Phytochemical Analysis of Anvillea garcinii Leaves: Identification of Garcinamines F–H and Their Antiproliferative Activities
Abstract
1. Introduction
2. Results and Discussion
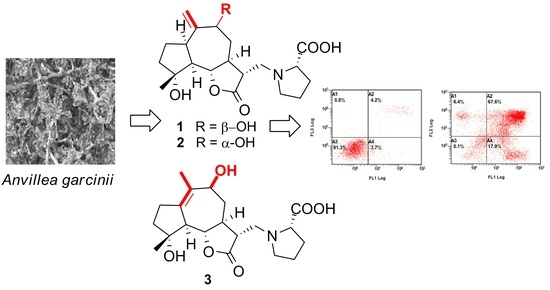
2.1. Structural Elucidation of Garcinamines F–H (1–3)
2.2. Biological Activity
3. Materials and Methods
3.1. General
3.2. Plant Material, Extraction and Isolation
3.3. Biological Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Center, M.M.; DeSantis, C.; Ward, E.M. Global Patterns of Cancer Incidence and Mortality Rates and Trends. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1893–1907. [Google Scholar] [CrossRef] [PubMed]
- Jazieh, A.R.; Da’ar, O.B.; Alkaiyat, M.; Zaatreh, Y.A.; Saad, A.A.; Bustami, R.; Alrujaib, M.; Alkattan, K. Cancer incidence trends from 1999 to 2015 and contributions of various cancer types to the overall burden: Projections to 2030 and extrapolation of economic burden in Saudi Arabia. Cancer Manag. Res. 2019, 11, 9665–9674. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Chen, S.S.; Wang, X.; Wu, J.M. Regulation of androgen receptor (AR) and prostate specific antigen (PSA) expres-sion in the androgen-responsive human prostate LNCaP cells by ethanolic extracts of the Chinese herbal preparation, PC-SPES. IUBMB Life 1997, 42, 535–544. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Boni, C.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Bonetti, A.; Clingan, P.; Bridgewater, J.; Rivera, F.; et al. Improved Overall Survival With Oxaliplatin, Fluorouracil, and Leucovorin As Adjuvant Treatment in Stage II or III Colon Cancer in the MOSAIC Trial. J. Clin. Oncol. 2009, 27, 3109–3116. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Luo, X.; Zhang, B.; Song, W.-X.; Ni, M.; Mehendale, S.; Xie, J.-T.; Aung, H.H.; He, T.-C.; Yuan, C.-S. Notoginseng enhances anti-cancer effect of 5-fluorouracil on human colorectal cancer cells. Cancer Chemother. Pharmacol. 2007, 60, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-C.; Ho, C.-T.; Pan, M.-H. Recent advances in cancer chemoprevention with phytochemicals. J. Food Drug Anal. 2020, 28, 14–37. [Google Scholar] [CrossRef] [PubMed]
- Aati, H.; El-Gamal, A.; Shaheen, H.; Kayser, O. Traditional use of ethnomedicinal native plants in the Kingdom of Saudi Arabia. J. Ethnobiol. Ethnomed. 2019, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tyson, R.L.; Chang, C.J.; McLaughlin, J.L.; Cassady, J.M. A novel sesquiterpene lactone from Anvillea garcinii (Burm.). J. Nat. Prod. 1979, 42, 680–681. [Google Scholar]
- Ulubelen, A.; Mabry, T.J.; Aynehchi, Y. Flavonoids of Anvillea garcinii. J. Nat. Prod. 1980, 42, 624–626. [Google Scholar] [CrossRef]
- Perveen, S.; Al-Taweel, A.M.; Yusufoglu, H.S.; Fawzy, G.A.; Foudah, A.; Abdel-Kader, M.S. Hepatoprotective and cytotoxic activities of Anvillea garcinii and isolation of four new secondary metabolites. J. Nat. Med. 2017, 72, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S.; Fawzi, G.A.; Al-Taweel, A.M.; Orfali, R.S.; Yusufoglu, H.S.; Abdel-Kader, M.S.; Al-Sabbagh, R.M. Antiulcer activ-ity of different extract of Anvillea garcinii and isolation of two new secondary metabolites. Open Chem. 2018, 16, 437–445. [Google Scholar] [CrossRef]
- Perveen, S.; Alqahtani, J.; Orfali, R.; Al-Taweel, A.M.; Yusufoglu, H.S.; Abdel-Kader, M.S.; Taglialatela-Scafati, O. Antimicro-bial guaianolide sesquiterpenoids from leaves of the Saudi Arabian plant Anvillea garcinii. Fitoterapia 2019, 134, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S.; Alqahtani, J.; Orfali, R.; Aati, H.Y.; Al-Taweel, A.M.; Ibrahim, T.A.; Khan, A.; Yusufoglu, H.S.; Abdel-Kader, M.S.; Taglialatela-Scafati, O. Antibacterial and Antifungal Sesquiterpenoids from Aerial Parts of Anvillea garcinii. Molecules 2020, 25, 1730. [Google Scholar] [CrossRef] [PubMed]
- Akram, G.A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.A.; Darwiche, N. Sesquiterpene lactones isolated from indigenous Middle Eastern plants inhibit tumor promoter-induced transformation of JB6 cells. Drug Discov. Today 2010, 15, 668–678. [Google Scholar]
- González, A.G.; Bermejo, J.; Breton, J.L.; Massanet, G.M.; Domínguez, B.; Amaro, J.M. The chemistry of the Compositae. Part XXX1. Absolute configuration of the sesquiterpene lactones centaurepensin (chlorohyssopifolin A), acroptilin (chlorohys-sopifolin C), and repin. J. Chem. Soc. Perkin Trans. I 1976, 115, 1663–1666. [Google Scholar] [CrossRef]
- El Bouakher, A.; Jismy, B.; Allouchi, H.; Duverger, E.; Barkaoui, L.; El Hakmaoui, A.; Daniellou, R.; Guillaumet, G.; Akssira, M. Synthetic Modification of 9α- and 9β-Hydroxyparthenolide by Heck or Acylation Reactions and Evaluation of Cytotoxic Activities. Planta Med. 2016, 83, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids Lactones: Benefits to Plants and People. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Hatakeyama, S.; Inoue, Y.; Yamahara, J. Saussureamines A, B, C, D, and E, new anti-ulcer principles from Chinese Saussureae Radix. Chem. Pharm. Bull. 1993, 41, 214–216. [Google Scholar] [CrossRef] [PubMed]

 ) NMR correlations of garcinamines F (1) and H (3).
) NMR correlations of garcinamines F (1) and H (3).



| Positions | Garcinamine F (1) | Garcinamine G (2) | Garcinamine H (3) |
|---|---|---|---|
| δH, type | δH, type | δH, type | |
| 1 | 35.4, CH | 35.4, CH | 136.2, C |
| 2 | 26.0, CH2 | 25.6, CH2 | 33.0, CH2 |
| 3 | 41.4, CH2 | 41.4, CH2 | 39.2, CH2 |
| 4 | 79.5, C | 79.6, C | 80.3, C |
| 5 | 55.1, CH | 54.9, CH | 56.6, CH |
| 6 | 84.1, CH | 84.6, CH | 83.3, CH |
| 7 | 43.0, CH | 41.4, CH | 47.0, CH |
| 8 | 39.5, CH2 | 38.5, CH2 | 35.7, CH2 |
| 9 | 74.6, CH | 73.2, CH | 71.4, CH |
| 10 | 152.6, C | 150.8, C | 131.3, C |
| 11 | 41.8, CH | 41.6, CH | 41.6, CH |
| 12 | 177.0, C | 177.1, C | 177.0, C |
| 13 | 53.0, CH2 | 53.1, CH2 | 52.5, CH2 |
| 14 | 108.5, CH2 | 111.1, CH2 | 15.0, CH3 |
| 15 | 22.5, CH3 | 22.5, CH3 | 21.8, CH3 |
| 1′ | 171.9, C | 171.9, C | 172.0, C |
| 2′ | 70.8, CH | 70.6, CH | 70.6, CH |
| 3′ | 28.6, CH2 | 28.6, CH2 | 28.6, CH2 |
| 4′ | 23.5, CH2 | 23.4, CH2 | 23.4, CH2 |
| 5′ | 54.1, CH2 | 54.1, CH2 | 54.2, CH2 |
| Positions | Garcinamine F (1) | Garcinamine G (2) | Garcinamine H (3) |
|---|---|---|---|
| δH (multiplicity, J in Hz) | δH (multiplicity, J in Hz) | δH (multiplicity, J in Hz) | |
| 1 | 3.04 (dd, 4.0, 11.0) | 3.56 m | - |
| 2a | 1.92 (m) | 1.90 (m) | 2.12 (m) |
| 2b | 1.83, overlapped | 1.78 (m) | 1.70 (m) |
| 3 | 1.83–1.85 (m) | 1.82 (m) | 1.76 (m) |
| 5 | 2.30 (t, 11.0) | 2.28 (t, 11.0) | 2.70 (d, 10.5) |
| 6 | 4.23 (d, 11.0) | 4.26 (d, 11.0) | 4.02 (bd, 10.5) |
| 7 | 2.30 (m) | 2.52 (m) | 2.31 (dd, 3.0, 13.0) |
| 8α | 2.39 (dd, 4.0, 9.0) | 2.24 (m) | 2.06 (d, 11.5) |
| 8β | 1.42 (m) | 1.65 (m) | 1.65 (d, 11.5) |
| 9 | 3.99 (dd, 4.0, 7.0) | 4.56 (bs) | 4.23 (bs) |
| 11 | 3.0 (m) | 3.07 (dd, 4.0, 11.5) | 3.13 (dd, 3.5, 12.5) |
| 13a | 3.63 (dd, 11.0, 12.5) | 3.60 (dd, 11.0, 12.5) | 3.65 (dd, 10.5, 12.5) |
| 13b | 3.53 (dd, 4.0, 12.5) | 3.45 (dd, 4.0, 12.5) | 3.57 (dd, 3.5, 10.5) |
| 14a | 5.51 (bs) | 5.15 (bs) | 1.77 (s) |
| 14b | 5.17 (bs) | 5.05 (bs) | - |
| 15 | 1.33 (s) | 1.30 (s) | 1.36 (s) |
| 2′ | 4.02 (dd, 5.0, 9.5) | 4.02 (dd, 5.0, 9.5) | 4.06 (dd, 6.0, 10.0) |
| 3′a | 2.46 (dd, 3.5, 9.5) | 2.45 (dd, 3.5, 9.5) | 2.46 (dd, 3.5, 10.0) |
| 3′b | 2.26 (dd, 5.0, 3.5) | 2.24 (dd, 5.0, 3.5) | 2.26 (m) |
| 4′a | 2.17 (m) | 2.15 (m) | 2.17 (m) |
| 4′b | 1.98 (m) | 1.97 (m) | 1.99 (m) |
| 5′a | 3.86 (dd, 4.0, 7.5) | 3.85 (dd, 4.0, 7.5) | 3.88 (dd, 4.0, 7.5) |
| 5′b | 3.25 (dd, 7.5, 10.5) | 3.24 (dd, 7.5, 10.5) | 3.27 (dd, 7.5, 10.5) |
| Compound | Cell Lines and IC50 (µM) | ||
|---|---|---|---|
| A549 | LoVo | MCF-7 | |
| 1 | NA | NA | NA |
| 2 | NA | NA | NA |
| 3 | NA | NA | NA |
| 4 | NA | NA | NA |
| 5 | 83.7 ± 0.3 | 121.4 ± 3.2 | 75.0 ± 5.3 |
| 6 | NA | NA | NA |
| 7 | 31.5 ± 3.1 | 39.2 ± 3.0 | 36.0 ± 3.4 |
| 8 | 75.8 ± 1.4 | 38.8 ± 2.1 | 71.6 ± 5.2 |
| Doxorubicin | 0.98 ± 0.02 | 5.5 ± 0.5 | 2.3 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aati, H.Y.; Perveen, S.; Orfali, R.; Al-Taweel, A.M.; Peng, J.; Tabassum, S.; Abdel-Kader, M.S.; Yusufoglu, H.S.; Taglialatela-Scafati, O. Phytochemical Analysis of Anvillea garcinii Leaves: Identification of Garcinamines F–H and Their Antiproliferative Activities. Plants 2021, 10, 1130. https://doi.org/10.3390/plants10061130
Aati HY, Perveen S, Orfali R, Al-Taweel AM, Peng J, Tabassum S, Abdel-Kader MS, Yusufoglu HS, Taglialatela-Scafati O. Phytochemical Analysis of Anvillea garcinii Leaves: Identification of Garcinamines F–H and Their Antiproliferative Activities. Plants. 2021; 10(6):1130. https://doi.org/10.3390/plants10061130
Chicago/Turabian StyleAati, Hanan Y., Shagufta Perveen, Raha Orfali, Areej M. Al-Taweel, Jiangnan Peng, Sobia Tabassum, Maged S. Abdel-Kader, Hasan Soliman Yusufoglu, and Orazio Taglialatela-Scafati. 2021. "Phytochemical Analysis of Anvillea garcinii Leaves: Identification of Garcinamines F–H and Their Antiproliferative Activities" Plants 10, no. 6: 1130. https://doi.org/10.3390/plants10061130
APA StyleAati, H. Y., Perveen, S., Orfali, R., Al-Taweel, A. M., Peng, J., Tabassum, S., Abdel-Kader, M. S., Yusufoglu, H. S., & Taglialatela-Scafati, O. (2021). Phytochemical Analysis of Anvillea garcinii Leaves: Identification of Garcinamines F–H and Their Antiproliferative Activities. Plants, 10(6), 1130. https://doi.org/10.3390/plants10061130