Impact of Commercial Seaweed Liquid Extract (TAM®) Biostimulant and Its Bioactive Molecules on Growth and Antioxidant Activities of Hot Pepper (Capsicum annuum)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seaweeds
2.2. Hot Pepper (Capsicum annuum) Methods
2.2.1. Soil
2.2.2. Experimental Design
2.3. Tested Parameters
2.3.1. Hot Pepper Capsicum annuum Growth Parameters
2.3.2. Capsicum annuum Yield and Fruit Parameters
2.3.3. Antioxidant Activities of TAM® and Capsicum annuum Fruits
2.4. Statistical Analysis
3. Results
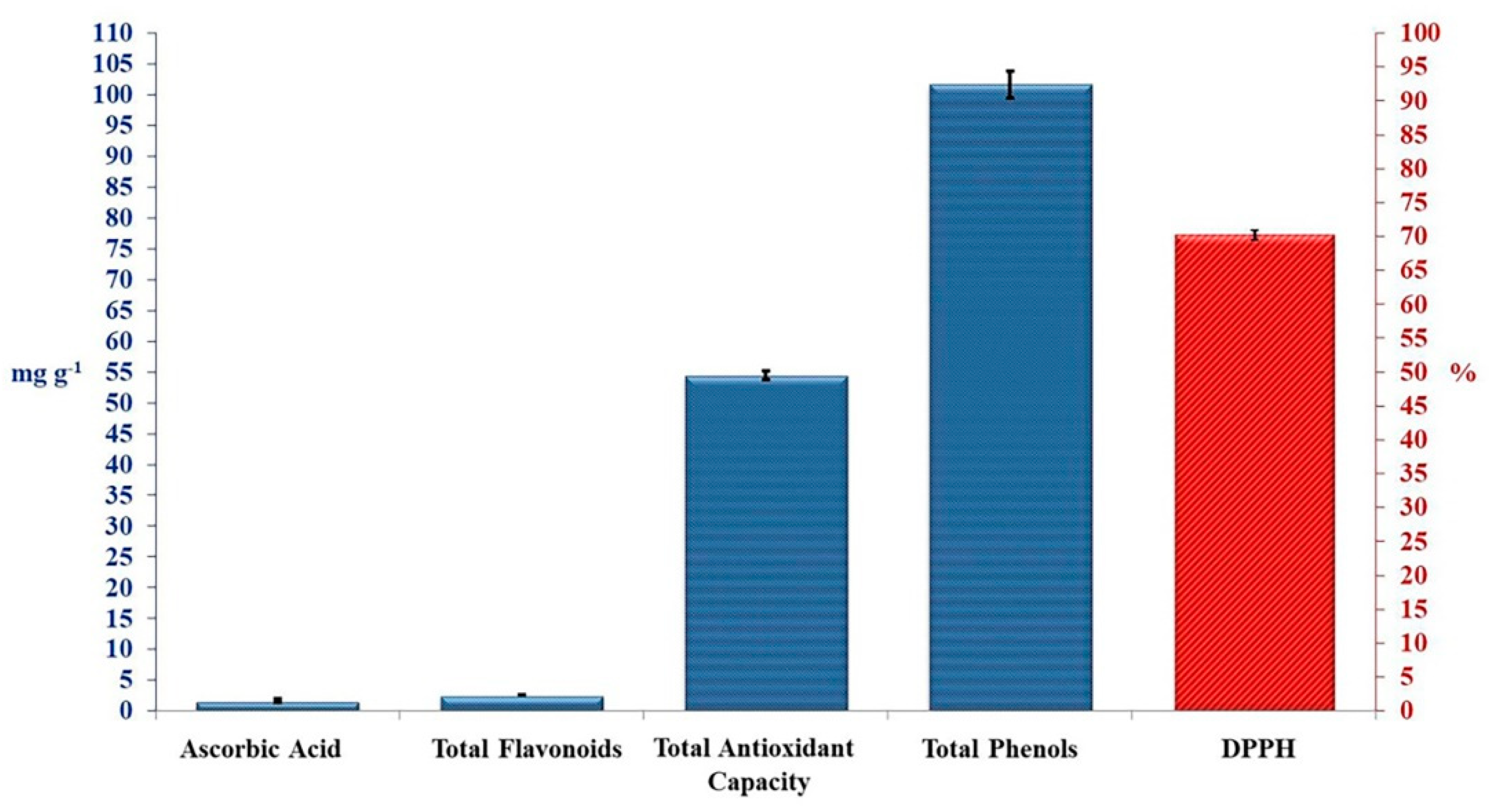
3.1. Antioxidant Activities of Crude TAM®
3.2. Hot Pepper C. annuum
3.2.1. Growth
3.2.2. Yield and Chemical Composition
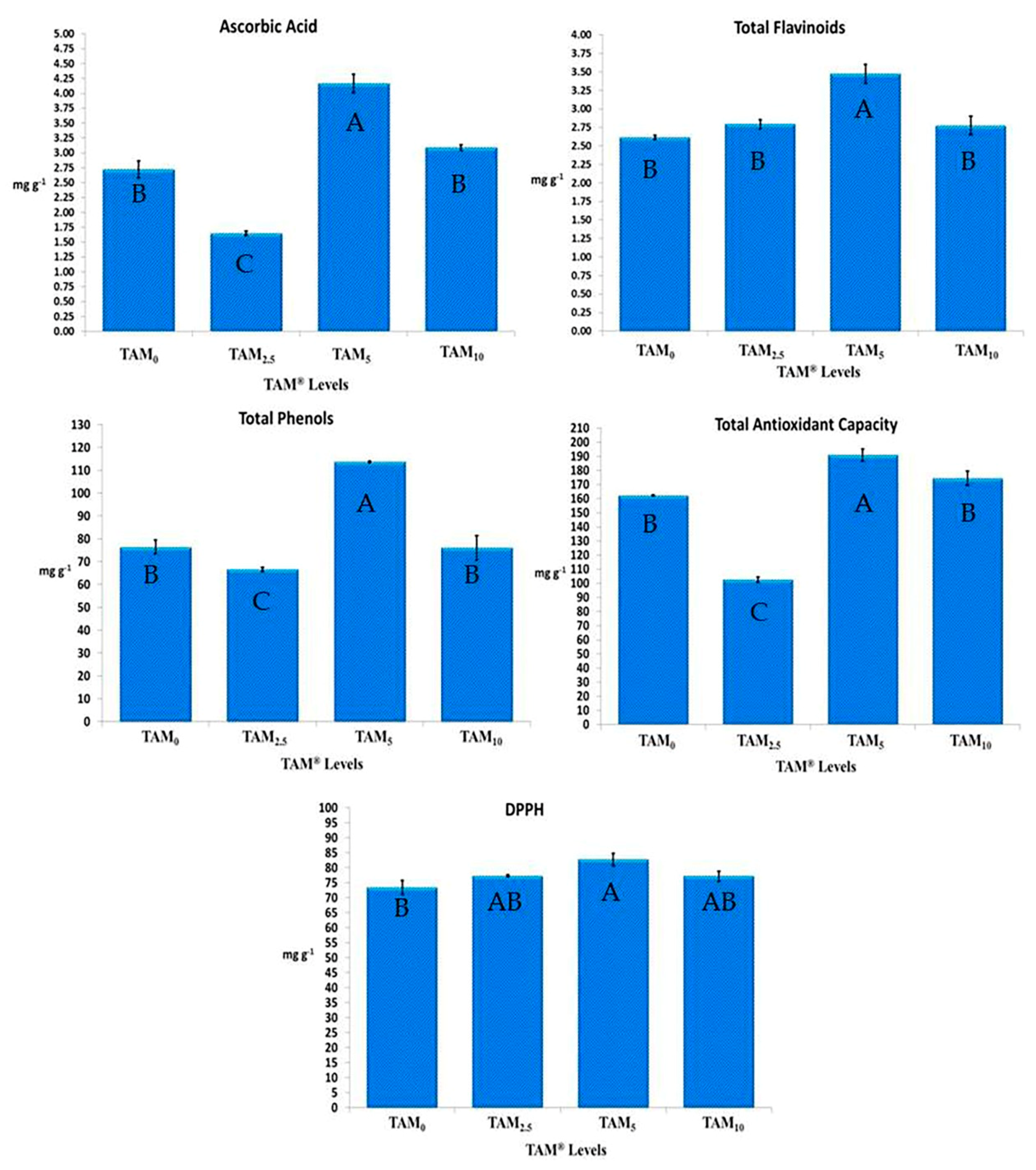
3.2.3. Antioxidant Activities of Colored Marketable Stage of Fruits of C. annuum
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benson, T.; Lavelle, F.; Bucher, T.; McCloat, A.; Mooney, E.; Egan, B.; Collins, C.; Dean, M. The Impact of Nutrition and Health Claims on Consumer Perceptions and Portion Size Selection: Results from a Nationally Representative Survey. Nutrients 2018, 10, 656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shambhavi, S.; Kumar, R.; Sharma, S.P.; Verma, G.; Sharma, R.P.; Sharma, S.K. Long-term effect of inorganic fertilizers and amendments on productivity and root dynamics under maize-wheat intensive cropping in an acid Alfisol. J. Appl. Nat. Sci. 2017, 9, 2004–2012. [Google Scholar] [CrossRef] [Green Version]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.; Ashour, M.; Soliman, A. Anticancer Activity, Antioxidant Activity, Mineral Contents, Vegetative and Yield of Eruca sativa Using Foliar Application of Autoclaved Cellular Extract of Spirulina platensis Extract, Comparing to N-P-K Fertilizers. J. Plant Prod. 2017, 8, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Ashour, M.; El-Shafei, A.A.; Khairy, H.M.; Abd-Elkader, D.Y.; Mattar, M.A.; Alataway, A.; Hassan, S.M. Effect of Pterocladia capillacea Seaweed Extracts on Growth Parameters and Biochemical Constituents of Jew’s Mallow. Agronomy 2020, 10, 420. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.H.; Ashour, M.; Soliman, A.A.F.; Hassanien, H.A.; Alsanie, W.F.; Gaber, A.; Elshobary, M.E. The potential of a new commercial seaweed extract in stimulating morphoagronomic and bioactive properties of Eruca vesicaria (L.) Cav. Sustianability 2021, 13, 4485. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ashour, M.; Sakai, N.; Zhang, L.; Hassanien, H.; Gaber, A.; Ammar, G. Impact of Seaweed Liquid Extract Biostimulant on Growth, Yield, and Chemical Composition of Cucumber (Cucumis sativus). Agriculture 2021, 11, 320. [Google Scholar] [CrossRef]
- El-Khodary, G.M.; El-Sayed, H.S.; Khairy, H.M.; El-Sheikh, M.A.; Qi, X.; Elshobary, M.E. Comparative study on growth, survival and pigmentation of Solea aegyptiaca larvae by using four different microalgal species with emphasize on water quality and nutritional value. Aquac. Nutr. 2020, anu.13211. [Google Scholar] [CrossRef]
- Ashour, M. Marine Microalgae: Aquaculture and Biodiesel Production; LAP LAMBERT Academic Publishing: Chișinău, Moldova, 2015; ISBN 3659691682. [Google Scholar]
- Sharawy, Z.Z.; Ashour, M.; Abbas, E.; Ashry, O.; Helal, M.; Nazmi, H.; Kelany, M.; Kamel, A.; Hassaan, M.; Rossi, W.; et al. Effects of dietary marine microalgae, Tetraselmis suecica, on production, gene expression, protein markers and bacterial count of Pacific white shrimp Litopenaeus vannamei. Aquac. Res. 2020, 51, 2216–2228. [Google Scholar] [CrossRef]
- Metwally, A.S.; El-Naggar, H.A.; El-Damhougy, K.A.; Bashar, M.A.; Ashour, M.; Abo-Taleb, H.A. GC-MS Analysis of bioactive components in six different crude extracts from the Soft Coral (Sinularia maxim) collected from Ras Mohamed, Aqaba Gulf, Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 2020, 24, 425–434. [Google Scholar] [CrossRef]
- Elshobary, M.E.; El-Shenody, R.A.; Ashour, M.; Zabed, H.M.; Qi, X. Antimicrobial and antioxidant characterization of bioactive components from Chlorococcum minutum. Food Biosci. 2020, 35, 100567. [Google Scholar] [CrossRef]
- Abo-Taleb, H.A.; Zeina, A.F.; Ashour, M.; Mabrouk, M.M.; Sallam, A.E.; El-Feky, M.M. Isolation and cultivation of the freshwater amphipod Gammarus pulex (Linnaeus, 1758), with an evaluation of its chemical and nutritional content. Egypt. J. Aquat. Biol. Fish. 2020, 24, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Ashour, M.; Kamel, A. Enhance Growth and Biochemical Composition of Nannochloropsis oceanica, Cultured under Nutrient Limitation, Using Commercial Agricultural Fertilizers. J. Marine Sci. Res. Dev. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2018, 8, e00162. [Google Scholar] [CrossRef]
- Ashour, M.; Elshobary, M.E.; El-Shenody, R.; Kamil, A.W.; Abomohra, A.E. Evaluation of a native oleaginous marine microalga Nannochloropsis oceanica for dual use in biodiesel production and aquaculture feed. Biomass Bioenergy 2019, 120, 439–447. [Google Scholar] [CrossRef]
- Hamed, S.M.; Abd El-Rhman, A.A.A.; Abdel-Raouf, N.; Ibraheem, I.B. Role of marine macroalgae in plant protection & improvement for sustainable agriculture technology. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 104–110. [Google Scholar] [CrossRef]
- Abo-Taleb, H.; Ashour, M.; El-Shafei, A.; Alataway, A.; Maaty, M.M. Biodiversity of Calanoida Copepoda in Different Habitats of the North-Western Red Sea (Hurghada Shelf). Water 2020, 12, 656. [Google Scholar] [CrossRef] [Green Version]
- El-Shenody, R.A.; Ashour, M.; Ghobara, M.M.E. Evaluating the chemical composition and antioxidant activity of three Egyptian seaweeds: Dictyota dichotoma, Turbinaria decurrens, and Laurencia obtusa. Braz. J. Food Technol. 2019, 22. [Google Scholar] [CrossRef] [Green Version]
- Elshobary, M.E.; El-Shenody, R.; Abomohra, A.E. Sequential biofuel production from seaweeds enhances the energy recovery: A case study for biodiesel and bioethanol production. Int. J. Energy Res. 2020, 1–11. [Google Scholar] [CrossRef]
- Osman, M.E.H.H.; Abu-Shady, A.M.; Elshobary, M.E. The Seasonal Fluctuation of the Antimicrobial Activity of Some Macroalgae Collected from Alexandria Coast, Egypt. In Salmonella—Distribution, Adaptation, Control Measures and Molecular Technologies; InTech: London, UK, 2012; pp. 173–186. [Google Scholar]
- Osman, M.E.H.; Abo-Shady, A.M.; Elshobary, M.E.; Abd El-Ghafar, M.O.; Abomohra, A.E. Screening of seaweeds for sustainable biofuel recovery through sequential biodiesel and bioethanol production. Environ. Sci. Pollut. Res. 2020, 27, 32481–32493. [Google Scholar] [CrossRef]
- Shabaka, S.H. Checklist of seaweeds and seagrasses of Egypt (Mediterranean Sea): A review. Egypt. J. Aquat. Res. 2018, 44, 203–212. [Google Scholar] [CrossRef]
- Osman, M.E.H.; Aboshady, A.M.; Elshobary, M.E. Production and characterization of antimicrobial active substance from some macroalgae collected from Abu-Qir bay (Alexandria) Egypt. Afr. J. Biotechnol. 2013, 12, 6847–6858. [Google Scholar] [CrossRef]
- Osman, M.E.H.; Abushady, A.M.; Elshobary, M.E. In vitro screening of antimicrobial activity of extracts of some macroalgae collected from Abu- Qir bay Alexandria, Egypt. Afr. J. Biotechnol. 2010, 9, 7203–7208. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Swieca, M.; Surdyka, M.; Gawlik-Dziki, U.; Złotek, U.; Baraniak, B. Antioxidant potential of fresh and stored lentil sprouts affected by elicitation with temperature stresses. Int. J. Food Sci. Technol. 2014, 49, 1811–1817. [Google Scholar] [CrossRef]
- Złotek, U.; Świeca, M.; Jakubczyk, A. Effect of abiotic elicitation on main health-promoting compounds, antioxidant activity and commercial quality of butter lettuce (Lactuca sativa L.). Food Chem. 2014, 148, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Świeca, M.; Baraniak, B. Nutritional and antioxidant potential of lentil sprouts affected by elicitation with temperature stress. J. Agric. Food Chem. 2014, 62, 3306–3313. [Google Scholar] [CrossRef]
- Ashour, M.; Mabrouk, M.M.; Ayoub, H.F.; El-Feky, M.M.M.; Sharawy, Z.Z.; Hoseinifar, S.H.; Rossi, W.; Van Doan, H.; El-Haroun, E.; Goda, A.M.-S. Effect of dietary seaweed extract supplementation on growth, feed utilization, hematological indices, and non-specific immunity of Nile Tilapia, Oreochromis niloticus challenged with Aeromonas hydrophila. J. Appl. Phycol. 2020, 32, 3467–3479. [Google Scholar] [CrossRef]
- Hassan, S.M.; El-Bebany, A.F.; Salem, M.Z.M.; Komeil, D.A. Productivity and post-harvest fungal resistance of hot pepper as affected by potassium silicate, clove extract foliar spray and nitrogen application. Plants 2021, 10, 662. [Google Scholar] [CrossRef]
- Hunziker, A.T. Genera Solanacearum: The Genera of Solanaceae Illustrated, Arranged according to a New System; ARG Gantner: Königstein, Germany, 2001; ISBN 3904144774. [Google Scholar]
- Basu, S.K.; De, A.K.; De, A. Capsicum: Historical and botanical perspectives. In Capsicum: The Genus Capsicum; CRC Press: Boca Raton, FL, USA, 2003; Volume 33, pp. 1–15. [Google Scholar]
- Perry, L.; Dickau, R.; Zarrillo, S.; Holst, I.; Pearsall, D.M.; Piperno, D.R.; Berman, M.J.; Cooke, R.G.; Rademaker, K.; Ranere, A.J.; et al. Starch fossils and the domestication and dispersal of chili peppers (Capsicum spp. L.) in the Americas. Science 2007, 315, 986–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aktas, H.; Abak, K.; Sensoy, S. Genetic diversity in some Turkish pepper (Capsicum annuum L.) genotypes revealed by AFLP analyses. Afr. J. Biotechnol. 2009, 8, 4378–4386. [Google Scholar]
- Gonzalez-Diaz, L.; Martínez-Jimenez, P.; Bastida, F.; Gonzalez-Andujar, J.L.; Milián, F.B. Expert system for integrated plant protection in pepper (Capsicum annuun L.). Expert Syst. Appl. 2009, 36, 8975–8979. [Google Scholar] [CrossRef]
- Helaly, A.A.; Hassan, S.M.; Craker, L.E.; Mady, E. Effects of growth-promoting bacteria on growth, yield and nutritional value of collard plants. Ann. Agric. Sci. 2020. [Google Scholar] [CrossRef]
- Ashour, M. Bioactive Compounds Extracted from Marine Algae Improve the Growth and Immunity of Plants, Fish and Marine crustaceans. Egypt Patent Application 2046/2019, 2019. (under processing). [Google Scholar]
- Hirayama, O.; Nakamura, K.; Hamada, S.; Kobayasi, Y. Singlet oxygen quenching ability of naturally occurring carotenoids. Lipids 1994, 29, 149–150. [Google Scholar] [CrossRef]
- Vassiliou, E.K.; Gonzalez, A.; Garcia, C.; Tadros, J.H.; Chakraborty, G.; Toney, J.H. Oleic acid and peanut oil high in oleic acid reverse the inhibitory effect of insulin production of the inflammatory cytokine TNF-α both in vitro and in vivo systems. Lipids Health Dis. 2009, 8, 10–25. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, C.; Cavia, M.D.M.; Alonso-Torre, S. Role of oleic acid in immune system; mechanism of action: A review. Nutr. Hosp. 2012, 27, 978–990. [Google Scholar] [CrossRef] [Green Version]
- Azam, M.M.; Waris, A.; Nahar, N. Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for use as biodiesel in India. Biomass Bioenergy 2005, 29, 293–302. [Google Scholar] [CrossRef]
- Agoramoorthy, G.; Chandrasekaran, M.; Venkatesalu, V.; Hsu, M. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Braz. J. Microbiol. 2007, 38, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Santos, I.; Coutinho, H.M.; Matias, E.F.; da Costa, J.M.; Alves, R.N.; Almeida, W.D.O. Antimicrobial activity of natural products from the skins of the semiarid living lizards Ameiva ameiva (Linnaeus, 1758) and Tropidurus hispidus (Spix, 1825). J. Arid Environ. 2012, 76, 138–141. [Google Scholar] [CrossRef]
- Page, A.L. (Ed.) Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 1965. [Google Scholar]
- Cottenie, A.; Verloo, M.; Kiekens, L.; Velghe, G.; Camerlynck, R. Chemical analysis of plants and soils. Lab. Agroch. State Univ. Gent Belgium 1982, 63. [Google Scholar] [CrossRef]
- Evenhuis, B.; de Waard, P.W.F. Paper 15 Principles and Practices in Plant Analysis. Soil Plant Test. Anal. 1980, 38, 152. [Google Scholar]
- Zaki, M.; Ashour, M.; Heneash, A.; Mabrouk, M.; Alprol, A.; Khairy, H.; Nour, A.; Mansour, A.; Hassanien, H.; Gaber, A.; et al. Potential Applications of Native Cyanobacterium Isolate (Arthrospira platensis NIOF17/003) for Biodiesel Production and Utilization of Its Byproduct in Marine Rotifer (Brachionus plicatilis) Production. Sustainability 2021, 13, 1769. [Google Scholar] [CrossRef]
- Abbas, E.M.; Ali, F.S.; Desouky, M.G.; Ashour, M.; El-Shafei, A.; Maaty, M.M.; Sharawy, Z.Z. Novel comprehensive molecular and ecological study introducing coastal mud shrimp (Solenocera crassicornis) recorded at the Gulf of suez, Egypt. J. Marine Sci. Eng. 2021, 9, 9. [Google Scholar] [CrossRef]
- Ashour, M.; Abo-Taleb, H.; Abou-Mahmoud, M.; El-Feky, M.M.M. Effect of the integration between plankton natural productivity and environmental assessment of irrigation water, El-Mahmoudia Canal, on aquaculture potential of Oreochromis niloticus. Turk. J. Fish. Aquat. Sci. 2018, 18. [Google Scholar] [CrossRef]
- Magouz, F.I.; Matter, M.; Essa, M.A.; El-shafei, A.; Tageldein, A.; Mahmoud, S.F.; Ashour, M. Effect of extended feeding with live copepods, Oithona nana, and artemia franciscana on the growth performance, intestine histolo- gy, and economic viability of European seabass (dicentrarchus labrax) postlarvae. Fresenius Environ. Bull. 2021, 30, 7106–7116. [Google Scholar]
- Magouz, F.I.; Essa, M.A.; Matter, M.; Mansour, A.T.; Gaber, A.; Ashour, M. Effect of different salinity levels on Copepoda (Oithona nana) population dynamics, production, and composition. Diversity 2021, 30, 190. [Google Scholar] [CrossRef]
- Magouz, F.I.; Essa, M.A.; Matter, M.; Mansour, A.T.; Alkafafy, M.; Ashour, M. Population Dynamics, Fecundity and Fatty Acid Composition of Oithona nana (Cyclopoida, Copepoda), Fed on Different Diets. Animals 2021, 11, 1188. [Google Scholar] [CrossRef]
- Arthur, G.; Stirk, W.; Van Staden, J.; Scott, P. Effect of a seaweed concentrate on the growth and yield of three varieties of Capsicum annuum. S. Afr. J. Bot. 2003, 69, 207–211. [Google Scholar] [CrossRef]
- Ashour, M.; Mabrouk, M.; Abo-Taleb, H.A.; Sharawy, Z.Z.; Ayoub, H.F.; Van Doan, H.; Davies, S.J.; El-Haroun, E.; Goda, A.A. A liquid seaweed extract (TAM®) improves aqueous rearing environment, diversity of zooplankton community, whilst enhancing growth and immune response of Nile tilapia, Oreochromis niloticus, challenged by Aeromonas hydrophila. Aquaculture 2021, 736915. [Google Scholar] [CrossRef]
- Marhoon, I.A.; Abbas, M.K. Effect of Foliar Application of Seaweed Extract and Amino Acids on Some Vegetative and Anatomical Characters of Two Sweet Pepper (Capsicum Annuum L.) Cultivars. Int. J. Res. Stud. Agric. Sci. 2015, 1, 35–44. [Google Scholar]
- Eris, A.; Sivritepe, H.; Sivritepe, N. The effect of seaweed (Ascophyllum nodosum) extract on yield and quality criteria in peppers. Acta Hortic. 1995, 185–192. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulatory activities of Ascophyllum nodosum extract in tomato and sweet pepper crops in a tropical environment. PLoS ONE 2019, 14, e0216710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yıldıztekın, M.; Tuna, A.L.; Kaya, C. Physiological effects of the brown seaweed (Ascophyllum nodosum) and humic substances on plant growth, enzyme activities of certain pepper plants grown under salt stress. Acta Biol. Hung. 2018, 69, 325–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| RT (min) | Phytochemical Compounds Name | Chemical Formula | Molecular Weight | Nature | Biological Properties | Literatures |
|---|---|---|---|---|---|---|
| 8.99 | 5-Silaspiro[4.4]nona-1,3,6,8-tetraene,3,8-bis(diet-hylboryl)-2,7-diethyl-1,4,6,9-tetraphenyl- | C44H50B2Si | 628.39 | Silicon-boron compound | Fish and plant growth regulator and immunity enhancer | [6,7,30] |
| 16.31 | Nonadecane | C19H40 | 268.31 | Alkane | Fish and plant immunity enhancer; antioxidant; antimicrobial; anti-inflammatory, | [6,7,30] |
| 19.45 | Rhodopin | C40H58O | 554.45 | Carotenoid | Fish and plant growth enhancer; antioxidant | [6,7,30,39] |
| 20.07 | Milbemycin-oxime | C32H44ClNO7 | 589.28 | Macrocyclic lactones | Fish and plant immunity enhancer; antiparasitic; antihelmintic; insecticidal | [6,7,30] |
| 20.90 | Tridecanoic acid methyl ester | C14H28O2 | 228.21 | Fatty acid methyl esters (FAMEs) | Antioxidant; herbicidal; antimicrobial; surfactants | [6,7,30,40] |
| 21.63 | Oleic Acid | C18H34O2 | 282.26 | Fatty acid | Fish and plant immunity enhancer; anti-inflammatory | [6,7,30,40,41] |
| 23.74 | γ-Linolenic acid methyl ester | C19H32O2 | 292.24 | FAMEs | Antioxidant; herbicidal; antimicrobial; surfactants | [6,7,30,41,42] |
| 24.02 | 9,12-Octadecadienoic acid methyl ester, (E,E)- | C19H34O2 | 294.26 | FAMEs | Antioxidant; herbicidal; antimicrobial; surfactants | [6,7,30,42,43] |
| 24.37 | Phytol | C20H40O | 296.31 | Diterpene alcohol | Antioxidant; plant growth enhancer | [30,39,44] |
| Soil Parameters | 2017 | 2018 |
|---|---|---|
| Particle size distribution | ||
| Sand (%) | 42.5 ± 3.6 | 39.8 ± 3.1 |
| Silt (%) | 25.2 ± 2.5 | 28.7 ± 2.2 |
| Clay (%) | 32.3 ± 1.6 | 31.5 ± 1.2 |
| Soil texture | Sandy loam | Sandy loam |
| pH | 7.45 ± 0.5 | 7.35 ± 0.3 |
| Chemical Characteristics | ||
| Soluble Cations (mmol g−1 soil) | ||
| Ca2+ | 1.44 ± 0.4 | 1.40 ± 0.5 |
| Mg2+ | 1.45 ± 0.4 | 0.98 ± 0.2 |
| Na+ | 3.63 ± 0.5 | 4.75 ± 0.7 |
| K+ | 0.54 ± 0.05 | 0.36 ± 0.03 |
| Soluble Anions (mmol L−1) | ||
| HCO3− | 1.66 ± 0.1 | 1.78 ± 0.2 |
| Cl− | 2.00 ± 0.3 | 1.80 ± 0.2 |
| SO42− | 1.70 ± 0.5 | 1.65 ± 0.6 |
| Total nitrogen (TN) (%) | 0.16 ± 0.03 | 0.15 ± 0.01 |
| Available phosphorus (mg L−1) | 0.32 ± 0.02 | 0.27 ± 0.01 |
| NPK Rate | Week after Transplanting | Calcium Nitrate (g 100 m−2) | Potassium Sulphate (g 100 m2) | Phosphoric Acid (cm3 100 m2) |
|---|---|---|---|---|
| 2% | 2 | 252 | 160 | 38 |
| 4% | 3 | 504 | 320 | 76 |
| 6% | 4 | 756 | 480 | 114 |
| 8% | 5 | 1008 | 640 | 152 |
| 12% | 6 | 1512 | 960 | 228 |
| 12% | 7 | 1512 | 960 | 228 |
| 12% | 8 | 1512 | 960 | 228 |
| 12% | 9 | 1512 | 960 | 228 |
| 8% | 10 | 1008 | 640 | 152 |
| 8% | 11 | 1008 | 640 | 152 |
| 8% | 12 | 1008 | 640 | 152 |
| 8% | 13 | 1008 | 640 | 152 |
| Treatments * | TAM0% (Control) | TAM0.25% | TAM0.5% | TAM1% | ||||
|---|---|---|---|---|---|---|---|---|
| Parameters | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 |
| plant Height (cm) | 67.33 ± 4.93 e | 70.00 ± 4.58 d | 81.00 ± 0.23 b | 80.33 ± 1.15 b | 88.00 ± 2.00 a | 80.33 ± 0.58 b | 72.67 ± 1.15 c | 74.00 ± 3.46 c |
| Increase/Decrease Rate (%) | (00.00) | (00.00) | (20.30) | (14.76) | (30.70) | (14.76) | (7.93) | (5.71) |
| Branches (No.) | 4.67 ± 0.58 b | 4.33 ± 0.58 b | 6.00 ± 0.65 a | 6.00 ± 0.53 a | 6.00 ± 0.80 a | 6.00 ± 0.83 a | 4.67 ± 0.58 b | 4.33 ± 0.58 b |
| Increase/Decrease Rate (%) | (00.00) | (00.00) | (28.48) | (38.57) | (28.48) | (38.57) | (00.00) | (00.00) |
| Chlorophyll (mg 100 g−1 FW) | 60.31 ± 2.05 a | 59.68 ± 1.67 c | 64.54 ± 2.44 a | 65.32 ± 2.09 b | 65.80 ± 5.03 a | 68.74 ± 0.81 a | 62.44 ± 4.83 a | 60.81 ± 1.07 c |
| Increase/Decrease Rate (%) | (00.00) | (00.00) | (7.03) | (9.46) | (9.11) | (15.19) | (3.54) | (1.90) |
| Dry matter (%) | 33.17 ± 1.73 c | 33.31 ± 0.87 b | 36.00 ± 1.52 ab | 35.90 ± 1.50 a | 36.80 ± 1.17 a | 36.54 ± 1.15 a | 34.11 ± 0.45 bc | 33.45 ± 0.88 b |
| Increase/Decrease Rate (%) | (00.00) | (00.00) | (8.53) | (7.77) | (10.94) | (9.70) | (2.83) | (0.42) |
| Leaf-N (mg 100 g−1 DW) | 1.32 ± 0.01 a | 1.35 ± 0.08 a | 1.19 ± 0.04 b | 1.22 ± 0.09 a | 1.21 ± 0.06 b | 1.17 ± 0.07 a | 1.16 ± 0.04 b | 1.23 ± 0.13 a |
| Increase/Decrease Rate (%) | (00.00) | (00.00) | (−9.87) | (−9.63) | (−8.10) | (−13.33) | (−13.09) | (−9.13) |
| Leaf-P (mg 100 g−1 DW) | 0.41 ± 0.01 b | 0.42 ± 0.03 b | 0.53 ± 0.03 a | 0.52 ± 0.02 a | 0.51 ± 0.03 a | 0.51 ± 0.02 a | 0.45 ± 0.04 b | 0.44 ± 0.01 b |
| Increase/Decrease Rate (%) | (00.00) | (00.00) | (29.27) | (23.81) | (25.20) | (20.64) | (10.56) | (4.76) |
| Leaf-K (mg 100 g−1 DW) | 3.97 ± 0.18 c | 4.13 ± 0.09 c | 5.49 ± 0.35 a | 5.21 ± 0.09 a | 5.26 ± 0.43 ab | 5.44 ± 0.21 a | 4.59 ± 0.44 bc | 4.49 ± 0.06 b |
| Increase/Decrease Rate (%) | (00.00) | (00.00) | (38.29) | (26.07) | (32.41) | (31.72) | (15.62) | (8.64) |
| Treatments * | TAM0% (Control) | TAM0.25% | TAM0.5% | TAM1% | ||||
|---|---|---|---|---|---|---|---|---|
| Parameters | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 |
| Total Yield (kg m2) | 3.27 ± 0.05 c | 3.12 ± 0.03 b | 3.75 ± 0.53 b | 3.76 ± 0.03 a | 4.32 ± 0.09 a | 3.58 ± 0.03 a | 3.5 ± 0.02 bc | 3.56 ± 0.33 a |
| Increase/Decrease Rate (%) | (00.00) | (00.00) | (14.67) | (17.50) | (32.22) | (23.20) | (7.30) | (14.00) |
| Fruit Length (cm) | 11.82 ± 0.32 b | 11.97 ± 0.07 a | 12.21 ± 025 ab | 12.19 ± 0.27 a | 12.60 ± 0.09 a | 15.68 ± 5.09 a | 11.88 ± 0.38 b | 12.03 ± 0.05 a |
| Increase/Decrease Rate (%) | (00.00) | (00.00) | (3.36) | (1.87) | (6.63) | (31.06) | (0.56) | (0.56) |
| Fruit Diameter (cm) | 1.16 ± 0.04 b | 1.11 ± 0.10 a | 1.23 ± 0.02 a | 1.17 ± 0.13 a | 1.24 ± 0.03 a | 1.27 ± 0.02 a | 1.17 ± 0.03 b | 1.11 ± 0.10 a |
| Increase/Decrease Rate (%) | (00.00) | (00.00) | (6.03) | (5.41) | (6.90) | (14.41) | (0.86) | (0.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashour, M.; Hassan, S.M.; Elshobary, M.E.; Ammar, G.A.G.; Gaber, A.; Alsanie, W.F.; Mansour, A.T.; El-Shenody, R. Impact of Commercial Seaweed Liquid Extract (TAM®) Biostimulant and Its Bioactive Molecules on Growth and Antioxidant Activities of Hot Pepper (Capsicum annuum). Plants 2021, 10, 1045. https://doi.org/10.3390/plants10061045
Ashour M, Hassan SM, Elshobary ME, Ammar GAG, Gaber A, Alsanie WF, Mansour AT, El-Shenody R. Impact of Commercial Seaweed Liquid Extract (TAM®) Biostimulant and Its Bioactive Molecules on Growth and Antioxidant Activities of Hot Pepper (Capsicum annuum). Plants. 2021; 10(6):1045. https://doi.org/10.3390/plants10061045
Chicago/Turabian StyleAshour, Mohamed, Shimaa M. Hassan, Mostafa E. Elshobary, Gamal A. G. Ammar, Ahmed Gaber, Walaa F. Alsanie, Abdallah Tageldein Mansour, and Rania El-Shenody. 2021. "Impact of Commercial Seaweed Liquid Extract (TAM®) Biostimulant and Its Bioactive Molecules on Growth and Antioxidant Activities of Hot Pepper (Capsicum annuum)" Plants 10, no. 6: 1045. https://doi.org/10.3390/plants10061045
APA StyleAshour, M., Hassan, S. M., Elshobary, M. E., Ammar, G. A. G., Gaber, A., Alsanie, W. F., Mansour, A. T., & El-Shenody, R. (2021). Impact of Commercial Seaweed Liquid Extract (TAM®) Biostimulant and Its Bioactive Molecules on Growth and Antioxidant Activities of Hot Pepper (Capsicum annuum). Plants, 10(6), 1045. https://doi.org/10.3390/plants10061045












