Selective BuChE Inhibitory Activity, Chemical Composition, and Enantiomeric Content of the Essential Oil from Salvia leucantha Cav. Collected in Ecuador
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical Properties of the EO
2.2. Chemical Composition of the EO
2.3. Enantiomeric Analysis of the EO
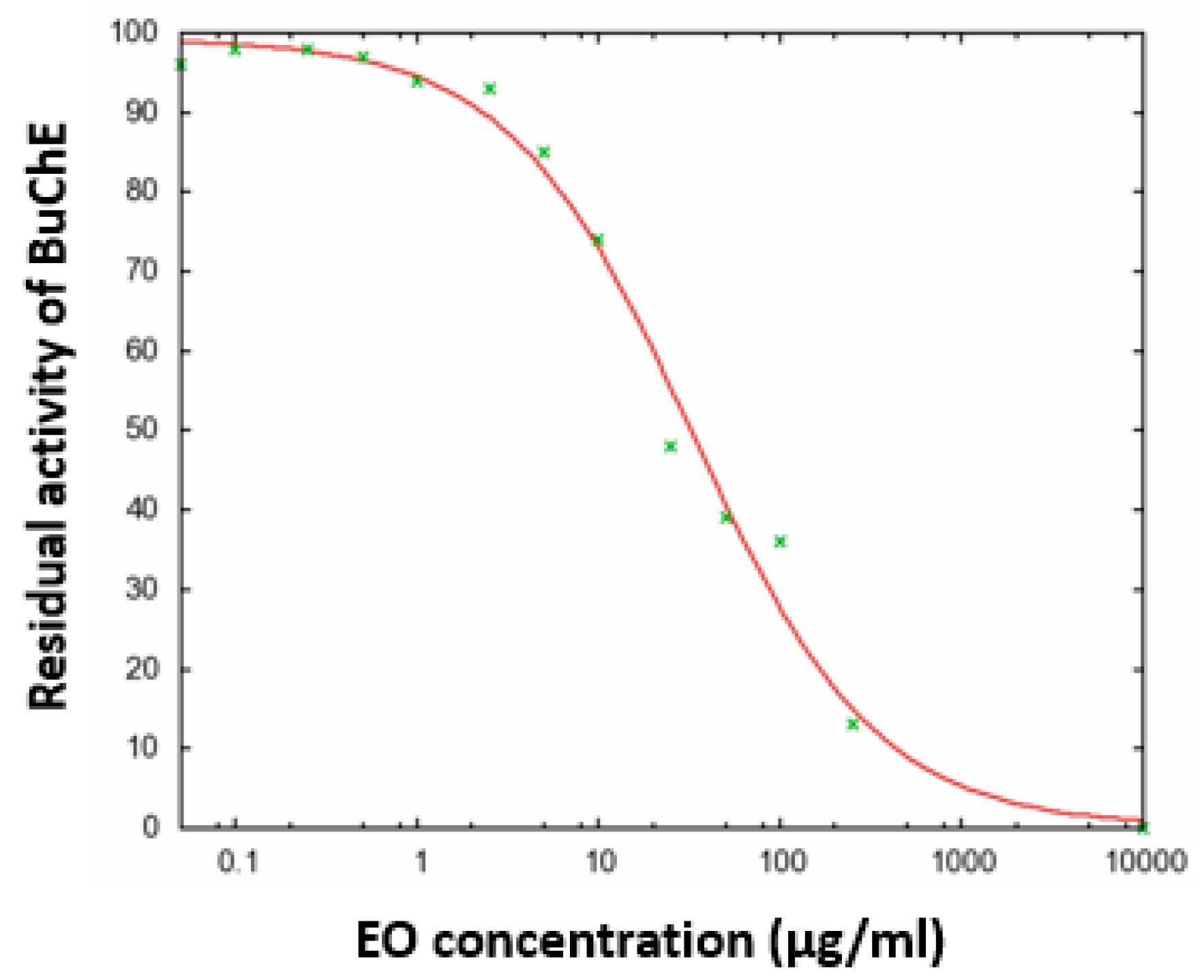
2.4. Cholinesterase (AChE and BuChE) Inhibitory Activity of the EO
3. Materials and Methods
3.1. Collection of Plant Material
3.2. Essential Oil Isolation
3.3. Physical Properties
3.4. Chemical Composition of the Essential Oil
3.4.1. Gas Chromatography Coupled to Mass Spectrometry (GC-MS)
3.4.2. Gas Chromatography Coupled to the Flame Ionization Detector (GC-FID)
3.5. Enantiomeric Analysis
3.6. Inhibition of Cholinesterase (AChE and BuChE)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Martínez, M.; Fragous, I.; Garcia, M.; Montiel, O. Géneros de Lamiaceae de México, diversidad y endemismo. Rev. Mex. Biodiv. 2013, 84, 30–86. [Google Scholar] [CrossRef] [Green Version]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Giovanelli, S.; Ruffoni, B.; D’Ascenzi, C.; Pistelli, L.; Mancianti, F. Activity of Salvia dolomitica and Salvia somalensis Essential oils against bacteria, molds and yeasts. Molecules 2018, 23, 396. [Google Scholar] [CrossRef] [Green Version]
- León, S.; Valencia, R.; Pitman, N.; Endara, L.; Ulloa, C.; Navarrete, H. Libro Rojo de las Plantas Endémicas del Ecuador, 2nd ed.; Herbario QCA, Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2011; p. 367. [Google Scholar]
- Carović, K.; Petek, M.; Grodyca, M.; Paint, J.; Bedekovic, D.; Herak, M.; Satovic, Z. Medicinal plants of the family Lamiaceae as functional foods—A review. Czech. J. Food Sci. 2016, 34, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Rojas, L.B.; Visbal, T.; Morillo, M.; de Rojas, Y.C.; Arzola, J.C.; Usubillaga, A. The volatile constituents of Salvia leucantha. Nat. Prod. Commun. 2010, 5, 937–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- INECOL. Cordón de Jesús. Available online: https://www.inecol.mx/inecol/index.php/es/ct-menu-item-25/planta-del-mes/37-planta-del-mes/602-cordon-de-jesus (accessed on 18 December 2020).
- Hosoki, T.; Tahara, Y. In vitro propagation of Salvia leucantha Cav. HortScience 1993, 28, 226. [Google Scholar] [CrossRef] [Green Version]
- Negi, A.; Javed, M.; Melkani, A.; Dev, V.; Beauchamp, P. Steam volatile terpenoids from Salvia leucantha. J. Essent. Oil Res. 2007, 19, 463–465. [Google Scholar] [CrossRef]
- Inderaja, B.; Pradhita, O.; Hanifah, R.; Manurung, R.; Abduh, M. Factors affecting biomass growth and production of essential oil from leaf and flower of Salvia leucantha Cav. J. Essen. Oil Bear. Plants 2018, 21, 1021–1029. [Google Scholar] [CrossRef]
- Skała, E.; Rijo, P.; Garcia, C.; Sitarek, P.; Kalemba, D.; Toma, M.; Szemraj, J.; Pytel, D.; Wysokińska, H.; Śliwiński, T. The essential oils of Rhaponticum carthamoides Hairy roots and roots of soil-grown plants: Chemical composition and antimicrobial, anti-inflammatory, and antioxidant activities. Oxid. Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Shaaban, H.A.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components. J. Essent. Oil Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.G. Plant essential oils as active antimicrobial agents. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential oils: Sources of antimicrobials and food preservatives. Front. Microbiol. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, T.C.; Gomes, T.; Pinto, B.; Camara, A.; Paes, A. Naturally occurring acetylcholinesterase inhibitors and their potential use for Alzheimer’s disease therapy. Front. Pharmacol. Res. 2018, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bartorelli, L.; Giraldi, C.; Saccardo, M.; Cammarata, S.; Bottini, G.; Fasanaro, A.; Trequattrini, A. Effects of switching from an AChE inhibitor to a dual AChE- BuChE inhibitor in patients with Alzheimer’ s disease. Curr. Med. Res. Opin. 2005, 21, 1809–1817. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatograpy/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Salinas, M.; Bec, N.; Calva, J.; Ramírez, J.; Andrade, J.; Vidari, G.; Larroque, C.; Armijos, C. Chemical composition and anticholinesterase activity of the essential oil from the Ecuadorian plant Salvia pichinchensis Benth. Rec. Nat. Prod. 2020, 14, 276–285. [Google Scholar] [CrossRef]
- Sandasi, M.; Kamatou, G.; Viljoen, A. Chemotaxonomic evidence suggests that Eriocephalus tenuifolius is the source of cape chamomile oil and not Eriocephalus punctulatus. Biochem. Syst. Ecol. 2011, 39, 328–338. [Google Scholar] [CrossRef]
- Saroglou, V.; Marin, P.D.; Rancic, A.; Veljic, M.; Skaltsa, H. Composition and antimicrobial activity of the essential oil of six Hypericum species from Serbia. Biochem. Syst. Ecol. 2007, 35, 146–152. [Google Scholar] [CrossRef]
- Bisio, A.; Ciarallo, G.; Romussi, G.; Fontana, N.; Mascolo, N.; Capasso, R.; Biscardi, D. Chemical composition of essential oils from some Salvia species. Phytother. Res. 1998, 12, S117–S120. [Google Scholar] [CrossRef]
- Valarezo, E.; Castillo, A.; Guaya, D.; Morocho, V.; Malagón, O. Chemical composition of essential oils of two species of the Lamiaceae family: Scutellaria volubilis and Lepechinia paniculata from Loja, Ecuador. J. Essent. Oil Res. 2012, 24, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Armijos, C.; Matailo, A.; Bec, N.; Salinas, M.; Aguilar, G.; Solano, N.; Calva, J.; Ludeña, C.; Larroque, C.; Vidari, G. Chemical composition and selective BuChE inhibitory activity of the essential oils from aromatic plants used to prepare the traditional Ecuadorian beverage horchata lojana. J. Ethnopharmacol. 2020, 263, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Demirci, B.; Başer, K.; Yildiz, B.; Bahçecioǧlu, Z. Composition of the essential oils of six endemic Salvia ssp. from Turkey. Flavour Fragr. J. 2003, 18, 116–121. [Google Scholar] [CrossRef]
- Paolini, J.; Muselli, A.; François, A.; Bighelli, A.; Casanova, J.; Costa, J. Thymol derivatives from essential oil of Doronicum corsicum L. Flavour Fragr. J. 2007, 22, 479–487. [Google Scholar] [CrossRef]
- Amiri, N.; Yadegari, M.; Hamedi, B. Essential oil composition of Cirsium arvense L. produced in different climate and soil properties. Rec. Nat. Prod. 2018, 12, 251–262. [Google Scholar] [CrossRef]
- Saïdana, D.; Mahjoub, S.; Boussaada, O.; Chriaa, J.; Mahjoub, M.; Chéraif, I.; Daami, M.; Mighri, Z.; Helal, A. Antibacterial and antifungal activities of the essential oils of two saltcedar species from Tunisia. J. Am. Oil Chem. Soc. 2008, 85, 817–826. [Google Scholar] [CrossRef]
- Van del Dool, H.; Kratz, P. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Upadhyaya, K.; Dixit, V.; Padalia, R.; Mathela, C. Terpenoid composition and antioxidant activity of essential oil from leaves of Salvia leucantha Cav. J. Essent. Oil-Bear Plants 2009, 12, 551–556. [Google Scholar] [CrossRef]
- Ali, A.; Tabanca, N.; Demirci, B.; Blythe, E.; Ali, Z.; Baser, K.; Khan, I. Chemical composition and biological activity of four Salvia essential oils and individual compounds against two species of mosquitoes. J. Agric. Food Chem. 2015, 63, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Castrillón, W.; Matulevich, J.; Rodríguez, J.; Silva, D. Composición química del aceite esencial de flores de Salvia leucantha Cav. (Lamiaceae). Rev. Fac. Cienc. Básicas 2019, 15, 41–47. [Google Scholar] [CrossRef]
- Oliveira, M.; Brugnera, D.; Cardoso, M.; Guimarães, L.; Piccoli, R. Rendimento, composição química e atividade antilisterial de óleos essenciais de espécies de Cymbopogon. Rev. Bras. Plantas Med. 2011, 13, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Al-Bayati, F.A. Synergistic antibacterial activity between Thymus vulgaris and Pimpinella anisum essential oils and methanol extracts. J. Ethnopharmacol. 2008, 116, 403–406. [Google Scholar] [CrossRef]
- Basha, R.H.; Sankaranarayanan, C. β-Caryophyllene, a natural sesquiterpene lactone attenuates hyperglycemia mediated oxidative and inflammatory stress in experimental diabetic rats. Chem. Biol. Interact. 2016, 245, 50–58. [Google Scholar] [CrossRef]
- Hernandez-Leon, A.; González-Trujano, M.E.; Narváez-González, F.; Pérez-Ortega, G.; Rivero-Cruz, F.; Aguilar, M.I. Role of β-caryophyllene in the antinociceptive and anti-Inflammatory effects of Tagetes lucida Cav. essential oil. Molecules 2020, 25, 675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fidyt, K.; Fiedorowicz, A.; Strzadala, L.; Szumny, A. β-Caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Prosser, I.; Altug, I.G.; Phillips, A.L.; König, W.A.; Bouwmeester, H.J.; Beale, M.H. Enantiospecific (+)- and (−)-germacrene D synthases, cloned from goldenrod, reveal a functionally active variant of the universal isoprenoid-biosynthesis aspartate-rich motif. Arch. Biochem. Biophys. 2004, 432, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Özek, G.; Özek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. Chemical composition and immunomodulatory activity of Hypericum perforatum essential oils. Biomolecules 2020, 10, 916. [Google Scholar] [CrossRef]
- Crock, J.; Wildung, M.; Croteau, R. Isolation and bacterial expression of a sesquiterpene synthase cDNA clone from peppermint (Mentha x piperita, L.) that produces the aphid alarm pheromone (E)-β-farnesene. Proc. Natl. Acad. Sci. USA 1997, 94, 12833–12838. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Qiao, H.; Ling, Y.; Yang, S.; Rui, C.; Pelosi, P.; Yang, X. New analogues of (E)-β-farnesene with insecticidal activity and binding affinity to aphid odorant-binding proteins. J. Agric. Food Chem. 2011, 59, 2456–2461. [Google Scholar] [CrossRef]
- Grecco, S.; Martins, E.G.; Girola, N.; de Figueiredo, C.R.; Matsuo, A.L.; Soares, M.G.; Bertoldo, B.; Sartorelli, P.; Lago, J.H. Chemical composition and in vitro cytotoxic effects of the essential oil from Nectandra leucantha leaves. Pharm. Biol. 2015, 53, 133–137. [Google Scholar] [CrossRef]
- Giuliani, C.; Pieraccini, G.; Santilli, C.; Tani, C.; Bottoni, M.; Schiff, S.; Fico, G.; Papini, A.; Falsini, S. Anatomical investigation and GC/MS analysis of ‘Coco de Mer’, Lodoicea maldivica (Arecaceae). Chem. Biodivers. 2020, 17, 1–23. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.Y.; Hong, C.Y.; Gwak, K.S.; Park, M.J.; Smith, D.; Choi, I.G. Whitening and antioxidant activities of bornyl acetate and nezukol fractionated from Cryptomeria japonica essential oil. Int. J. Cosmet. Sci. 2013, 35, 484–490. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, R.; Chen, H.; Jia, P.; Bao, L.; Tang, H. Bornyl acetate has an anti-inflammatory effect in human chondrocytes via induction of IL-11. IUBMB Life 2014, 66, 854–859. [Google Scholar] [CrossRef]
- Chen, N.; Sun, G.; Yuan, X.; Hou, J.; Wu, Q.; Soromou, L.W.; Feng, H. Inhibition of lung inflammatory responses by bornyl acetate is correlated with regulation of myeloperoxidase activity. J. Surg. Res. 2014, 186, 436–445. [Google Scholar] [CrossRef]
- Feng, Y.X.; Wang, Y.; Chen, Z.Y.; Guo, S.S.; You, C.X.; Du, S.S. Efficacy of bornyl acetate and camphene from Valeriana officinalis essential oil against two storage insects. Environ. Sci. Pollut. Res. Int. 2019, 26, 16157–16165. [Google Scholar] [CrossRef] [PubMed]
- Matailo, A.; Bec, N.; Calva, J.; Ramírez, J.; Andrade, J.M.; Larroque, C.; Vidari, G.; Armijos, C. Selective BuChE inhibitory activity, chemical composition, and enantiomer content of the volatile oil from the Ecuadorian plant Clinopodium brownei. Rev. Bras. Farmacogn. 2019, 29, 749–750. [Google Scholar] [CrossRef]
- Zeb, A.; Hameed, A.; Khan, L.; Khan, I.; Dalvandi, K.; Choudhary, M.I.; Basha, F.Z. Quinoxaline derivatives: Novel and selective butyrylcholinesterase inhibitors. Med. Chem. 2014, 10, 724–729. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Yu, Q.; Zhu, X.; Holloway, H.W.; Perry, T.; Lee, B.; Ingram, D.K.; Lahiri, D.K. A new therapeutic target in Alzheimer’s disease treatment: Attention to butyrylcholinesterase. Curr. Med. Res. Opin. 2001, 17, 159–165. [Google Scholar] [CrossRef]
- Ayaz, M.; Junaid, M.; Ullah, F.; Sadiq, A.; Khan, M.A.; Ahmad, W.; Shah, M.R.; Imran, M.; Ahmad, S. Comparative chemical profiling, cholinesterase inhibitions and anti-radicals properties of essential oils from Polygonum Hydropiper L: A preliminary anti-Alzheimer’s study. Lipids Health Dis. 2015, 14, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Rashed, A.A.; Rahman, A.Z.A.; Rathi, D.N.G. Essential oils as a potential neuroprotective remedy for age-related neurodegenerative diseases: A review. Molecules 2021, 26, 1107. [Google Scholar] [CrossRef] [PubMed]
- Savelev, S.U.; Okello, E.J.; Perry, E.K. Butyryl- and acetyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother. Res. 2004, 18, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Bosak, A.; Ramić, A.; Šmidlehner, T.; Hrenar, T.; Primožič, I.; Kovarik, Z. Design evaluation of selective butyrylcholinesterase inhibitors based on Cinchona alkaloid scaffold. PLoS ONE 2018, 13, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Rhee, I.K.; Van Rijn, R.M.; Verpoorte, R. Qualitative determination of false-positive effects in the acetylcholinesterase assay using thin layer chromatography. Phytochem. Anal. 2003, 14, 127–131. [Google Scholar] [CrossRef] [PubMed]
- McGleenon, B.; Dynan, K.; Passmore, A. Acetylcholinesterase inhibitors and Alzheimer’s disease. Br. J. Clin. Pharmacol. 1999, 48, 471–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Component | DB-5ms | HP-INNOWax | ||||||
|---|---|---|---|---|---|---|---|---|
| LRI a | LRI b [16] | % c | S.D d | LRI a | LRI b | % c | S.D d | |
| α-Pinene | 942 | 932 | 3.31 | 0.68 | 1055 | 1066 [17] | 3.70 | 0.31 |
| Camphene | 956 | 946 | 3.03 | 0.55 | 1079 | 1084 [17] | 3.33 | 0.27 |
| Sabinene | 975 | 969 | 0.48 | 0.07 | 1120 | 1117 [18] | 0.51 | 0.06 |
| β-Pinene | 979 | 974 | 2.01 | 0.50 | 1108 | 1104 [18] | 2.15 | 0.17 |
| Myrcene | 990 | 988 | 0.17 | 0.02 | - | - | - | - |
| Limonene | 1028 | 1024 | 0.35 | 0.07 | 1199 | 1199 [17] | 0.29 | 0.03 |
| γ-Terpinene | 1056 | 1054 | 0.13 | 0.02 | - | - | - | - |
| α-Phellandrene | - | - | - | - | 1162 | 1160 [19] | 0.15 | 0.01 |
| 3-Methyl-3-butenyl, 3-methyl- butanoate | 1112 | 1112 | 0.12 | 0.00 | - | - | - | - |
| Borneol | 1171 | 1165 | 0.14 | 0.04 | - | - | - | - |
| Terpinene-4-ol | 1178 | 1174 | 0.12 | 0.04 | - | - | - | - |
| Bornyl acetate | 1286 | 1284 | 14.74 | 0.31 | 1577 | 1580 [20] | 13.26 | 0.22 |
| Humulene | - | - | - | - | 1658 | 1660 [20] | 0.75 | 0.01 |
| δ-Elemene | 1333 | 1335 | 0.54 | 0.01 | 1466 | 1460 [20] | 1.30 | 0.18 |
| α-Copaene | 1372 | 1374 | 0.36 | 0.01 | 1483 | 1493 [18] | 0.35 | 0.00 |
| β-Bourbonene e | 1379 | 1387 | 0.35 | 0.00 | 1509 | 1519 [21] | 0.36 | 0.01 |
| β-Elemene | 1386 | 1389 | 0.42 | 0.01 | - | - | - | - |
| (E)-Caryophyllene | 1418 | 1417 | 16.80 | 0.16 | 1588 | 1590 [20] | 17.56 | 0.20 |
| β-Copaene | 1425 | 1430 | 0.14 | 0.01 | - | - | - | - |
| Aromadendrene | 1433 | 1439 | 0.35 | 0.00 | 1613 | 1613 [22] | 1.74 | 0.01 |
| 6,9-Guaiadiene | 1442 | 1442 | 19.14 | 0.29 | 1600 | 1617 [23] | 17.8 | 0.17 |
| Allo-aromadendrene | 1447 | 1458 | 1.88 | 0.04 | 1633 | 1633 [17] | 0.64 | 0.01 |
| (E)-β-Farnesene | 1455 | 1454 | 10.00 | 0.47 | 1670 | 1665 [24] | 9.11 | 0.16 |
| Germacrene D | 1481 | 1480 | 10.22 | 0.11 | 1699 | 1700 [20] | 12.5 | 0.20 |
| trans-Cadina-1(6).4-diene | 1482 | 1475 | 0.53 | 0.00 | - | - | - | - |
| β-Selinene | 1485 | 1489 | 0.29 | 0.16 | 1706 | 1708 [24] | 0.38 | 0.04 |
| Bicyclogermacrene | 1494 | 1500 | 7.52 | 0.07 | 1724 | 1723 [17] | 6.23 | 0.04 |
| n-Pentadecane | 1500 | 1500 | 0.16 | 0.01 | 1500 | 1500 [25] | 0.26 | 0.01 |
| γ-Cadinene | 1509 | 1513 | 1.07 | 0.02 | 1750 | 1750 [17] | 0.47 | 0.01 |
| δ-Amorphene | 1511 | 1511 | 1.31 | 0.05 | - | - | - | - |
| Furopelargone A e | 1527 | 1538 | 0.39 | 0.02 | - | - | - | - |
| Germacrene B | 1553 | 1559 | 0.41 | 0.03 | 1815 | 1811 [26] | 0.24 | 0.02 |
| Spatulenol | 1573 | 1577 | 0.55 | 0.00 | 2118 | 2118 [17] | 1.90 | 0.07 |
| Cubeban-11-ol e | 1577 | 1595 | 0.62 | 0.01 | - | - | - | - |
| Cadinol | 1638 | 1638 | 0.14 | 0.00 | - | - | - | - |
| Mint sulfide e | 1730 | 1740 | 0.29 | 0.06 | - | - | - | - |
| Sclarene | 1977 | 1974 | 1.05 | 0.15 | - | - | - | - |
| Caryophyllene oxide | - | - | - | - | 1967 | 1967 [17] | 0.87 | 0.01 |
| α-Cadinol | - | - | - | - | 2229 | 2220 [21] | 0.22 | 0.01 |
| trans-α-Bergamotol | - | - | - | - | 2249 | 2247 [23] | 1.98 | 0.19 |
| Monoterpene hydrocarbons (%) | 9.48 | 10.13 | ||||||
| Oxygenated monoterpenes (%) | 15.12 | 13.26 | ||||||
| Sesquiterpene hydrocarbons (%) | 71.49 | 69.69 | ||||||
| Oxygenated sesquiterpenes (%) | 1.70 | 4.97 | ||||||
| Others (%) | 1.34 | - | ||||||
| Total identified (%) | 99.13 | 98.05 | ||||||
| Enantiomer | LRI a | Enantiomeric Distribution (%) | ee%b |
|---|---|---|---|
| (+)-α-Pinene | 926 | 4.32 | |
| 91.36 | |||
| (−)-α-Pinene | 932 | 95.68 | |
| (+)-Sabinene | 973 | 29.98 | |
| 40.04 | |||
| (−)-Sabinene | 975 | 70.02 | |
| (+)-Aromadendrene | 1426 | 19.15 | |
| 61.70 | |||
| (−)-Aromadendrene | 1440 | 80.85 | |
| (+)-Germacrene D | 1475 | 1.65 | |
| 96.70 | |||
| (−)-Germacrene D | 1489 | 98.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalta, G.; Salinas, M.; Calva, J.; Bec, N.; Larroque, C.; Vidari, G.; Armijos, C. Selective BuChE Inhibitory Activity, Chemical Composition, and Enantiomeric Content of the Essential Oil from Salvia leucantha Cav. Collected in Ecuador. Plants 2021, 10, 1169. https://doi.org/10.3390/plants10061169
Villalta G, Salinas M, Calva J, Bec N, Larroque C, Vidari G, Armijos C. Selective BuChE Inhibitory Activity, Chemical Composition, and Enantiomeric Content of the Essential Oil from Salvia leucantha Cav. Collected in Ecuador. Plants. 2021; 10(6):1169. https://doi.org/10.3390/plants10061169
Chicago/Turabian StyleVillalta, Gabriela, Melissa Salinas, James Calva, Nicole Bec, Christian Larroque, Giovanni Vidari, and Chabaco Armijos. 2021. "Selective BuChE Inhibitory Activity, Chemical Composition, and Enantiomeric Content of the Essential Oil from Salvia leucantha Cav. Collected in Ecuador" Plants 10, no. 6: 1169. https://doi.org/10.3390/plants10061169







