Comparative Study on Phytochemical Profile and Antioxidant Activity of an Epiphyte, Viscum album L. (White Berry Mistletoe), Derived from Different Host Trees
Abstract
1. Introduction
2. Materials and Methods
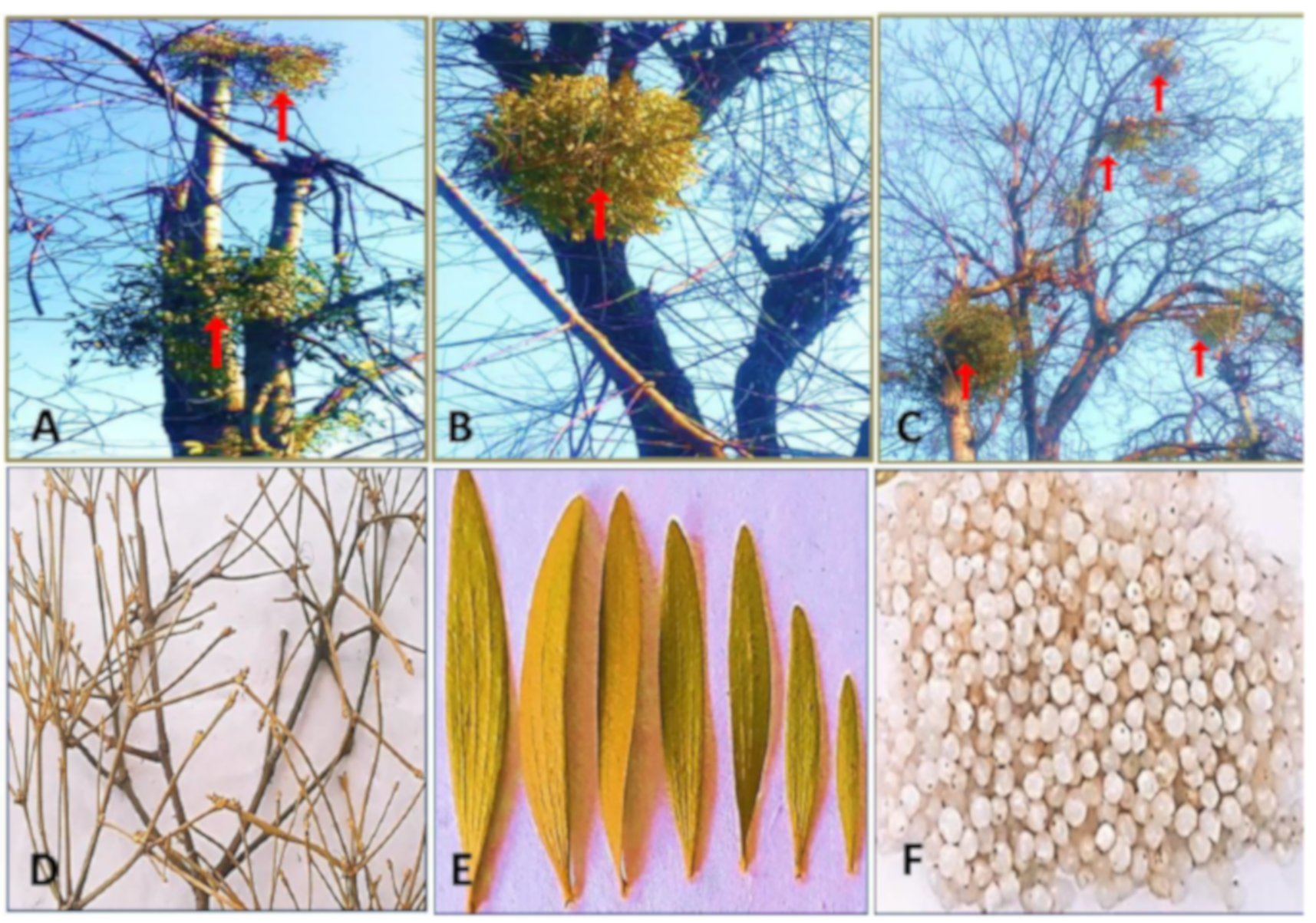
2.1. Plant Material Collection and Processing
2.2. Extraction Procedure
2.3. Phytochemical Profiling
2.3.1. Total Phenolic Content (TPC)
2.3.2. Total Flavonoid Content (TFC)
2.3.3. Antioxidant Activity based on the Total Reducing Power (TRP), Ferric Reducing Antioxidant Power(FRAP), 1, 1-diphenyl 1-2-picryl-hydrazyl (DPPH), Superoxide Radical Scavenging (SOR), and Hydroxyl Radical Scavenging (OH-) Assays
2.4. Statistical Analysis
3. Results
3.1. Effect of Host Plants on the Phytochemical Content
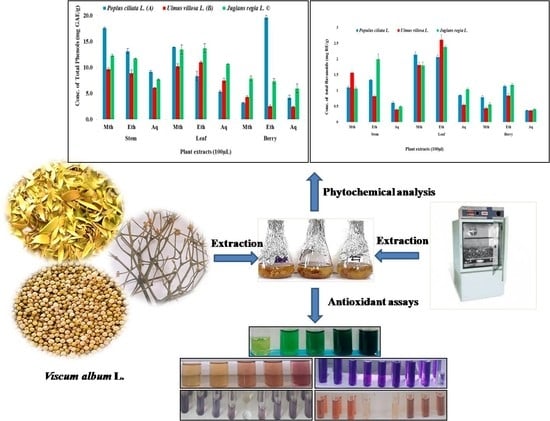
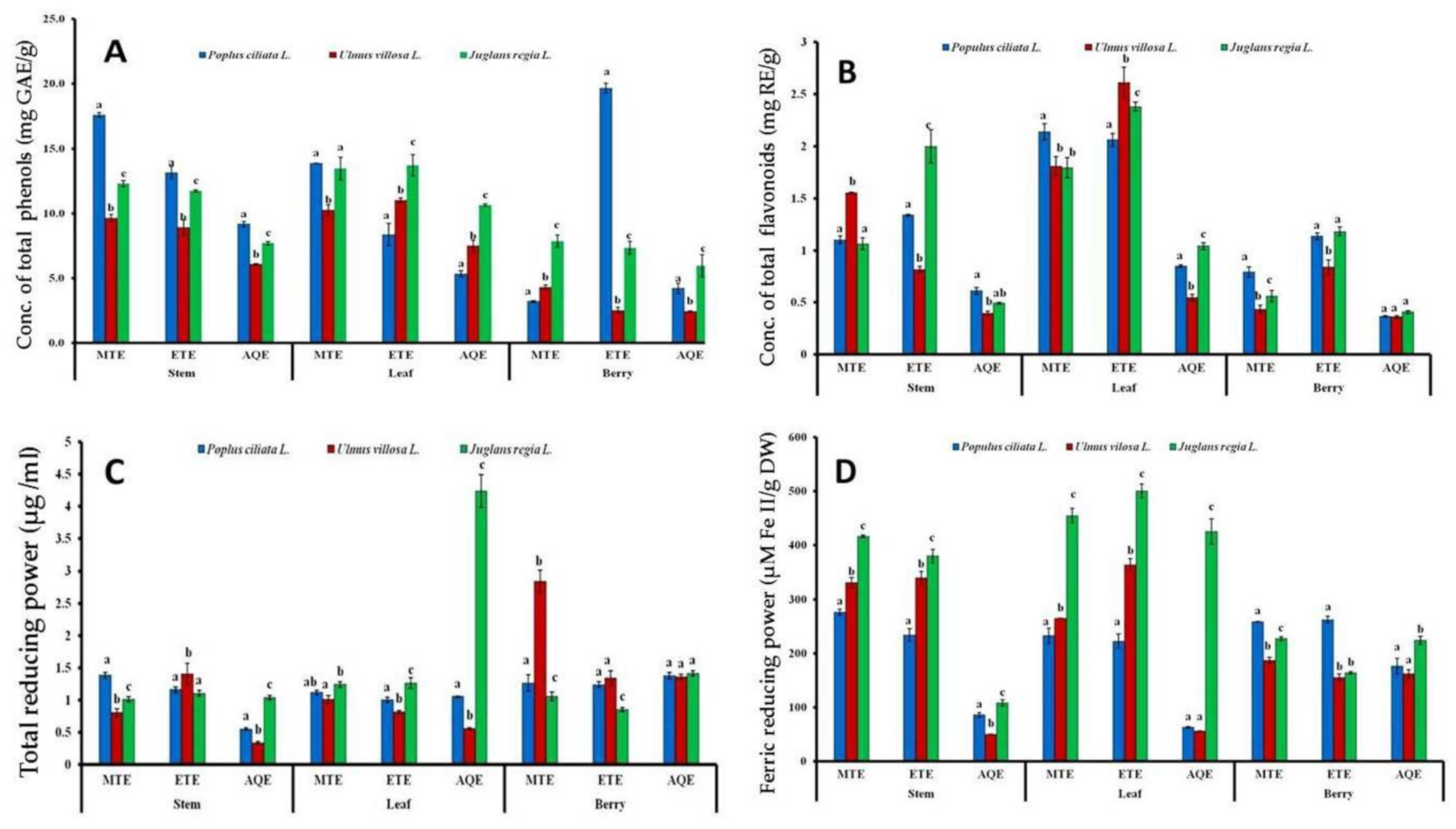
3.1.1. Total Phenolic Content (TPC)
3.1.2. Total Flavonoid Content (TFC)
3.1.3. Total Reducing Power (TRP) Assay
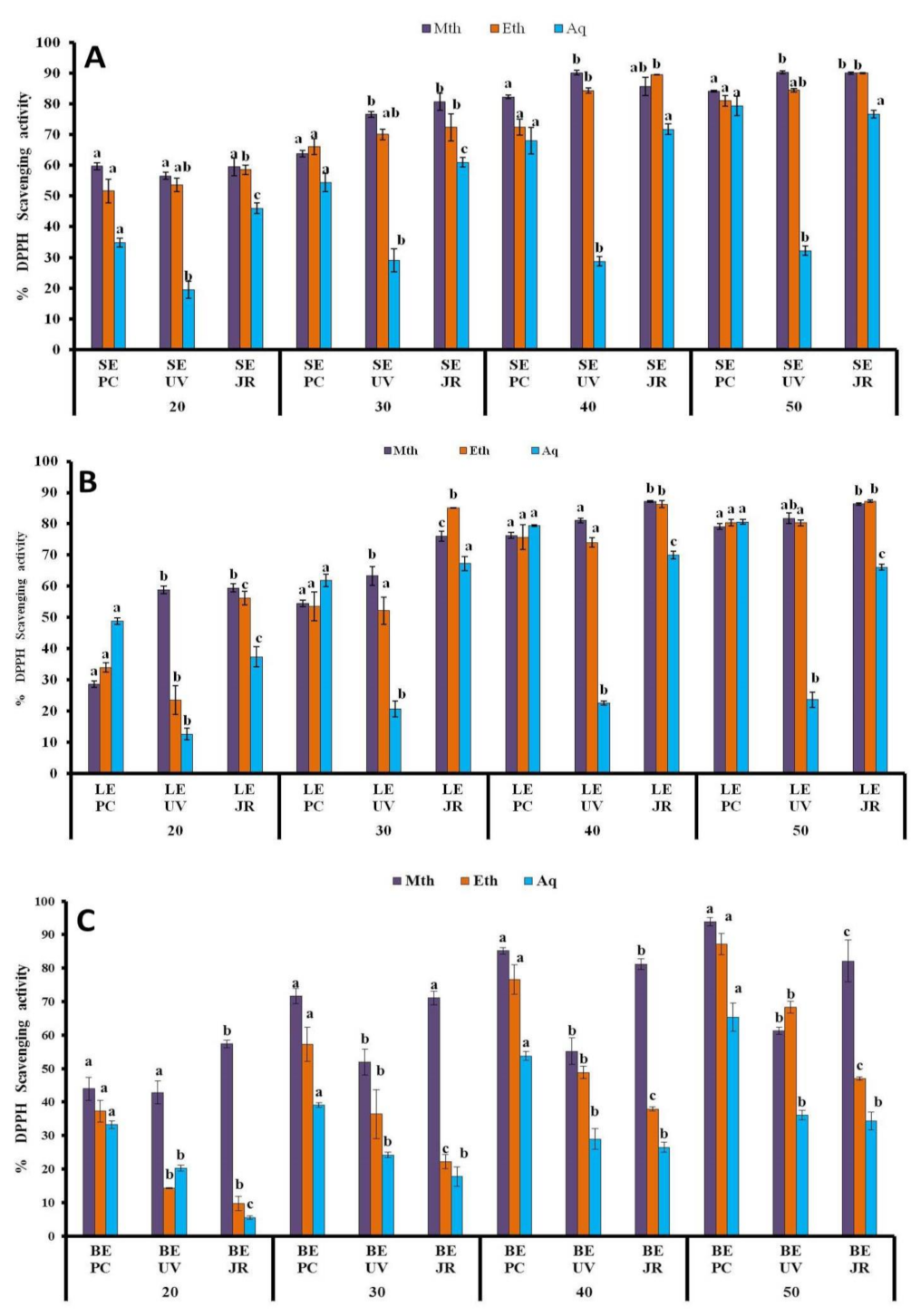
3.1.4. DPPH Assay
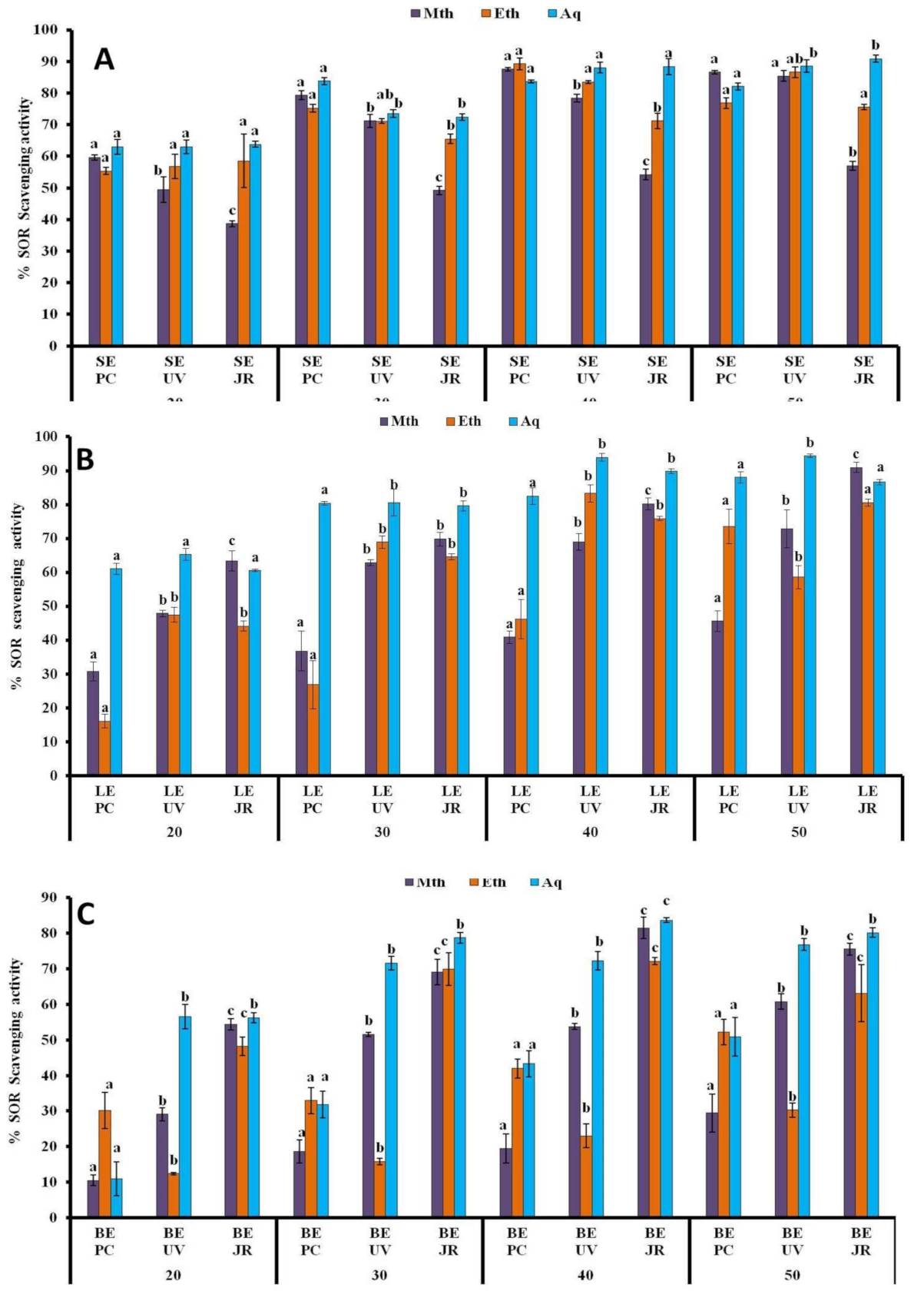
3.1.5. SOR Assay
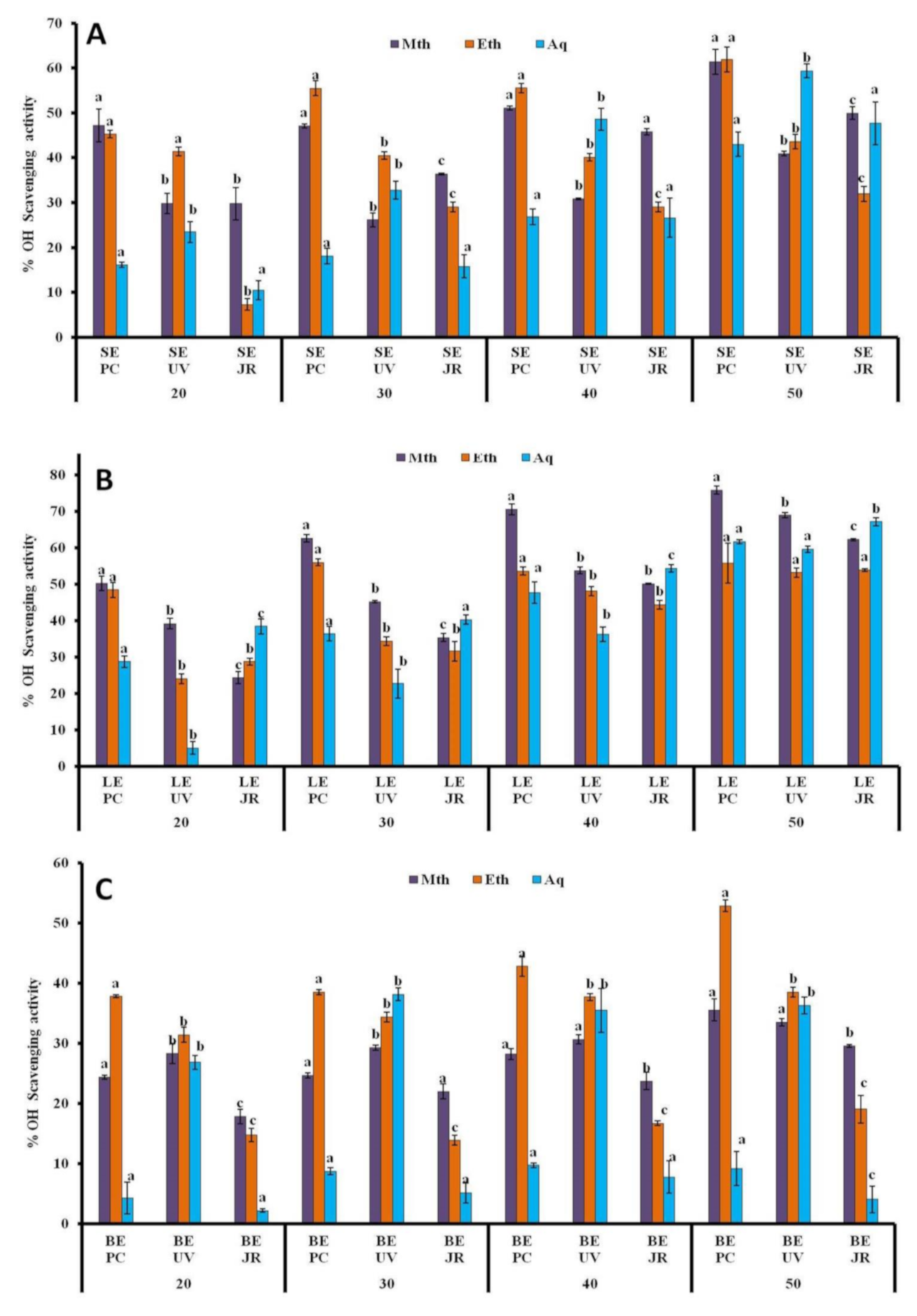
3.1.6. •OH Assay
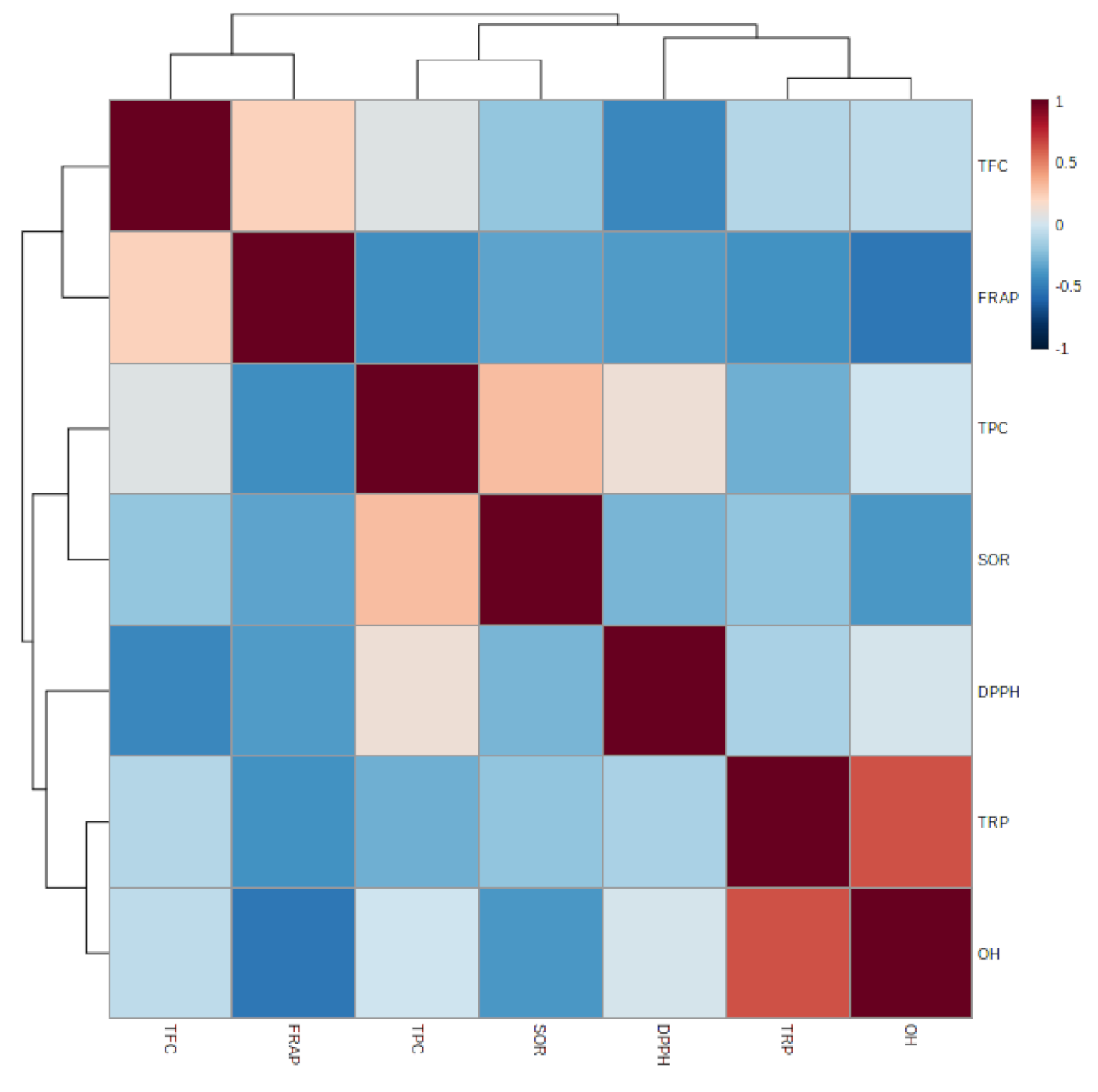
3.1.7. Relationship between Epiphyte–Host Plants in Total Phenolic, Flavonoid, and Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rice-Evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. Betalains: Natural plant pigments with potential application in functional foods. LWT Food Sci. Technol. 2015, 64, 645–649. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Biological activities of plant pigments betalains. Cri. Rev. Food Sci. Nutr. 2016, 56, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Pohl, F.; Thoo Lin, P.K. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: In vitro, in vivo and clinical trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef]
- Hrelia, S.; Angeloni, C. New Mechanisms of Action of Natural Antioxidants in Health and Disease. Antioxidants 2020, 9, 344. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signallingmolecules? Free Rad. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Kumar, S. Free Radicals and Antioxidants: Human and Food System. Adv. Appl. Sci. Res. 2011, 2, 129–135. [Google Scholar]
- Al-Rasheed, N.M.; Fadda, L.M.; Ali, H.M.; Abdel Baky, N.A.; El-Orabi, N.F.; Al-Rasheed, N.M.; Yacoub, H.I. New mechanism in the modulation of carbon tetrachloride hepatotoxicity in rats using different natural antioxidants. Toxicol. Mech. Meth. 2016, 26, 243–250. [Google Scholar] [CrossRef]
- Angeloni, C.; Malaguti, M.; Rizzo, B.; Barbalace, M.C.; Fabbri, D.; Hrelia, S. Neuroprotective Effect of Sulforaphane against Methylglyoxal Cytotoxicity. Chem. Res. Toxicol. 2015, 28, 1234–1245. [Google Scholar] [CrossRef]
- Giusti, L.; Angeloni, C.; Barbalace, M.C.; Lacerenza, S.; Ciregia, F.; Ronci, M.; Urbani, A.; Manera, C.; Digiacomo, M.; Macchia, M.; et al. A proteomic approach to uncover neuroprotective mechanisms of oleocanthal against oxidative stress. Int. J. Mol. Sci. 2018, 19, 2329. [Google Scholar] [CrossRef]
- Izzo, S.; Naponelli, V.; Bettuzzi, S. Flavonoids as Epigenetic Modulators for Prostate Cancer Prevention. Nutrients 2020, 12, 1010. [Google Scholar] [CrossRef]
- Carrera, I.; Martínez, O.; Cacabelos, R. Neuroprotection with Natural Antioxidants and Nutraceuticals in the Context of Brain Cell Degeneration: The Epigenetic Connection. Curr. Top. Med. Chem. 2019, 19, 2999–3011. [Google Scholar] [CrossRef]
- Khan, M.S.; Ikram, M.; Park, J.S.; Park, T.J.; Kim, M.O. Gut Microbiota, Its Role in Induction of Alzheimer’s Disease Pathology, and Possible Therapeutic Interventions: Special Focus on Anthocyanins. Cells 2020, 9, 853. [Google Scholar] [CrossRef]
- Zengin, G.; Cakmak, Y.S.; Guler, G.O.; Aktumsek, A. Antioxidant properties of methanolic extract and fatty acid composition of Centaurea urvillei DC. subsp. hayekianaWagenitz. Rec. Nat. Prod. 2011, 5, 123–132. [Google Scholar]
- Wannes, W.A.; Mhamdi, B.; Sriti, J.; Jemia, M.B.; Ouchikh, O.; Hamdaoui, G. Antioxidant activities of the essential oil and methanol extracts from myrtle (Myrtus communis var. italica L.) leaf, stem and flower. Food Chem. Toxicol. 2010, 48, 1362–1370. [Google Scholar] [CrossRef]
- Naz, R.; Roberts, T.H.; Bano, A.; Nosheen, A.; Yasmin, H.; Hassan, M.N.; Anwar, Z. GC-MS analysis, antimicrobial, antioxidant, antilipoxygenase and cytotoxic activities of Jacaranda mimosifolia methanol leaf extracts and fractions. PLoS ONE 2020, 15, e0236319. [Google Scholar] [CrossRef]
- Szurpnicka, A.; Kowalczuk, A.; Szterk, A. Biological activity of mistletoe: In vitro and in vivo studies and mechanisms of action. Arch. Pharm. Res. 2020, 43, 593–629. [Google Scholar] [CrossRef]
- Bussing, A.; Schietzel, M. Apoptosis-inducing properties of Viscum album L. extracts from different host trees, correlate with their content of toxic mistletoe lectins. Anticancer Res. 1999, 19, 23–28. [Google Scholar]
- Glatzel, G.; Geils, B.W. Mistletoe ecophysiology: Host–parasite interactions. Bot 2009, 87, 10–15. [Google Scholar] [CrossRef]
- Bussing, A. Mistletoe: The Genus Viscum; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Bujor, O. Ghidul Plantelor Medicinale si Aromatice de la A la Z; DHARANA: Bucharest, Romania, 2003; 232p. [Google Scholar]
- Vicas, S.I.; Rugina, D.; Pantea, S.; Socaciu, C. The Morphological Features and UV-VIS Analysis of Some Taxonomic Markers of Genus Viscum. Bull UASMV Agric. 2009, 66, 193–200. [Google Scholar]
- Zuber, D. Biological flora of Central Europe: Viscum album L. Flora-Morphology, Distribution. Fun Ecol. Plants 2004, 199, 181–203. [Google Scholar] [CrossRef]
- Idris, O.A.; Wintola, O.A.; Afolayan, A.J. Phytochemical and antioxidant activities of Rumex crispus L. in treatment of gastrointestinal helminths in Eastern Cape Province, South Africa. APJ Trop. Biomed. 2017, 7, 1071–1078. [Google Scholar] [CrossRef]
- Ohikhena, F.U.; Wintola, O.A.; Afolayan, A.J. Quantitative phytochemical constituents and antioxidant activities of the mistletoe, phragmanthera capitata (sprengel) balle extracted with different solvents. Pharm. Res. 2018, 10, 16. [Google Scholar]
- Moein, M.R.; Moein, S.; Ahmadizadeh, S. Radical scavenging and reducing power of Salvia mirzayanii subfractions. Molecules 2008, 13, 2804–2813. [Google Scholar] [CrossRef]
- Pang, Y.; Ahmed, S.; Xu, Y.; Beta, T.; Zhu, Z.; Shao, Y.; Bao, J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2018, 240, 212–221. [Google Scholar] [CrossRef]
- Nickavar, B.; Kamalinejad, M.; Mohandesi, S. Comparison of the components of the essential oils from leaves and fruits of Grammosciadium platycarpum. Chem. Nat. Compd. 2006, 42, 686–688. [Google Scholar] [CrossRef]
- Liu, F.; Ng, T.B. Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci. 2000, 66, 725–735. [Google Scholar] [CrossRef]
- Zhao, T.; Yang, H.; Li, Y.F.; Xu, J.L.; Yu, M.Q. In vitro studies on the radical Scavenging activity of hulless barley pigment. Adv. Mater. Res. 2011, 183, 145–150. [Google Scholar] [CrossRef]
- Yoder, J.I. Parasitic plant responses to host plant signals: A model for subterranean plant–plant interactions. Curr. Opin. Plant Biol. 1999, 2, 265–270. [Google Scholar] [CrossRef]
- Bowie, M.; Ward, D. Water and nutrient status of the mistletoe Plicosepalus acacia parasitic on isolated Negev Desert populations of Acacia raddiana differing in level of mortality. J. Arid. Environ. 2004, 56, 487–508. [Google Scholar] [CrossRef]
- Piwowarczyk, R.; Ochmian, I.; Lachowicz, S.; Kapusta, I.; Sotek, Z.; Blaszak, M. Phytochemical parasite-host relations and interactions: A Cistanche armena case study. Sci. Total Environ. 2020, 716, 137071. [Google Scholar] [CrossRef] [PubMed]
- Vicaş, S.I.; Rugin, D.; Leopold, L.; PInTEA, A.; Socaciu, A. HPLC Fingerprint of bioactive compounds and antioxidant activities of Viscum album from different host trees. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 48–57. [Google Scholar] [CrossRef]
- Yismairai, E.; Hemelda, N.M.; Yasman; Handayani, W. Antioxidant activity of extract of Mistletoe, Dendrophthoe pentandra (L.) Miq., lived in three different host plants, collected from Kampus UI, Depok. AIP Conf. Proc. 2019, 2168. [Google Scholar] [CrossRef]
- Oche, J.R.I.; Johnson, T.O.; Akinsanmi, A.O.; Jaryum, K.H.; Francis, T. In vitro Antioxidant Activity and Inhibition of Fe2+ and SNP Lipid Peroxidation of African Mistletoes (Tapinanthus globiferus) from Three Selected Host Plants in Jos Plateau State Nigeria. J. Appl. Life Sci. Int. 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Durazzo, A. Study approach of antioxidant properties in foods: Update and considerations. Foods 2017, 6, 17. [Google Scholar] [CrossRef]
- Apak, R. Current issues in antioxidant measurement. J. Agric. Food Chem. 2019, 67, 9187–9202. [Google Scholar] [CrossRef]
- Finley, J.W.; Kong, A.N.; Hintze, K.J.; Jeffery, E.H.; Ji, L.L.; Lei, X.G. Antioxidants in foods: State of the science important to the food industry. J. Agric. Food Chem. 2011, 59, 6837–6846. [Google Scholar] [CrossRef]
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the determination of biological antioxidant capacity in vitro: A review. J. Sci. Food Agric. 2006, 86, 2046–2056. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Therapeutic uses of Amaranthus caudatus L. Trop. Biomed. 2019, 36, 1038–1053. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Do Nascimento, K.S.; Sattler, J.A.G.; Macedo, L.F.L.; González, C.V.S.; de Melo, I.L.P.; da Silva Araújo, E.; de Almeida-Muradian, L.B. Phenolic compounds, antioxidant capacity and physicochemical properties of Brazilian Apis mellifera honeys. LWT 2018, 91, 85–94. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Botany 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Olowokudejo, J.D. Leaf epidermal morphology and petiole anatomy of the genus AnthocleistaAfzel. ex R. Br. (Gentianaceae). J. Trop. Agric. 2018, 55, 121–133. [Google Scholar]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Riipi, M.; Ossipov, V.; Lempa, K.; Haukioja, E.; Koricheva, J.; Ossipova, S.; Pihlaja, K. Seasonal changes in birch leaf chemistry: Are there trade-offs between leaf growth and accumulation of phenolics? Oecologia 2002, 130, 380–390. [Google Scholar] [CrossRef]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of Ethanol/Water Solvents on Phenolic Profiles and Antioxidant Properties of Beijing Propolis Extracts. Evid. Based Complement. Altern. Med. 2015, 595393, 1–9. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Alia, M.; Horcajo, C.; Bravo, L.; Goya, L. Effect of grape antioxidant dietary fiber on the total antioxidant capacity and the activity of liver antioxidant enzymes in rats. Nutr. Res. 2003, 23, 1251–1267. [Google Scholar] [CrossRef]
- Amic, D.; Davidović-Amić, D.; Bešlo, D.; Trinajstić, N. Structure-radical scavenging activity relationships of flavonoids. Croat. Chemica Acta 2003, 76, 55–61. [Google Scholar]
- Kiliçgün, H.; Altiner, D. Correlation between antioxidant effect mechanisms and polyphenol content of Rosa canina. Pharmacogn. Mag. 2010, 6, 238–241. [Google Scholar] [CrossRef]
- Song, F.L.; Gan, R.Y.; Zhang, Y.; Xiao, Q.; Kuang, L.; Li, H.B. Total phenolic contents and antioxidant capacities of selected chinese medicinal plants. Int. J. Mol. Sci. 2010, 11, 2362–2372. [Google Scholar] [CrossRef]
- Tosun, M.; Ercisli, S.; Sengul, M.; Ozer, H.; Polat, T.; Ozturk, E. Antioxidant properties and total phenolic content of eight Salvia species from Turkey. Biol. Res. 2009, 42, 175–181. [Google Scholar] [CrossRef]
- Alali, F.Q.; Tawaha, K.; El-Elimat, T.; Syouf, M.; El-Fayad, M.; Abulaila, K.; Nielsen, S.J.; Wheaton, W.D.; Falkinham, J.O., 3rd; Oberlies, N.H. Antioxidant activity and total phenolic content of aqueous and methanolic extracts of Jordanian plants: An ICBG project. Nat. Prod. Res. 2007, 21, 1121–1231. [Google Scholar] [CrossRef]
- Lin, S.; Guo, H.; Gong, J.D.B.; Lu, M.; Lu, M.Y.; Wang, L.; Qin, W. Phenolic profiles, β-glucan contents, and antioxidant capacities of colored Qingke (Tibetan hulless barley) cultivars. J. Cere. Sci. 2018, 81, 69–75. [Google Scholar] [CrossRef]
- Yang, X.J.; Dang, B.; Fan, M.T. Free and bound phenolic compound content and antioxidant activity of different cultivated blue highland barley varieties from the qinghai-tibet plateau. Molecules 2018, 23, 879. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Jing, L.; Zhao, K.; Su, C.; Zhang, B.; Zhang, Q.; Li, W. The phenolic compounds profile, quantitative analysis and antioxidant activity of four naked barley grains with different color. Food Chem. 2021, 335, 127655. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef]
- Vicaş, S.I.; Rugină, D.; Socaciu, C. Comparative study about antioxidant activities of Viscum album from different host trees, harvested in different seasons. J. Med. Plants Res. 2011, 5, 2237–2244. [Google Scholar]
- Urech, K.; Baumgartner, S. Chemical constituents of Viscum album L.: Implications for the pharmaceutical preparation of mistletoe. Mistletoe: From mythology to evidence-based medicine. Transl. Res. Biomed. 2015, 4, 11–23. [Google Scholar]
- Sultana, F.B.; Anwar, M. Ashraf Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Hsu, B.; Coupar, I.M.; Ng, K. Antioxidant activity of hot water extract from the fruit of the Doum palm, Hyphaene thebaica. Food Chem. 2006, 98, 317–328. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Onay Ucar, E.; Karagoz, A.; Arda, N. Antioxidant activity of Viscum album ssp. album. Fitoterapia 2006, 77, 556–560. [Google Scholar]
- Oluwaseun, A.A.; Ganiyu, O. Antioxidant properties of methanolic extracts of mistletoes (Viscum album) from cocoa and cashew trees in Nigeria. Afr. J. Biotech. 2008, 7, 17. [Google Scholar]
- Vicas, S.; Prokish, J.; Rugina, O.D.; Socaciu, C. Hydrophilic and Lipophilic Antioxidant Activities of Mistletoe (Viscum album) as determined by FRAP method. Not. Bot. Horti. Agrobot. Cluj-Napoca 2009, 37, 112–116. [Google Scholar]
- Peschel, W.; Sanchez-Rabaneda, F.; Dn, W.; Plescher, A.; Gartzia, I.; Jimenez, D.; Lamuela-Raventos, R.; Buxaderas, S.; Condina, C. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem. 2006, 97, 137–150. [Google Scholar] [CrossRef]
- Lahbib, K.; Dabbou, S.; ELBok, S.; Pandino, G.; Lombardo, S.; Gazzah, M.E.L. Variation of biochemical andantioxidant activity with respect to the part of Capsicum annuum fruit from Tunisian autochthonous cultivars. Ind. Crops Prod. 2017, 104, 164–170. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. The effect on tuber quality of an organic versus a conventional cultivation system in the early crop potato. J. Food Compos. Anal. 2017, 62, 189–196. [Google Scholar] [CrossRef]
- Pandino, G.; Meneghini, M.; Tavazza, R.; Lombardo, S.; Mauromicale, G. Phytochemicals accumulation and antioxidant activity in callus and suspension cultures of Cynara scolymus L. Plant Cell Tissue Org. Cult. 2017, 128, 223–230. [Google Scholar] [CrossRef]
- Baumann, J.; Wurm, G.; Bruchhausen, F.V. Prostaglandin synthetase inhibition by flavonoids and phenolic compounds in relation to their O2-scavenging properties. Arch. Pharm. Pharm. Med. Chem. 1980, 313, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. IJBS 2008, 4, 89–96. [Google Scholar] [PubMed]
- Rahman, M.; Islam, B.; Biswas, M.; Alam, A.H.M.K. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar]
- Lee, S.; Park, Y.; Zuidema, M.Y.; Hannink, M.; Zhang, C. Effects of interventions on oxidative stress and inflammation of cardiovascular diseases. World J. Cardiol. 2011, 3, 18–24. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Abrha, B.; Chaithanya Krishna, K.; Gopalakrishnan, V.K.; Hagos, Z.; Hiruy, M.; Devaki, K. Phytochemical screening and in vitro antioxidants activities of ethanolic extract of Acokantheraschimperi leaves. J. Pharm. Res. 2018, 12, 660. [Google Scholar]
- Packer, L.; Weber, S.U.; Rimbach, G. Molecular aspects of alpha-tocotrienol antioxidant action and cell signaling. J. Nutri. 2001, 131, 369S–373S. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Mandal, M.S.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Sig. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef]
- Samak, G.; Shenoy, P.R.; Manjunatha, M.S.; Vinayak, K.S. Superoxide and hydroxyl radical scavenging actions of botanical extracts of Wagatea spicata. Food Chem. 2009, 115, 631–634. [Google Scholar] [CrossRef]
- Bryant, J. Psychological effects of dietary components of tea: Caffeine and Ltheanine. Nutr. Rev. 2008, 66, 82–90. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafa, H.Z.E.; Rahmat, A.; Rahman, Z.A. Effects of nitrogen fertilization on synthesis of primary and secondary metabolites in three varieties of kacip fatimah (Labisia pumila blume). Int. J. Mol. Sci. 2011, 12, 5238–5254. [Google Scholar]
- Sultana, B.; Anwar, F.; Przybylski, R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. trees. Food Chem. 2007, 104, 1106–1114. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Valentim, I.B.; Silva, C.A.; Bechara, E.J.H.; Barros, M.P.; Mano, C.M.; Goulart, M.O.F. Total phenolic content and free radical scavenging activities of methanolic extract powders of tropical fruit residues. Food Chem. 2009, 115, 469–475. [Google Scholar] [CrossRef]

| Populus ciliataL. | ||||||
| DPPH (A) | IC50 M | R2 | IC50 E | R2 | IC50 A | R2 |
| Stem | 0.0471 * | 0.8954 | 0.617 | 0.97 | 1.879 | 0.9841 |
| Leaf | 1.9433 | 0.9122 | 1.823 | 0.9453 | 0.933 | 0.919 |
| Berry | 1.0492 | 0.9331 | 1.6369 | 0.9828 | 2.6944 | 0.9759 |
| SOR (B) | IC50 M | R2 | IC50 E | R2 | IC50 A | R2 |
| Stem | 0.6698 | 0.7896 | 0.5783 | 0.5219 | 2.4171 | 0.5318 |
| Leaf | 44.6118 | 0.9937 | 2.2375 | 0.9641 | 0.8714 | 0.8318 |
| Berry | 7.796 | 0.9199 | 3.9222 | 0.9487 | 3.7001 | 0.9493 |
| OH (C) | IC50 M | R2 | IC50 E | R2 | IC50 A | R2 |
| Stem | 1.5902 * | 0.8799 | 2.1375 | 0.8029 | 5.183 | 0.8876 |
| Leaf | 0.7505 * | 0.9653 | 0.7357 * | 0.5177 | 3.0822 | 0.9835 |
| Berry | 3.9131 | 0.8461 | 8.3729 | 0.8455 | 47.3906 | 0.6563 |
| Ulmus villosaL. | ||||||
| DPPH (A) | IC50 M | R2 | IC50 E | R2 | IC50 A | R2 |
| Stem | 0.0244 * | 0.8664 | 0.3317 | 0.8845 | 8.4543 | 0.7898 |
| Leaf | 0.0424 * | 0.8827 | 2.1093 | 0.9344 | 11.1628 | 0.8141 |
| Berry | 2.0207 | 0.9657 | 2.9606 | 0.9898 | 6.8233 | 0.9809 |
| SOR (B) | IC50 M | R2 | IC50 E | R2 | IC50 A | R2 |
| Stem | 0.6678 | 0.9117 | 0.0955 * | 0.9383 | 0.5958 | 0.9088 |
| Leaf | 0.874 | 0.9074 | 0.5531 | 0.1637 | 0.8363 | 0.8913 |
| Berry | 2.6237 | 0.8388 | 7.3755 | 0.9765 | 0.6575 | 0.8092 |
| OH (C) | IC50 M | R2 | IC50 E | R2 | IC50 A | R2 |
| Stem | 7.2738 | 0.5988 | 15.9993 | 0.2713 | 3.2238 | 0.9908 |
| Leaf | 2.3186 | 0.9565 | 3.497 | 0.9731 | 3.577 | 0.9891 |
| Berry | 13.9943 | 0.9388 | 8.3498 | 0.9515 | 8.6177 | 0.4364 |
| Juglans regiaL. | ||||||
| DPPH (A) | IC50 M | R2 | IC50 E | R2 | IC50 A | R2 |
| Stem | 0.5006 | 0.8485 | 0.0251 * | 0.906 | 1.1501 | 0.9539 |
| Leaf | 0.4733 | 0.8451 | 0.5471 | 0.6555 | 1.3503 | 0.5632 |
| Berry | 0.2172 | 0.8926 | 4.1274 | 0.9906 | 5.5522 | 0.9886 |
| SOR (B) | IC50 M | R2 | IC50 E | R2 | IC50 A | R2 |
| Stem | 2.5363 | 0.9205 | 0.6081 | 0.99 | 0.4696 | 0.9386 |
| Leaf | 0.7908 | 0.7583 | 1.1484 | 0.9211 | 0.3061 * | 0.9882 |
| Berry | 0.1589 | 0.7049 | 0.3438 * | 0.3148 | 0.7142 | 0.6294 |
| OH (C) | IC50 M | R2 | IC50 E | R2 | IC50 A | R2 |
| Stem | 3.8609 | 0.981 | 5.9722 | 0.6975 | 4.5285 | 0.9223 |
| Leaf | 3.0419 | 0.9972 | 3.6744 | 0.9521 | 1.9956 | 0.9281 |
| Berry | 46.197 | 0.961 | 24.0373 | 0.7804 | 57.8761 | 0.2045 |
| TFC | FRAP | TPC | SOR | DPPH | TRP | OH | |
|---|---|---|---|---|---|---|---|
| TFC | |||||||
| FRAP | 0.231 | ||||||
| TPC | 0.060 | −0.414 | |||||
| SOR | −0.192 | −0.331 | 0.305 | ||||
| DPPH | −0.448 | −0.362 | 0.130 | −0.265 | |||
| TRP | −0.093 | −0.398 | −0.282 | −0.196 | −0.122 | ||
| OH | −0.058 | −0.520 * | −0.007 | −0.375 | 0.018 | 0.636 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majeed, M.; Pirzadah, T.B.; Mir, M.A.; Hakeem, K.R.; Alharby, H.F.; Alsamadany, H.; Bamagoos, A.A.; Rehman, R.U. Comparative Study on Phytochemical Profile and Antioxidant Activity of an Epiphyte, Viscum album L. (White Berry Mistletoe), Derived from Different Host Trees. Plants 2021, 10, 1191. https://doi.org/10.3390/plants10061191
Majeed M, Pirzadah TB, Mir MA, Hakeem KR, Alharby HF, Alsamadany H, Bamagoos AA, Rehman RU. Comparative Study on Phytochemical Profile and Antioxidant Activity of an Epiphyte, Viscum album L. (White Berry Mistletoe), Derived from Different Host Trees. Plants. 2021; 10(6):1191. https://doi.org/10.3390/plants10061191
Chicago/Turabian StyleMajeed, Mahak, Tanveer Bilal Pirzadah, Manzoor Ahmad Mir, Khalid Rehman Hakeem, Hesham F. Alharby, Hameed Alsamadany, Atif A. Bamagoos, and Reiaz Ul Rehman. 2021. "Comparative Study on Phytochemical Profile and Antioxidant Activity of an Epiphyte, Viscum album L. (White Berry Mistletoe), Derived from Different Host Trees" Plants 10, no. 6: 1191. https://doi.org/10.3390/plants10061191
APA StyleMajeed, M., Pirzadah, T. B., Mir, M. A., Hakeem, K. R., Alharby, H. F., Alsamadany, H., Bamagoos, A. A., & Rehman, R. U. (2021). Comparative Study on Phytochemical Profile and Antioxidant Activity of an Epiphyte, Viscum album L. (White Berry Mistletoe), Derived from Different Host Trees. Plants, 10(6), 1191. https://doi.org/10.3390/plants10061191