] [Green Version]
- Julkowska, M.M.; McLoughlin, F.; Galvan-Ampudia, C.S.; Rankenberg, J.M.; Kawa, D.; Klimecka, M.; Haring, M.A.; Munnik, T.; Kooijman, E.E.; Testerink, C. Identification and functional characterization of the A rabidopsis Snf 1-related protein kinase SnRK2.4 phosphatidic acid-binding domain. Plant Cell Environ. 2015, 38, 614–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.J.; Guo, X.; Zhao, D.; Zhang, Q.; Jiang, Y.Z.; Wang, Y.T.; Peng, X.; Wei, Y.; Zhai, Z.F.; Zhao, W.; et al. Cloning and expression profiling of the PacSnRK2 and PacPP2C gene families during fruit development, ABA treatment, and dehydration stress in sweet cherry. Plant Physiol. Bioch. 2017, 119, 275–285. [Google Scholar] [CrossRef]
- Rehman, S.U.; Wang, J.Y.; Chang, X.P.; Zhang, X.Y.; Mao, X.G.; Jin, R.L. A wheat protein kinase gene TaSnRK2.9-5A associated with yield contributing traits. Theor. Appl. Genet. 2019, 132, 907–919. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.J.; Mao, X.G.; Zhang, H.Y.; Chen, S.S.; Zhai, C.C.; Yang, S.M.; Jin, R.L. Cloning and characterization of TaSnRK2. 3, a novel SnRK2 gene in common wheat. J. Exp. Bot. 2013, 64, 2063–2080. [Google Scholar] [CrossRef] [Green Version]
- McLoughlin, F.; Galvan-Ampudia, C.S.; Julkowska, M.M.; Caarls, L.; Does, D.V.D.; Laurière, C.; Munnik, T.; Haring, M.A.; Testerink, C. The Snf1-related protein kinases SnRK2. 4 and SnRK2. 10 are involved in maintenance of root system architecture during salt stress. Plant J. 2012, 72, 436–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.H. Ammopiptanthus Cheng, F. a new genus of leguminosae from central asia. J. Bot. USSR 1959, 44, 1381–1386. [Google Scholar]
- Jia, X.H.; Li, X.R.; Zhang, J.G.; Zhang, Z.S. Analysis of spatial variability of the fractal dimension of soil particle size in Ammopiptanthus mongolicus’ desert habitat. Environ. Geol. 2009, 58, 953–962. [Google Scholar] [CrossRef]
- Fei, Y.B.; Cao, P.X.; Gao, S.Q.; Wang, B.; Wei, L.B.; Zhao, J.; Chen, G.; Wang, B.H. Purification and structure analysis of antifreeze proteins from Ammopiptanthus mongolicus. Prep. Biochem. Biotech. 2008, 38, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Qin, Y.; Zou, Y.J.; Ma, F.W. Genome-wide identification and expression profiling of the SnRK2 gene family in Malus prunifolia. Gene 2014, 552, 87–97. [Google Scholar] [CrossRef]
- Umezawa, T.; Nakashima, K.; Miyakawa, T.; Kuromori, T.; Tanokura, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular basis of the core regulatory network in ABA responses: Sensing, signaling and transport. Plant Cell Physiol. 2010, 51, 1821–1839. [Google Scholar] [CrossRef] [PubMed]
- Ruvinsky, A.; Eskesen, S.T.; Eskesen, F.N.; Hurst, L.D. Can codon usage bias explain intron phase distributions and exon symmetry? J. Mol. Evol. 2005, 60, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Boudsocq, M.; Droillard, M.J.; Barbier-Brygoo, H.; Laurière, C. Different phosphorylation mechanisms are involved in the activation of sucrose non-fermenting 1 related protein kinases 2 by osmotic stresses and abscisic acid. Plant Mol. Biol. 2007, 63, 491–503. [Google Scholar] [CrossRef]
- Huai, J.L.; Wang, M.; He, J.G.; Zheng, J.; Dong, Z.G.; Lv, H.K.; Zhao, J.F.; Wang, G.Y. Cloning and characterization of the SNRK2 gene gamily from Zea mays. Plant Cell Rep. 2008, 27, 1861–1868. [Google Scholar] [CrossRef]
- Li, L.B.; Zhang, Y.R.; Liu, K.C.; Ni, Z.F.; FANG, Z.J.; Sun, Q.X.; GAO, J.W. Identification and Bioinformatics Analysis of SnRK2 and CIPK Family Genes in Sorghum. Agric. Sci. China 2010, 9, 19–30. [Google Scholar] [CrossRef]
- Huang, Z.; Tang, J.; Duan, W.; Wang, Z.; Song, X.; Hou, X. Molecular evolution, characterization, and expression analysis of SnRK2 gene family in Pak-choi (Brassica rapa ssp. chinensis). Front. Plant Sci. 2015, 6, 879–892. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Wang, J.; Qiao, X.; Jin, C.; Wu, J. Genome-wide survey of sucrose non-fermenting 1-related protein kinase 2 in Rosaceae and expression analysis of PbrSnRK2 in response to ABA stress. BMC Genom. 2020, 21, 781. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.M.; Cheng, J.W.; Hu, F.; Qin, C.; Xu, X.W.; Hu, K.L. The SnRK2 family in pepper (Capsicum annuum L.): Genome-wide identification and expression analyses during fruit development and under abiotic stress. Genes Genom. 2020, 42, 1117–1130. [Google Scholar] [CrossRef]
- Bai, J.P.; Mao, J.; Yang, H.Y.; Khan, A.; Fan, A.; Liu, S.; Zhang, J.L.; Wang, D.; Gao, H.J.; Zhang, J.L. Sucrose non-ferment 1 related protein kinase 2 (SnRK2) genes could mediate the stress responses in potato (Solanum tuberosum L.). BMC Genet. 2017, 18, 41. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Cheng, Y.H.; Zhang, C.; Shen, X.J.; You, Q.B.; Guo, W.; Li, X.; Song, X.J.; Zhou, X.A.; Jiao, Y.Q. Genome-wide identification and characterization of the GmSnRK2 family in soybean. Int. J. Mol. Sci. 2017, 18, 1834–1855. [Google Scholar] [CrossRef] [Green Version]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.X.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.J.; Thelen, J.J.; Cheng, J.L.; et al. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Ali, G.M.; Komastu, S. Proteomic analysis of rice leaf sheath during drought stress. J. Proteome Res. 2006, 5, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ge, X.; Yang, Z.; Liu, Z.; Ge, X.; Yang, Z.; Zhang, C.; Zhao, G.; Chen, E.; Liu, J.; et al. Genome-wide identification and characterization of SnRK2 gene family in cotton (Gossypium hirsutum L.). BMC Genet. 2017, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Diédhiou, C.J.; Popova, O.V.; Dietz, K.J.; Golldack, D. The SNF1- type serine-threonine protein kinase SAPK4 regulates stress-responsive gene expression in rice. BMC Plant Biol. 2008, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Wang, J.; Gou, J.; Wang, X.; Sun, S. Overexpression of NtSnRK2.2 enhances salt tolerance in Nicotiana tabacum by regulating carbohydrate metabolism and lateral root development. Funct. Plant Biol. 2020, 47, 537–543. [Google Scholar] [CrossRef]
- Gao, F.; Wang, X.; Li, X.; Xu, M.; Li, H.; Abla, M.; Sun, H.; Wei, S.; Feng, J.; Zhou, Y. Long-read sequencing and de novo genome assembly of Ammopiptanthus nanus, a desert shrub. GigaScience 2018, 7, giy074. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Guo, X.; Wang, K.X.; Pang, H.W.; Liu, Y.; Yang, Q.Q.; Fu, F.L.; Li, W.C.; Yu, H.Q. Genome-wide analysis of BES1/BZR1 transcription factors and their responses to osmotic stress in Ammopiptanthus nanus. J. Forest Res-JPN. 2020, 1, 1–9. [Google Scholar] [CrossRef]
- Yu, H.Q.; Yong, T.M.; Li, H.J.; Liu, Y.P.; Zhou, S.F.; Fu, F.L.; Li, W.C. Overexpression of a phospholipase Dα gene from Ammopiptanthus nanus enhances salt tolerance of phospholipase Dα1-deficient Arabidopsis mutant. Planta 2015, 242, 1495–1509. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Q.; Zhang, Y.Y.; Yong, T.M.; Liu, Y.P.; Zhou, S.F.; Fu, F.L.; Li, W.C. Cloning and functional validation of molybdenum cofactor sulfurase gene from Ammopiptanthus nanus. Plant Cell Rep. 2015, 34, 1165–1176. [Google Scholar] [CrossRef]
- Yu, H.Q.; Feng, W.Q.; Sun, F.A.; Zhang, Y.Y.; Qu, J.T.; Liu, B.L.; Lu, F.Z.; Yang, L.; Fu, F.L.; Li, W.C. Cloning and characterization of BES1/BZR1 transcription factor genes in maize. Plant Growth Regul. 2018, 86, 235–249. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Gene ID | CDs(bp) | Amino Acid | Isoelectric Point | Molecular Weight (KDa) | Introns | Grand Average Hydropathy | Subcellular Localization (Probability) |
|---|---|---|---|---|---|---|---|---|
| AnSnRK2.1 | EVM0003959.1 | 1152 | 383 | 5.92 | 43.07 | 8 | −0.25 | chloroplast |
| AnSnRK2.2 | EVM0011565.1 | 1062 | 353 | 5.81 | 40.52 | 8 | −0.53 | cytoskeleton |
| AnSnRK2.3 | EVM0013530.1 | 1071 | 356 | 6.21 | 40.71 | 8 | −0.47 | cytoskeleton |
| AnSnRK2.4 | EVM0016282.1 | 1098 | 365 | 4.89 | 41.55 | 8 | −0.34 | cytoskeleton |
| AnSnRK2.5 | EVM0017722.1 | 1059 | 352 | 5.95 | 40.60 | 8 | −0.50 | cytoskeleton |
| AnSnRK2.6 | EVM0020312.1 | 1074 | 357 | 5.69 | 40.43 | 7 | −0.19 | cytoplasm |
| AnSnRK2.7 | EVM0025729.1 | 1062 | 353 | 5.00 | 40.22 | 8 | −0.35 | cytoskeleton |
| AnSnRK2.8 | EVM0028012.1 | 1029 | 342 | 5.24 | 38.76 | 8 | −0.31 | cytoplasm |
| AnSnRK2.9 | EVM0029135.1 | 1035 | 344 | 5.23 | 38.96 | 8 | −0.3 | cytoskeleton |
| AnSnRK2.10 | EVM0032503.1 | 1017 | 338 | 5.3 | 38.42 | 8 | −0.2 | cytoplasm |
| AnSnRK2.11 | EVM0034033.1 | 1089 | 362 | 4.71 | 41.16 | 8 | −0.27 | cytoskeleton |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Lu, F.; Feng, W.; Liu, Y.; Cao, Y.; Li, W.; Fu, F.; Yu, H. Genome-Wide Identification and Expression Analyses of AnSnRK2 Gene Family under Osmotic Stress in Ammopiptanthus nanus. Plants 2021, 10, 882. https://doi.org/10.3390/plants10050882
Tang Y, Lu F, Feng W, Liu Y, Cao Y, Li W, Fu F, Yu H. Genome-Wide Identification and Expression Analyses of AnSnRK2 Gene Family under Osmotic Stress in Ammopiptanthus nanus. Plants. 2021; 10(5):882. https://doi.org/10.3390/plants10050882
Chicago/Turabian StyleTang, Yueming, Fengzhong Lu, Wenqi Feng, Yuan Liu, Yang Cao, Wanchen Li, Fengling Fu, and Haoqiang Yu. 2021. "Genome-Wide Identification and Expression Analyses of AnSnRK2 Gene Family under Osmotic Stress in Ammopiptanthus nanus" Plants 10, no. 5: 882. https://doi.org/10.3390/plants10050882






