Cathodic Water Enhances Seedling Emergence and Growth of Controlled Deteriorated Orthodox Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Acquisition of Plant Materials
2.2. Preparation of Cathodic Water
2.3. Seed Priming, Plant Management and Data Collection
2.3.1. Emergence
2.3.2. Leaf Chlorophyll Content and Chlorophyll Fluorescence
2.3.3. Photosynthetic Capacity—Steady-State Gas Exchange
2.3.4. Harvesting and Post-Harvest Data Collection
2.4. Determination of MDA and 4HNE Contents in Seeds
2.5. Determination of Seeds Membrane Stability Index
2.6. Statistical Analysis
3. Results
3.1. Effect of Cathodic Water on Seedling Emergence
3.2. Effect of Priming on Seedling Growth
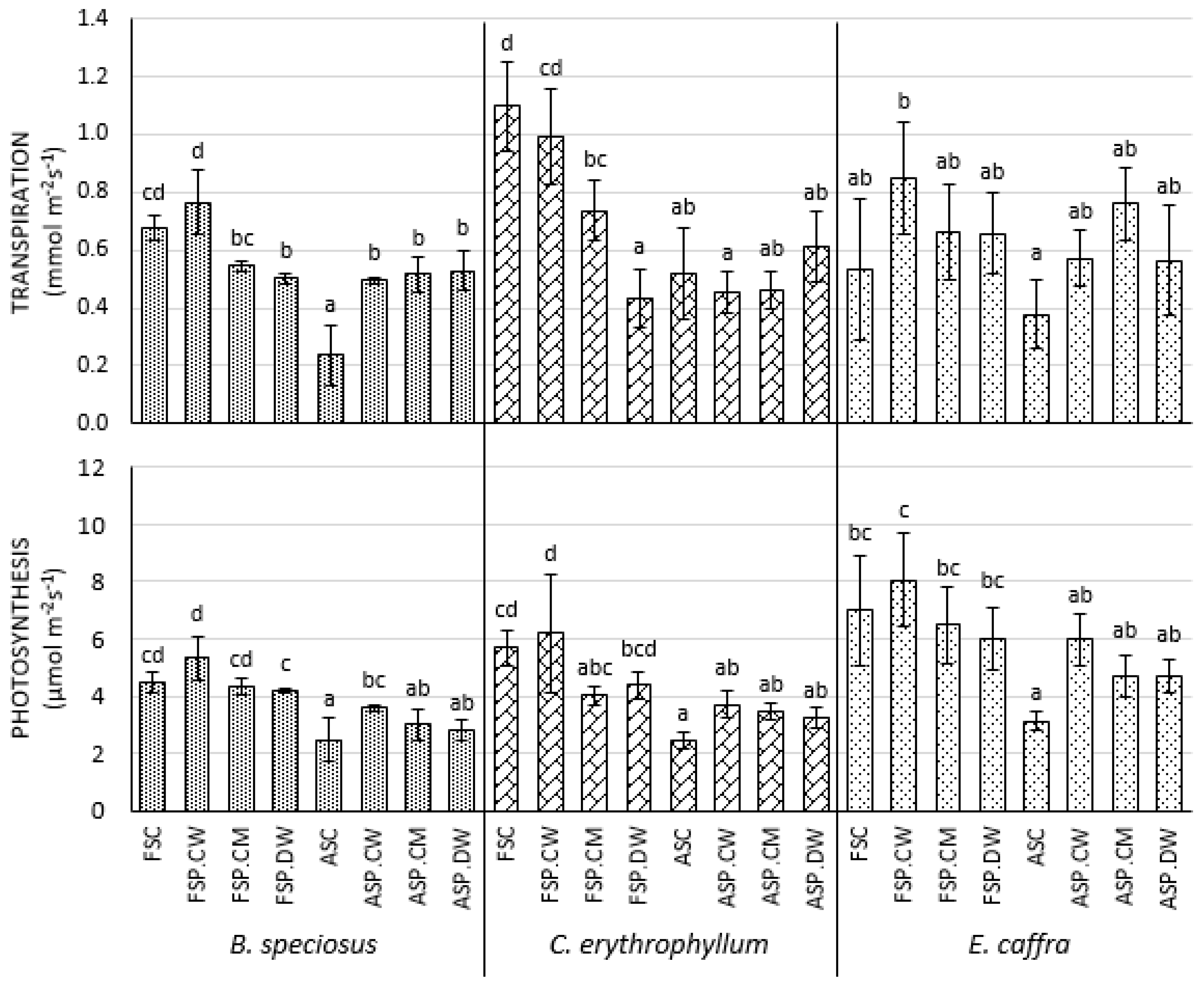
3.3. Effect of Priming on Photosynthetic Parameters
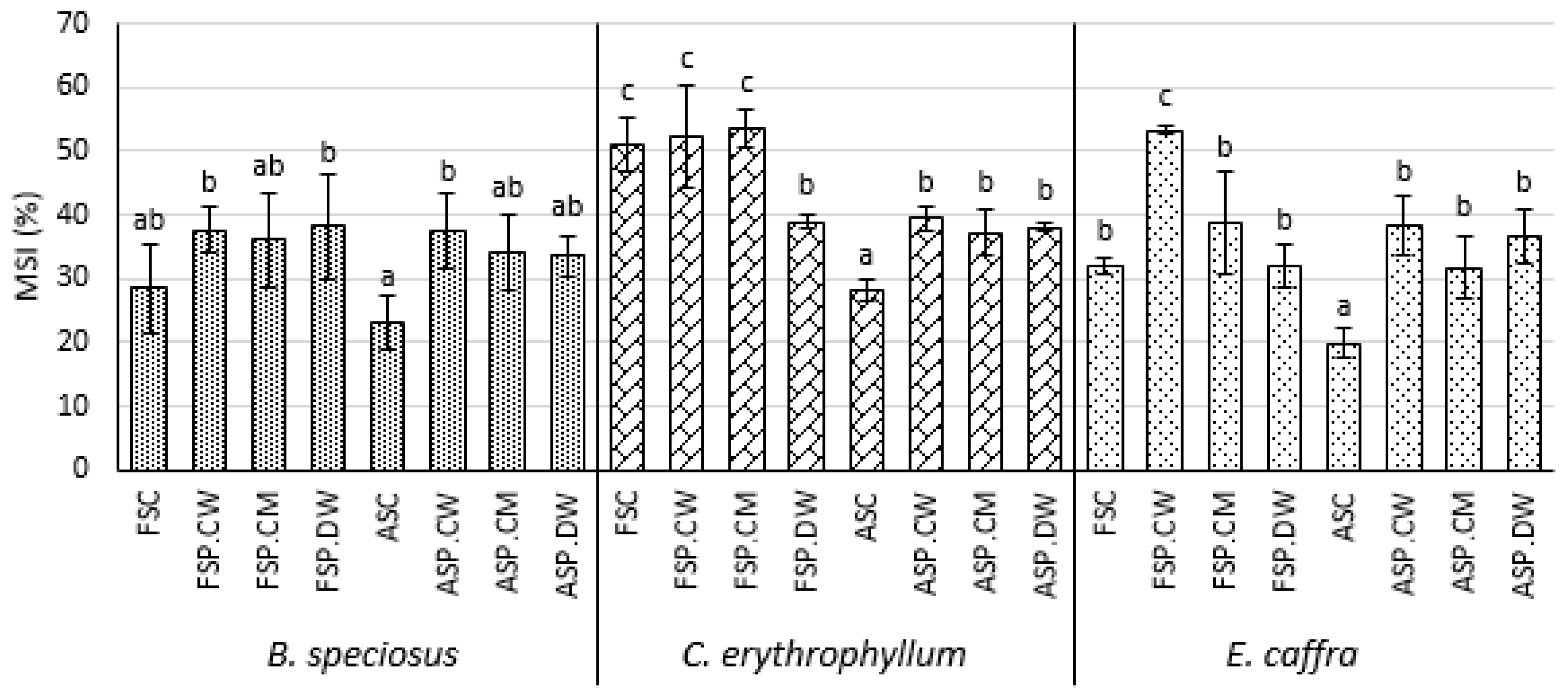
3.4. Effect of Priming on Membrane Stability and the Levels of Oxidized Lipids
4. Discussion
4.1. Effects of Priming on Emergence
4.2. Effect of Priming on Seedling Growth
4.3. Effects of Priming on Photosynthetic Parameters
4.4. Effects on Membrane Leakage and Lipid Peroxidation Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farooq, M.; Usman, M.; Nadeem, F.; Rehman, H.U.; Wahid, A.; Basra, S.M.A.; Siddique, K.H.M. Seed priming in field crops: Potential benefits, adoption and challenges. Crop. Pasture Sci. 2019, 70, 731–771. [Google Scholar] [CrossRef]
- Sreepriya, S.; Girija, T. Seed invigoration- a technique for improving vigour and productivity of sesame (Sesamum indicum L.) variety Thilak. J. Trop. Agric. 2019, 57, 10. [Google Scholar]
- Sahu, B.; Sahu, A.K.; Thomas, V.; Naithani, S.C. Reactive oxygen species, lipid peroxidation, protein oxidation and anti-oxidative enzymes in dehydrating Karanj (Pongamia pinnata) seeds during storage. S. Afr. J. Bot. 2017, 112, 383–390. [Google Scholar] [CrossRef]
- Barrera, G.; Pizzimenti, S.; Daga, M.; Dianzani, C.; Arcaro, A.; Cetrangolo, G.P.; Giordano, G.; Cucci, M.A.; Graf, M.; Gentile, F. Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehyde in Aging-Related Disorders. Antioxidants 2018, 7, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berjak, P.; Pammenter, N.W. From Avicennia to Zizania: Seed Recalcitrance in Perspective. Ann. Bot. 2007, 101, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Ardebili, Z.M.; Abbaspour, H.; Afshari, R.T.; Kalat, S.M.N. Evaluation of Germination and Antioxidant Activity in GA3-Primed Deteriorated Wheat Seed. Russ. J. Plant Physiol. 2019, 66, 958–965. [Google Scholar] [CrossRef]
- Smith, M.T.; Berjak, P. Deteriorative changes associated with the loss of viability of stored desiccation tolerant and desiccation sensitive seeds. In Seed Development and Germination; Kigel, J., Galili, G., Eds.; Marcel Dekker: New York, NY, USA, 1995; pp. 701–746. [Google Scholar]
- Finchsavage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.G.; Allen, P.S.; Bennett, M.A.; Bradford, K.J.; Burris, J.S.; Misra, M.K. Seed enhancements. Seed Sci. Res. 1998, 8, 245–256. [Google Scholar] [CrossRef]
- Basra, S.; Afzal, I.; Anwar, S.; Anwar-ul-Haq, M.; Shafiq, M.; Majeed, K. Alleviation of salinity stress by seed invigoration techniques in wheat (Triticum aestivum). Seed Sci. Technol. 2006, 28, 36–46. [Google Scholar]
- Farooq, M.; Basra, S.M.A.; Ahmad, N.; Hafeez, K. Thermal Hardening: A New Seed Vigor Enhancement Tool in Rice. J. Integr. Plant Biol. 2005, 47, 187–193. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Ahmad, N. Improving the performance of transplanted rice by seed priming. Plant Growth Regul. 2007, 51, 129–137. [Google Scholar] [CrossRef]
- Song, W.J.; Hu, J.; Qiu, J.; Geng, H.Y.; Wang, R.M. Primary study on the development of special seed coating agents and their application in rice (Oryza saliva L.) cultivated by direct seeding. J. Zhejiang Univ. 2005, 31, 368–373. [Google Scholar]
- Ella, E.S.; Dionisio-Sese, M.L.; Ismail, A.M. Seed pre-treatment in rice reduces damage, enhances carbohydrate mobilization and improves emergence and seedling establishment under flooded conditions. AoB PLANTS 2011, 2011, plr007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heydecker, W.; Coolbear, P. Seed treatments for improved performance-survey and attempted prognosis. Seed Sci. Technol. 1977, 5, 353–425. [Google Scholar]
- McDonald, M.B. Seed priming. In Seed Technology and Its Biological Basis; Black, M., Bewley, J.D., Eds.; Sheffield Academic Press: Sheffield, UK, 2000; pp. 287–325. [Google Scholar]
- McDonald, M.B. Orthodox seed deterioration and its repair. In Handbook of Seed Physiology: Applications to Agriculture; Benech-Arnold, R.L., Sanchez, R.A., Eds.; Food Products Press: New York, NY, USA, 2004; pp. 273–304. [Google Scholar]
- Eskandari, H.; Kazemi, K. Effect of Seed Priming on Germination Properties and Seedling Establishment of Cowpea (Vigna sinensis). Not. Sci. Biol. 2011, 3, 113–116. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.; Basra, S.M.A.; Rehman, H.; Saleem, B.A. Seed Priming Enhances the Performance of Late Sown Wheat (Triticum aestivum L.) by Improving Chilling Tolerance. J. Agron. Crop. Sci. 2008, 194, 55–60. [Google Scholar] [CrossRef]
- Arun, M.; Hebbar, S.S.; Bhanuprakas, K.; Senthivel, T. Seed priming improves irrigation water use efficiency, yield and yield components of summer cowpea under limited water conditions. Legum. Res. Int. J. 2017, 40, 864–871. [Google Scholar] [CrossRef]
- Berjak, P.; Sershen; Varghese, B.; Pammenter, N. Cathodic amelioration of the adverse effects of oxidative stress accompanying procedures necessary for cryopreservation of embryonic axes of recalcitrant-seeded species. Seed Sci. Res. 2011, 21, 187–203. [Google Scholar] [CrossRef]
- Fatokun, K.; Beckett, R.P.; Varghese, B.; Pammenter, N.W. Germination indices of orthodox seeds as influ-enced by controlled deterioration and cathodic water seed invigoration. J. Environ. Biol. 2020, 41, 1105–1111. [Google Scholar] [CrossRef]
- Gondwe, D.; Berjak, P.; Pammenter, N.; Sershen; Varghese, B. Effect of priming with cathodic water and subsequent storage on invigoration of Pisum sativum, Cucurbita maxima and Lycopersicon esculentum seeds. Seed Sci. Technol. 2016, 44, 370–381. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Seeds Physiology of Development and Germination (The Language of Science); Plenum Press: New York, NY, USA, 1994; p. 230. [Google Scholar]
- Bhattacharya, S.; Guha, P.; Mandal, A.K. Deteriorative changes in enzyme activity of non-invigorated and invigorated soybean seeds (Glycine max [L.] Merrill, cv. Soyamax). Legum. Res. Int. J. 2018, 42, 633–639. [Google Scholar] [CrossRef] [Green Version]
- Mycock, D.J. Addition of calcium and magnesium to a glycerol and sucrose cryoprotectant solution improves the quality of plant embryo recovery from cryostorage. Cryo Lett. 1999, 20, 77–82. [Google Scholar]
- Ellis, R.A.; Roberts, E.R. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Czabator, F.J. Germination value: An index combining speed and completeness of pine seed germination. For. Sci. 1962, 8, 386–396. [Google Scholar]
- Abdollahi, M.; Eshghi, S.; Tafazzoli, E.; Moosavi, N. Effects of paclobutrazol, boric acid and zinc sulfate on vegetative and reproductive growth of strawberry cv. Selva. J. Agric. Sci. Technol. 2012, 14, 357–363. [Google Scholar]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Ray, S.; Roy, K.; Sengupta, C. Evaluation of protective effects of water extract of Spirulina platensis on cisplatin-induced lipid peroxidation. Indian J. Pharm. Sci. 2007, 69, 378–383. [Google Scholar]
- Yuan, L.; Zhu, S.; Li, S.; Shu, S.; Sun, J.; Guo, S. 24-Epibrassinolide regulates carbohydrate metabolism and increases polyamine content in cucumber exposed to Ca NO3)2 stress. Acta Physiol. Plant. 2014, 36, 2845–2852. [Google Scholar] [CrossRef]
- Wang, W.; He, A.; Peng, S.; Huang, J.; Cui, K.; Nie, L. The Effect of Storage Condition and Duration on the Deterioration of Primed Rice Seeds. Front. Plant Sci. 2018, 9, 172. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farshbaf-Jafari, S.; Shafagh-Kolvanagh, J. Seed Priming and Field Performance of Soybean (Glycine max L.) in Response to Water Limitation. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 186–189. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.; Basra, S.; Khalid, M.; Tabassum, R.; Mahmood, T. Nutrient homeostasis, metabolism of reserves, and seedling vigor as affected by seed priming in coarse rice. Can. J. Bot. 2006, 84, 1196–1202. [Google Scholar] [CrossRef]
- Andreev, I.O.; Spiridonova, E.V.; Kunakh, V.A.; Solov’Yan, V.T. Aging and Loss of Germination in Rye Seeds Is Accompanied by a Decreased Fragmentation of Nuclear DNA at Loop Domain Boundaries. Russ. J. Plant Physiol. 2004, 51, 241–248. [Google Scholar] [CrossRef]
- Kranner, I.; Chen, H.; Birtiæ, S.; Pritchard, H. Mechanisms of viability loss in dry orthodox seeds and during imbibition. S. Afr. J. Bot. 2007, 73, 489. [Google Scholar] [CrossRef] [Green Version]
- Pawar, V.A.; Laware, S.L. Seed priming: A critical review. Int. J. Sci. Res. Biol. Sci. 2018, 31, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Anwar, A.; Xianchang, Y.U.; Yansu, L.I. Seed priming as a promising technique to improve growth, chlorophyll, photosynthesis and nutrient contents in cucumber seedlings. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Osmond, B.; Badger, M.; Maxwell, K.; Björkman, O.; Leegood, R. Too many photons: Photorespiration, photoinhibition and photooxidation. Trends Plant Sci. 1997, 2, 119–121. [Google Scholar] [CrossRef]
- Simon, E.W. Phospholipids and plant membrane permeability. New Phytol. 1974, 73, 377–420. [Google Scholar] [CrossRef]
- Lutts, S.; Benincasa, P.; Wojtyla, L.; Kubala, S.; Pace, R.; Lechowska, K.; Garnczarska, M. Seed priming: New comprehensive approaches for an old empirical technique. In New Challenges in Seed Biology: Basic and Translational Research Driving Seed Technology; Araújo, S., Balestrazzi, A., Eds.; IntechOpen: Rijeka, Croatia, 2016; pp. 1–46. [Google Scholar]
- Kubala, S.; Wojtyla, L.; Garnczarska, M. Seed priming improves salt stress tolerance during germination by modulation of antioxidative capacity. BioTechnologia 2013, 94, 223. [Google Scholar]
- Kubala, S.; Garnczarska, M.; Wojtyla, Ł.; Clippe, A.; Kosmala, A.; Żmieńko, A.; Lutts, S.; Quinet, M. Deciphering priming-induced improvement of rapeseed (Brassica napus L.) germination through an integrated transcriptomic and proteomic approach. Plant Sci. 2015, 231, 94–113. [Google Scholar] [CrossRef] [Green Version]
- Kibinza, S.; Bazin, J.; Bailly, C.; Farrant, J.M.; Corbineau, F.; El-Maarouf-Bouteau, H. Catalase is a key enzyme in seed recovery from ageing during priming. Plant Sci. 2011, 181, 309–315. [Google Scholar] [CrossRef]
- Fallah, S.; Malekzadeh, S.; Pessarakli, M.; Fallah, S. Seed Priming Improves Seedling Emergence and Reduces Oxidative Stress in Nigella Sativa under Soil Moisture Stress. J. Plant Nutr. 2017, 41, 29–40. [Google Scholar] [CrossRef]
- Bharuth, V.; Naidoo, C. Responses to cryopreservation of recalcitrant seeds of Ekebergia capensis from different provenances. S. Afr. J. Bot. 2020, 132, 1–14. [Google Scholar] [CrossRef]
- Pammenter, N.W.; Adamson, J.H.; Berjak, P. Viability of Stored Seed: Extension by Cathodic Protection. Science 1974, 186, 1123–1124. [Google Scholar] [CrossRef] [PubMed]
- Berjak, P. Viability extension and improvement of stored seeds. S. Afr. J. Sci. 1978, 74, 365–368. [Google Scholar]
- Naidoo, S. Studies on Factors Influencing Viability after Cryopreservation of Excised Zygotic Embryos from Recalcitrant Seeds of Two Amaryllid Species. Ph.D. Dissertation, University of KwaZulu-Natal, Durban, South Africa, 2010. [Google Scholar]
- Gebashe, F.C. Studies on the Cryopreservation of Shoot Apices from Recalcitrant-Seeded Trichilia emetica Vahl. and Trichilia dregeana Sond. Ph.D. Dissertation, University of KwaZulu Natal, Durban, South Africa, 2015. [Google Scholar]
- Zhang, H.; Mittal, N.; Leamy, L.J.; Barazani, O.; Song, B.-H. Back into the wild-Apply untapped genetic diversity of wild relatives for crop improvement. Evol. Appl. 2016, 10, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Jorgenson, A.K.; Burns, T.J. Effects of Rural and Urban Population Dynamics and National Development on Deforestation in Less-Developed Countries, 1990–2000. Sociol. Inq. 2007, 77, 460–482. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2018. Building Climate Resilience for Food Security and Nutrition; FAO: Rome, Italy, 2018. [Google Scholar]


| Emergence | FSC | FSP.CW | FSP.CM | FSP.DW | ASC | ASP.CW | ASP.CM | ASP.DW | LSD | |
|---|---|---|---|---|---|---|---|---|---|---|
| First day of emergence | B. speciosus | 6.0 ± 0.8 abc | 4.5 ± 0.5 a | 5.0 ± 0.6 ab | 7.0 ± 0.6 abcd | 9.5 ± 1.0 d | 7.0 ± 1.0 abcd | 8.0 ± 0.8 bcd | 8.5 ± 0.5 cd | 2.2 ± 1.05 |
| Emergence% | 85.0 ± 5.0 cd | 100.0 ± 0.0 d | 90.0 ± 5.8 d | 90.0 ± 5.8 d | 30.0 ± 5.8 a | 60.0 ± 8.2 bc | 50.0 ± 5.8 ab | 50.0 ± 5.8 ab | 16.6 ± 8.04 | |
| Mean emergence time | 3.3 ± 0.1 c | 4.6 ± 0.041 d | 3.6 ± 0.2 c | 3.3 ± 0.2 c | 0.7 ± 0.2 a | 2.3 ± 0.3 b | 1.5 ± 0.3 ab | 1.6 ± 0.2 ab | 0.6 ± 0.30 | |
| Emergence index | 1.6 ± 0.1 d | 2.6 ± 0.1 e | 1.7 ± 0.2 d | 1.4 ± 0.1 cd | 0.3 ± 0.1 a | 1.0 ± 0.1 bc | 0.6 ± 0.1 ab | 0.6 ± 0.1 ab | 0.3 ± 0.16 | |
| Uniformity of emergence | 0.023 ± 0.001 d | 0.037 ± 0.001 e | 0.024 ± 0.001 d | 0.021 ± 0.002 cd | 0.010 ± 0.001 a | 0.016 ± 0.001 bc | 0.012 ± 0.002 ab | 0.012 ± 0.001 ab | 0.004 ± 0.002 | |
| First day of emergence | C. erythrophyllum | 12.5 ± 0.5 a | 11.5 ± 0.3 a | 12.8 ± 0.6 a | 11.8 ± 0.5 a | 15.8 ± 1.1 b | 13.0 ± 0.6 ab | 13.0 ± 0.4 ab | 12.8 ± 0.6 a | 1.8 ± 0.88 |
| Emergence% | 60.0 ± 8.2 bc | 70.0 ± 5.8 c | 60.0 ± 8.2 bc | 45.0 ± 9.6 abc | 25.0 ± 5.0 a | 45.0 ± 5.0 abc | 35.0 ± 5.0 ab | 30.0 ± 5.8 ab | 19.6 ± 9.57 | |
| Mean emergence time | 2.3 ± 0.3 bc | 2.8 ± 0.3 c | 2.5 ± 0.4 bc | 1.9 ± 0.5 abc | 0.7 ± 0.229 a | 1.5 ± 0.2 abc | 1.3 ± 0.2 abc | 1.2 ± 0.3 ab | 0.9 ± 0.43 | |
| Emergence index | 1.2 ± 0.2 bc | 1.5 ± 0.2 c | 1.3 ± 0.2 bc | 1.1 ± 0.3 abc | 0.3 ± 0.111 a | 0.7 ± 0.095 abc | 0.7 ± 0.128 abc | 0.6 ± 0.144 ab | 0.5 ± 0.25 | |
| Uniformity of emergence | 0.005 ± 0.0002 b | 0.006 ± 0.0003 b | 0.005 ± 0.0004 b | 0.005 ± 0.0005 b | 0.004 ± 0.0003 a | 0.004 ± 0.0002 ab | 0.004 ± 0.0002 ab | 0.005 ± 0.0002 ab | 0.001 ± 0.0004 | |
| First day of emergence | E. caffra | 5.3 ± 0.3 ab | 4.5 ± 0.3 a | 4.8 ± 0.3 ab | 4.8 ± 0.3 ab | 6.8 ± 0.3 c | 5.8 ± 0.3 bc | 6.5 ± 0.3 c | 6.8 ± 0.3 c | 0.8 ± 0.37 |
| Emergence% | 100.0 ± 0.0 b | 100.0 ± 0.0 b | 100.0 ± 0.0 b | 100.0 ± 0.0 b | 40.0 ± 0.0 a | 50.0 ± 5.8 a | 45.0 ± 5.0 a | 50.0 ± 5.8 a | 9.9 ± 4.79 | |
| Mean emergence time | 3.6 ± 0.1 b | 4.6 ± 0.1 c | 4.2 ± 0.2 bc | 4.1 ± 0.1 bc | 1.0 ± 0.074 a | 1.5 ± 0.1 a | 1.4 ± 0.2 a | 1.3 ± 0.2 a | 0.4 ± 019 | |
| Emergence index | 2.5 ± 0.1 b | 4.1 ± 0.2 d | 3.3 ± 0.3 c | 3.2 ± 0.2 bc | 0.6 ± 0.060 a | 1.0 ± 0.092 a | 0.9 ± 0.139 a | 0.8 ± 0.119 a | 0.4 ± 0.22 | |
| Uniformity of emergence | 0.059 ± 0.004 b | 0.136 ± 0.012 d | 0.095 ± 0.013 c | 0.086 ± 0.007 bc | 0.020 ± 0.001 a | 0.025 ± 0.001 a | 0.023 ± 0.002 a | 0.022 ± 0.002 a | 0.02 ± 0.01 |
| Treatment | Bolusanthus speciosus | Combretum erythrophyllum | Erythrina caffra | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root Length (cm) | Stem Length (cm) | Number of Leaves | Leaf Area (cm2) | Root Length (cm) | Stem Length (cm) | Number of Leaves (cm) | Leaf Area (cm2) | Root Length (cm) | Stem Length (cm) | Number of Leaves | Leaf Area (cm2) | |
| FSC | 34.3 ± 0.6 d | 13.0 ± 0.8 c | 13.0 ± 0.7 bc | 58.3 ± 4.2 bc | 36.0 ± 1.1 bcd | 16.5 ± 1.7 c | 15.0 ± 1.1 ab | 59.0 ± 1.1 d | 25.8 ± 1.1 c | 17.5 ± 1.0 cde | 12.8 ± 0.5 b | 716.2 ± 15.4 d |
| FSP.CW | 33 ± 1.1 cd | 12.8 ± 0.3 c | 16.0 ± 0.7 d | 112.4 ± 5.3 e | 41.3 ± 0.3 d | 21.5 ± 1.3 d | 22.5 ± 0.6 c | 78.8 ± 0.6 f | 28.5 ± 0.6 cd | 20.9 ± 1.4 e | 16.0 ± 0.4 c | 779.4 ± 17.2 d |
| FSP.CM | 33 ± 0.7 cd | 14.0 ± 0.7 c | 12.0 ± 0.4 bc | 72.6 ± 4.2 cd | 32.8 ± 1.0 bc | 16.6 ± 0.7 c | 16.5 ± 0.6 b | 77.9 ± 2.0 f | 24.8 ± 0.5 c | 17.0 ± 0.7 cd | 13.8 ± 0.3 bc | 739.8 ± 26.0 d |
| FSP.DW | 36.5 ± 2 d | 14.3 ± 0.2 c | 13.8 ± 0.6 cd | 74.1 ± 2 d | 36.3 ± 1.4 cd | 12.5 ± 0.6 bc | 15.3 ± 0.3 ab | 69.3 ± 1.4 e | 30.5 ± 1.0 d | 19.3 ± 0.6 de | 15.0 ± 0.4 bc | 736.2 ± 14.6 d |
| ASC | 18.3 ± 1.1 a | 7.5 ± 0.2 a | 8.5 ± 0.6 a | 21.2 ± 1.3 a | 18.5 ± 0.6 a | 7.2 ± 0.2 a | 11.5 ± 0.6 a | 20.5 ± 0.5 a | 11.9 ± 0.9 a | 9.9 ± 0.4 a | 7.3 ± 0.5 a | 86.3 ± 0.6 a |
| ASP.CW | 31.3 ± 1.3 bcd | 12.5 ± 1.2 c | 11.5 ± 0.6 bc | 58.0 ± 1.8 bc | 30.8 ± 0.5 b | 12.1 ± 1.1 bc | 14.3 ± 0.8 ab | 45.9 ± 1.7 c | 26.3 ± 0.6 c | 14.3 ± 0.5 bc | 13.5 ± 0.6 b | 642.7 ± 9.8 c |
| ASP.CM | 28.5 ± 1.2 bc | 9.0 ± 0.9 ab | 10.5 ± 0.3 ab | 31.0 ± 2.1 a | 21.9 ± 2.2 a | 11.4 ± 0.7 ab | 14.2 ± 0.8 ab | 27.0 ± 1.7 b | 18.8 ± 1.0 b | 12.6 ± 0.6 ab | 8.5 ± 0.6 a | 122.7 ± 1.2 a |
| ASP.DW | 25.7 ± 1.2 b | 12.0 ± 0.4 bc | 8 ± 0.4 a | 50.5 ± 2 b | 23.9 ± 1.1 a | 8.5 ± 0.6 ab | 13.5 ± 1.4 ab | 21.4 ± 1.3 ab | 18.5 ± 1.0 b | 12.0 ± 0.5 ab | 9.5 ± 0.6 a | 214.1 ± 17.1 b |
| LSD0.05 | 3.6 ± 1.7 | 2.0 ± 1.0 | 1.7 ± 0.8 | 9.3 ± 4.5 | 3.4 ± 1.6 | 2.9 ± 1.4 | 2.4 ± 1.2 | 4.0 ± 2.0 | 2.5 ± 1.2 | 2.3 ± 1.1 | 1.5 ± 0.7 | 43.9 ± 21.3 |
| Dry Mass (g Plant−1) | FSC | FSP.CW | FSP.CM | FSP.DW | ASC | ASP.CW | ASP.CM | ASP.DW | LSD0.05 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Root mass | B. speciosus | 0.12 ± 0.004 cd | 0.20 ± 0.013 e | 0.15 ± 0.013 de | 0.18 ± 0.015 e | 0.04 ± 0.004 a | 0.12 ± 0.009 bcd | 0.07 ± 0.008 ab | 0.10 ± 0.011 bc | 0.03 ± 0.01 |
| Stem mass | 0.13 ± 0.008 b | 0.20 ± 0.003 c | 0.13 ± 0.004 b | 0.12 ± 0.030 b | 0.05 ± 0.001 a | 0.11 ± 0.004 b | 0.09 ± 0.002 ab | 0.10 ± 0.002 ab | 0.03 ± 0.02 | |
| Leaf mass | 0.19 ± 0.006 b | 0.28 ± 0.016 c | 0.31 ± 0.007 c | 0.30 ± 0.036 c | 0.10 ± 0.004 a | 0.19 ± 0.007 b | 0.11 ± 0.003 a | 0.16 ± 0.006 ab | 0.04 ± 0.02 | |
| Shoot mass | 0.32 ± 0.012 cd | 0.48 ± 0.014 e | 0.44 ± 0.009 e | 0.42 ± 0.066 de | 0.16 ± 0.004 a | 0.30 ± 0.009 bc | 0.20 ± 0.003 ab | 0.26 ± 0.004 abc | 0.07 ± 0.03 | |
| Total biomass | 0.45 ± 0.014 c | 0.68 ± 0.023 d | 0.59 ± 0.019 d | 0.59 ± 0.068 d | 0.20 ± 0.007 a | 0.41 ± 0.011 c | 0.27 ± 0.009 ab | 0.37 ± 0.012 bc | 0.08 ± 0.04 | |
| Shoot/root ratio | 2.70 ± 0.091 a | 2.44 ± 0.158 a | 2.93 ± 0.198 a | 2.38 ± 0.371 a | 3.60 ± 0.318 a | 2.60 ± 0.260 a | 2.91 ± 0.357 a | 2.69 ± 0.298 a | 0.80 ± 0.39 | |
| Root mass | C. erythrophyllum | 0.34 ± 0.035 c | 0.68 ± 0.008 e | 0.53 ± 0.018 d | 0.33 ± 0.031 c | 0.08 ± 0.009 a | 0.25 ± 0.012 c | 0.16 ± 0.009 ab | 0.16 ± 0.004 b | 0.05 ± 0.03 |
| Stem mass | 0.20 ± 0.025 b | 0.51 ± 0.041 d | 0.39 ± 0.018 c | 0.25 ± 0.019 b | 0.06 ± 0.004 a | 0.19 ± 0.006 b | 0.09 ± 0.006 a | 0.05 ± 0.003 a | 0.06 ± 0.03 | |
| Leaf mass | 0.35 ± 0.018 b | 0.65 ± 0.02 c | 0.65 ± 0.016 c | 0.37 ± 0.016 b | 0.067 ± 0.004 a | 0.29 ± 0.032 b | 0.13 ± 0.011 a | 0.12 ± 0.009 a | 0.05 ± 0.02 | |
| Shoot mass | 0.55 ± 0.027 bc | 1.16 ± 0.036 e | 1.04 ± 0.022 d | 0.62 ± 0.015 c | 0.13 ± 0.007 a | 0.48 ± 0.034 b | 0.23 ± 0.016 a | 0.18 ± 0.008 a | 0.07 ± 0.03 | |
| Total biomass | 0.90 ± 0.061 cd | 1.84 ± 0.043 f | 1.57 ± 0.036 e | 0.96 ± 0.039 d | 0.20 ± 0.009 a | 0.74 ± 0.025 c | 0.38 ± 0.025 b | 0.34 ± 0.007 ab | 0.10 ± 0.05 | |
| Shoot/root ratio | 1.66 ± 0.096 ab | 1.70 ± 0.037 ab | 1.95 ± 0.048 b | 1.91 ± 0.177 b | 1.74 ± 0.328 ab | 1.93 ± 0.224 b | 1.45 ± 0.024 ab | 1.07 ± 0.070 a | 0.47 ± 0.23 | |
| Root mass | E. caffra | 0.87 ± 0.106 cd | 0.82 ± 0.029 bcd | 0.85 ± 0.072 cd | 1.08 ± 0.153 d | 0.26 ± 0.025 a | 0.73 ± 0.035 bcd | 0.55 ± 0.085 abc | 0.46 ± 0.058 ab | 0.24 ± 0.12 |
| Stem mass | 1.06 ± 0.074 bcd | 1.56 ± 0.149 d | 1.30 ± 0.181 cd | 1.37 ± 0.144 cd | 0.20 ± 0.038 a | 0.93 ± 0.170 bc | 0.27 ± 0.038 a | 0.56 ± 0.069 ab | 0.35 ± 0.17 | |
| Leaf mass | 2.02 ± 0.175 bc | 3.31 ± 0.202 d | 2.26 ± 0.163 bc | 2.70 ± 0.302 cd | 0.21 ± 0.041 a | 1.46 ± 0.247 a | 0.42 ± 0.064 b | 0.58 ± 0.075 a | 0.53 ± 0.26 | |
| Shoot mass | 3.08 ± 0.208 bc | 4.88 ± 0.306 d | 3.56 ± 0.308 bc | 4.07 ± 0.434 cd | 0.41 ± 0.068 a | 2.39 ± 0.292 b | 0.69 ± 0.101 a | 1.15 ± 0.140 a | 0.76 ± 0.37 | |
| Total biomass | 3.95 ± 0.225 bc | 5.70 ± 0.278 d | 4.41 ± 0.355 bcd | 5.15 ± 0.579 cd | 0.68 ± 0.089 a | 3.12 ± 0.274 b | 1.24 ± 0.159 a | 1.61 ± 0.197 a | 0.88 ± 0.43 | |
| Shoot/root ratio | 3.75 ± 0.596 c | 6.01 ± 0.594 d | 4.20 ± 0.293 cd | 3.83 ± 0.245 c | 1.57 ± 0.176 ab | 3.34 ± 0.524 bc | 1.32 ± 0.190 a | 2.50 ± 0.086 abc | 1.13 ± 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatokun, K.; Beckett, R.P.; Varghese, B.; Pammenter, N.W. Cathodic Water Enhances Seedling Emergence and Growth of Controlled Deteriorated Orthodox Seeds. Plants 2021, 10, 1170. https://doi.org/10.3390/plants10061170
Fatokun K, Beckett RP, Varghese B, Pammenter NW. Cathodic Water Enhances Seedling Emergence and Growth of Controlled Deteriorated Orthodox Seeds. Plants. 2021; 10(6):1170. https://doi.org/10.3390/plants10061170
Chicago/Turabian StyleFatokun, Kayode, Richard P. Beckett, Boby Varghese, and Norman W. Pammenter. 2021. "Cathodic Water Enhances Seedling Emergence and Growth of Controlled Deteriorated Orthodox Seeds" Plants 10, no. 6: 1170. https://doi.org/10.3390/plants10061170






