Growth and Element Uptake by Salt-Sensitive Crops under Combined NaCl and Cd Stresses
Abstract
:1. Introduction
2. Results and Discussion
2.1. Vegetative Growth and Fresh Yield Parameters under Applied Treatments
2.2. Dry Matter Content and Na and Cl Uptake as Influenced by the Applied Treatments
2.3. Altered K/Na, Ca/Na and Mg/Na Concentration Ratios Are Strong Indicators of Na-Stress Exposure
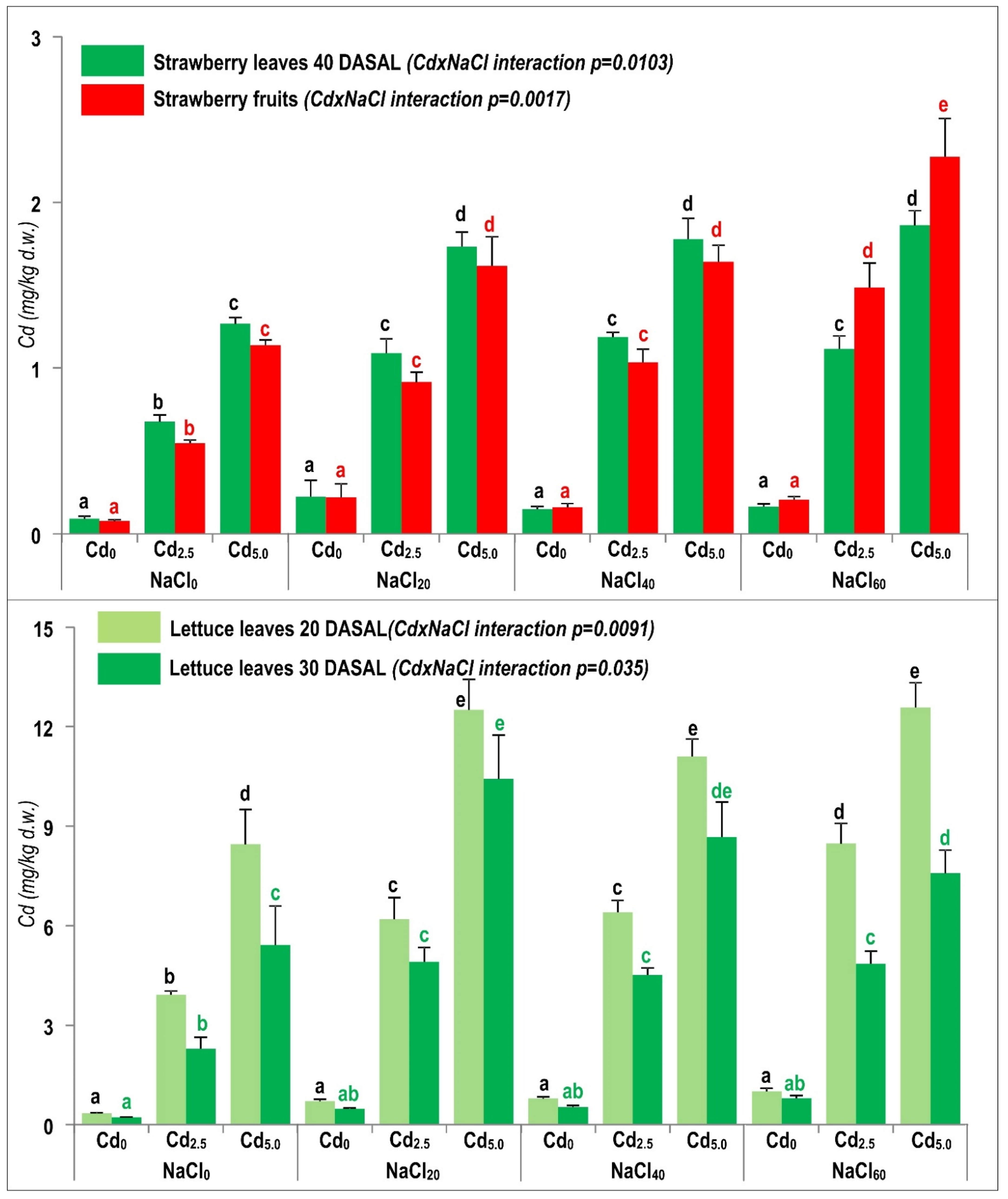
2.4. Enhanced Cd Accumulation under Combined NaCl and Cd Exposure
2.4.1. Interaction of NaCl and Cd Stresses as Influenced by the Specific Properties of Tested Crops
2.4.2. Biogeochemistry of NaCl and Cd in Organically Rich Soil
3. Materials and Methods
3.1. Experimental Setup and Conditions
3.2. Data Collection, Sampling and Chemical Analyses
3.3. Experimental Design and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ondrasek, G.; Bakić Begić, H.; Romić, D.; Brkić, Ž.; Husnjak, S.; Bubalo Kovačić, M. A novel LUMNAqSoP approach for prioritising groundwater monitoring stations for implementation of the Nitrates Directive. Environ. Sci. Eur. 2021, 33, 23. [Google Scholar] [CrossRef]
- Nosek, M.; Kaczmarczyk, A.; Jędrzejczyk, R.J.; Supel, P.; Kaszycki, P.; Miszalski, Z. Expression of Genes Involved in Heavy Metal Trafficking in Plants Exposed to Salinity Stress and Elevated Cd Concentrations. Plants 2020, 9, 475. [Google Scholar] [CrossRef] [Green Version]
- Ondrasek, G. Water Scarcity and Water Stress in Agriculture. In Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment; Springer: New York, NY, USA, 2014; Volume 1, pp. 75–96. ISBN 9781461485919. [Google Scholar]
- Ondrasek, G.; Rengel, Z. Environmental salinization processes: Detection, implications & solutions. Sci. Total Environ. 2021, 754, 142432. [Google Scholar] [CrossRef]
- Nawab, J.; Khan, N.; Ahmed, R.; Khan, S.; Ghani, J.; Rahman, Z.; Khan, F.; Wang, X.; Muhammad, J.; Sher, H. Influence of different organic geo-sorbents on Spinacia oleracea grown in chromite mine-degraded soil: A greenhouse study. J. Soils Sediments 2019, 19, 2417–2432. [Google Scholar] [CrossRef]
- Egene, C.E.; Van Poucke, R.; Ok, Y.S.; Meers, E.; Tack, F.M.G. Impact of organic amendments (biochar, compost and peat) on Cd and Zn mobility and solubility in contaminated soil of the Campine region after three years. Sci. Total Environ. 2018, 626, 195–202. [Google Scholar] [CrossRef]
- Dell’Aversana, E.; Hessini, K.; Ferchichi, S.; Fusco, G.M.; Woodrow, P.; Ciarmiello, L.F.; Abdelly, C.; Carillo, P. Salinity Duration Differently Modulates Physiological Parameters and Metabolites Profile in Roots of Two Contrasting Barley Genotypes. Plants 2021, 10, 307. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulaudzi, T.; Hendricks, K.; Mabiya, T.; Muthevhuli, M.; Ajayi, R.F.; Mayedwa, N.; Gehring, C.; Iwuoha, E. Calcium Improves Germination and Growth of Sorghum bicolor Seedlings under Salt Stress. Plants 2020, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.F.S.; da Silva Filho, J.B.; Liu, X.; Sandhu, D. Spinach Plants Favor the Absorption of K+ over Na+ Regardless of Salinity, and May Benefit from Na+ When K+ is Deficient in the Soil. Plants 2020, 9, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ondrasek, G. The responses of salt-affected plants to cadmium. In Salt Stress in Plants: Signalling, Omics and Adaptations; Springer: New York, NY, USA, 2013; Volume 9781461461, ISBN 9781461461081. [Google Scholar] [CrossRef]
- Filipović, L.; Romić, M.; Romić, D.; Filipović, V.; Ondrašek, G. Organic matter and salinity modify cadmium soil (phyto)availability. Ecotoxicol. Environ. Saf. 2018, 147. [Google Scholar] [CrossRef] [PubMed]
- Ondrasek, G.; Romic, D.; Rengel, Z. Interactions of humates and chlorides with cadmium drive soil cadmium chemistry and uptake by radish cultivars. Sci. Total Environ. 2020, 702, 134887. [Google Scholar] [CrossRef] [PubMed]
- Pelinsom Marques, J.; Silvestre Rodrigues, V.G.; Monici Raimondi, I.; Zanin Lima, J. Increase in Pb and Cd Adsorption by the Application of Peat in a Tropical Soil. Water Air Soil Pollut. 2020, 231, 136. [Google Scholar] [CrossRef]
- Fine, P.; Scagnossi, A.; Chen, Y.; Mingelgrin, U. Practical and mechanistic aspects of the removal of cadmium from aqueous systems using peat. Environ. Pollut. 2005, 138, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Bartczak, P.; Norman, M.; Klapiszewski, Ł.; Karwańska, N.; Kawalec, M.; Baczyńska, M.; Wysokowski, M.; Zdarta, J.; Ciesielczyk, F.; Jesionowski, T. Removal of nickel(II) and lead(II) ions from aqueous solution using peat as a low-cost adsorbent: A kinetic and equilibrium study. Arab. J. Chem. 2018, 11, 1209–1222. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.F.S.; Liu, X.; Suarez, D.L. Fruit yield and survival of five commercial strawberry cultivars under field cultivation and salinity stress. Sci. Hortic. 2019, 243, 401–410. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Jagendorf, A.; Zhu, J.-K. Understanding and Improving Salt Tolerance in Plants. Crop Sci. 2005, 45, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Ul Haq, T.; Akhtar, J.; Steele, K.A.; Munns, R.; Gorham, J. Reliability of ion accumulation and growth components for selecting salt tolerant lines in large populations of rice. Funct. Plant Biol. 2014, 41, 379–390. [Google Scholar] [CrossRef] [Green Version]
- Ondrasek, G.; Davor, R.; Zed, R.; Marija, R.; Monika, Z. Cadmium accumulation by muskmelon under salt stress in contaminated organic soil. Sci. Total Environ. 2009, 407, 2175–2182. [Google Scholar] [CrossRef]
- Garg, N.; Chandel, S. Role of Arbuscular Mycorrhizal (AM) Fungi on Growth, Cadmium Uptake, Osmolyte, and Phytochelatin Synthesis in Cajanus cajan (L.) Millsp. Under NaCl and Cd Stresses. J. Plant Growth Regul. 2012, 31, 292–308. [Google Scholar] [CrossRef]
- Akrami, M.; Arzani, A. Inheritance of fruit yield and quality in melon (Cucumis melo L.) grown under field salinity stress. Sci. Rep. 2019, 9, 7249. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Higgs, D.; Saltali, K.; Gezerel, O. Response of Strawberry Grown at High Salinity and Alkalinity to Supplementary Potassium. J. Plant Nutr. 2002, 25, 1415–1427. [Google Scholar] [CrossRef]
- Bose, J.; Munns, R.; Shabala, S.; Gilliham, M.; Pogson, B.; Tyerman, S.D. Chloroplast function and ion regulation in plants growing on saline soils: Lessons from halophytes. J. Exp. Bot. 2017, 68, 3129–3143. [Google Scholar] [CrossRef]
- Tester, M.; Davenport, R. Na+ Tolerance and Na+ Transport in Higher Plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Jawad Hassan, M.; Ali Raza, M.; Ur Rehman, S.; Ansar, M.; Gitari, H.; Khan, I.; Wajid, M.; Ahmed, M.; Abbas Shah, G.; Peng, Y.; et al. Effect of Cadmium Toxicity on Growth, Oxidative Damage, Antioxidant Defense System and Cadmium Accumulation in Two Sorghum Cultivars. Plants 2020, 9, 1575. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Khan, A.L.; Kim, D.-H.; Lee, S.-Y.; Kim, K.-M.; Waqas, M.; Jung, H.-Y.; Shin, J.-H.; Kim, J.-G.; Lee, I.-J. Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativalow silicon genes, and endogenous phytohormones. BMC Plant Biol. 2014, 14, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Bao, M.; Wang, L.; Khan, I.; Ullah, E.; Tung, S.A.; Samad, R.A.; Shahzad, B. Cadmium toxicity in Maize (Zea mays L.): Consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ. Sci. Pollut. Res. 2015, 22, 17022–17030. [Google Scholar] [CrossRef]
- Pang, Q.; Chen, X.; Lv, J.; Li, T.; Fang, J.; Jia, H. Triacontanol Promotes the Fruit Development and Retards Fruit Senescence in Strawberry: A Transcriptome Analysis. Plants 2020, 9, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2011; ISBN 0123849063. [Google Scholar]
- Furumoto, T.; Yamaguchi, T.; Ohshima-Ichie, Y.; Nakamura, M.; Tsuchida-Iwata, Y.; Shimamura, M.; Ohnishi, J.; Hata, S.; Gowik, U.; Westhoff, P.; et al. A plastidial sodium-dependent pyruvate transporter. Nature 2011, 476, 472–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geilfus, C.-M. Chloride: From Nutrient to Toxicant. Plant Cell Physiol. 2018, 59, 877–886. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Chloride in soils and its uptake and movement within the plant: A review. Ann. Bot. 2001, 88, 967–988. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Magen, H.; Tarchitzky, J.; Kafkafi, U. Advances in Chloride Nutrition of Plants. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: London, UK, 1999; Volume 68, pp. 97–150. [Google Scholar]
- Sun, Y.; Niu, G.; Wallace, R.; Masabni, J.; Gu, M. Relative Salt Tolerance of Seven Strawberry Cultivars. Horticulturae 2015, 1, 27–43. [Google Scholar] [CrossRef] [Green Version]
- Grattan, S.; Grieve, C. Salinity–mineral nutrient relations in horticultural crops. Sci. Hortic. 1998, 78, 127–157. [Google Scholar] [CrossRef]
- Al-Khateeb, S.A. Effect of Calcium/Sodium Ratio on Growth and Ion Relations of Alfalfa (Medicago sativa L.) Seedling Grown under Saline Condition. J. Agron. 2006, 5, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Fuad Mondal, M.; Asaduzzaman, M.; Ueno, M.; Kawaguchi, M.; Yano, S.; Ban, T.; Tanaka, H.; Asao, T. Reduction of Potassium (K) Content in Strawberry Fruits through KNO3 Management of Hydroponics. Hortic. J. 2017, 86, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na+ and K+ Transporters in Salt Stress Adaptation in Glycophytes. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Lee, B.; Wu, S.-J.; Zhu, J.-K. Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat. Biotechnol. 2003, 21, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Horie, T. Sodium Transporters in Plants. Diverse Genes and Physiological Functions. Plant Physiol. 2004, 136, 2457–2462. [Google Scholar] [CrossRef] [Green Version]
- Hauser, F.; Horie, T. A conserved primary salt tolerance mechanism mediated by HKT transporters: A mechanism for sodium exclusion and maintenance of high K +/Na + ratio in leaves during salinity stress. Plant. Cell Environ. 2010, 33, 552–565. [Google Scholar] [CrossRef]
- Rengel, Z. The role of calcium in salt toxicity. Plant Cell Environ. 1992, 15, 625–632. [Google Scholar] [CrossRef]
- Pottosin, I.; Dobrovinskaya, O. Ion Channels in Native Chloroplast Membranes: Challenges and Potential for Direct Patch-Clamp Studies. Front. Physiol. 2015, 6, 396. [Google Scholar] [CrossRef] [Green Version]
- Khorshidi, M.B.; Yarnia, M.; Hassanpanah, D. Salinity effect on nutrients accumulation in alfalfa shoots in hydroponic condition. J. Food Agric. Environ. 2009, 7, 787–790. [Google Scholar]
- Khayyat, M.; Rajaee, S.; Abdoreza, S.; Eshghi, S.; Tafazoli, E. Calcium effects on changes in chlorophyll contents, dry weight and micronutrients of strawberry (Fragaria × ananassa Duch.) plants under salt-stress conditions. Fruits 2009, 64, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Meriño-Gergichevich, C.; Ondrasek, G.; Zovko, M.; Šamec, D.; Alberdi, M.; Reyes-Díaz, M. Comparative study of methodologies to determine the antioxidant capacity of Al-toxified blueberry amended with calcium sulfate. J. Soil Sci. Plant Nutr. 2015, 15. [Google Scholar] [CrossRef] [Green Version]
- Tahjib-Ul-Arif, M.; Roy, P.R.; Al Mamun Sohag, A.; Afrin, S.; Rady, M.M.; Hossain, M.A. Exogenous Calcium Supplementation Improves Salinity Tolerance in BRRI Dhan28; a Salt-Susceptible High-Yielding Oryza Sativa Cultivar. J. Crop Sci. Biotechnol. 2018, 21, 383–394. [Google Scholar] [CrossRef]
- Hayes, P.E.; Clode, P.L.; Guilherme Pereira, C.; Lambers, H. Calcium modulates leaf cell-specific phosphorus allocation in Proteaceae from south-western Australia. J. Exp. Bot. 2019, 70, 3995–4009. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.-J.; Mimura, T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant. Cell Environ. 2016, 39, 1112–1126. [Google Scholar] [CrossRef]
- Cabrera, D.; Young, S.D.; Rowell, D.L. The toxicity of cadmium to barley plants as affected by complex formation with humic acid. Plant Soil 1988, 105, 195–204. [Google Scholar] [CrossRef]
- Mühling, K.H.; Läuchli, A. Interaction of NaCl and Cd stress on compartmentation pattern of cations, antioxidant enzymes and proteins in leaves of two wheat genotypes differing in salt tolerance. Plant Soil 2003, 253, 219–231. [Google Scholar] [CrossRef]
- Ishikawa, S.; Ae, N.; Sugiyama, M.; Murakami, M.; Arao, T. Genotypic Variation in Shoot Cadmium Concentration in Rice and Soybean in Soils with Different Levels of Cadmium Contamination. Soil Sci. Plant Nutr. 2005, 51, 101–108. [Google Scholar] [CrossRef]
- Clarke, B.B.; Brennan, E. Differential Cadmium Accumulation and Phytotoxicity in Sixteen Tobacco Cultivars. JAPCA 1989, 39, 1319–1322. [Google Scholar] [CrossRef]
- Florijn, P.J.; Nelemans, J.A.; Beusichem, M.L. van Cadmium uptake by lettuce varieties. Neth. J. Agric. Sci. 1991, 39, 103–114. [Google Scholar] [CrossRef]
- Cristiano, G.; Vuksani, G.; Tufarelli, V.; De Lucia, B. Response of Weeping Lantana (Lantana montevidensis) to Compost-Based Growing Media and Electrical Conductivity Level in Soilless Culture: First Evidence. Plants 2018, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Lee, S.-J.; Lee, M.-E.; Chung, J.W. Comparison of heavy metal immobilization in contaminated soils amended with peat moss and peat moss-derived biochar. Environ. Sci. Process. Impacts 2016, 18, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Ondrasek, G.; Rengel, Z. The Role of Soil Organic Matter in Trace Element Bioavailability and Toxicity. In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2012; pp. 403–423. ISBN 9781461406341. [Google Scholar]
- Lee, S.-J.; Lee, M.-E.; Chung, J.W.; Park, J.H.; Huh, K.Y.; Jun, G.-I. Immobilization of Lead from Pb-Contaminated Soil Amended with Peat Moss. J. Chem. 2013, 2013, 509520. [Google Scholar] [CrossRef] [Green Version]
- López-Chuken, U.J.; López-Domínguez, U.; Parra-Saldivar, R.; Moreno-Jiménez, E.; Hinojosa-Reyes, L.; Guzmán-Mar, J.L.; Olivares-Sáenz, E. Implications of chloride-enhanced cadmium uptake in saline agriculture: Modeling cadmium uptake by maize and tobacco. Int. J. Environ. Sci. Technol. 2012, 9, 69–77. [Google Scholar] [CrossRef]
- Smolders, E. Effect of Cl on Cd uptake by Swiss chard in nutrient solutions. Plant Soil 1996, 179, 57–64. [Google Scholar] [CrossRef]
- Milner, M.J.; Seamon, J.; Craft, E.; Kochian, L. V Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 2013, 64, 369–381. [Google Scholar] [CrossRef] [Green Version]
- SAS Institution Inc. SAS v. 9.1.3; SAS Institution Inc.: Cary, NC, USA, 2004. [Google Scholar]


| Treatment | Strawberry | Lettuce | ||||
|---|---|---|---|---|---|---|
| Marketable Fruit Number/Plant | Total Marketable Fruit g/Plant | Total Runners Number/Plant | The Longest Runner cm | Leaf Area cm2 | Head Weight g/Plant | |
| Cd0 | 7.8(0.67) a | 62(6.5) a | 2.6(0.37) a | 54(5.3) a | 171(13) a | 399(13) a |
| Cd2.5 | 7.3(0.56) a | 63(6.3) a | 2.0(0.31) ab | 48(3.8) ab | 174(13) a | 376(14) ab |
| Cd5.0 | 8.3(0.58) a | 74(7.3) a | 1.9(0.28) b | 46(4.6) b | 164(10) a | 359(11) b |
| NaCl0 | 10(0.54) a | 96(6.0) a | 3.5(0.29) a | 71(2.6) a | 235(8.0) a | 422(10) a |
| NaCl20 | 7.8(0.57) b | 69(5.8) b | 2.7(0.21) b | 54(3.8) b | 180(8.0) b | 411(7.0) a |
| NaCl40 | 7.5(0.49) bc | 63(4.8) b | 1.9(0.21) b | 44(1.9) c | 154(5.4) c | 367(10) b |
| NaCl60 | 5.7(0.44) c | 39(3.9) c | 0.5(0.08) c | 27(2.4) d | 111(3.0) d | 311(8.0) c |
| NaClxCd | ns | ns | ns | ns | ns | ns |
| Treatment | Strawberry Leaves 40 DASAL | Lettuce Leaves 20 DASAL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DM % | Na | Cl | Cu | Zn | DM % | Na | Cl | Cu | Zn | |
| g/kg | mg/kg | g/kg | mg/kg | |||||||
| Cd0 | 23(1.0) a | 5.0(1.2) a | 26(3.75) a | 4.7(0.28) a | 33(2.0) a | 4.9(0.07) a | 21(3.2) a | 47(6.9) a | 4.9(0.30) a | 77(3.7) a |
| Cd2.5 | 22(0.73) b | 4.0(0.87) b | 23(3.40) b | 4.4(0.28) a | 31(1.7) ab | 5.0(0.07) a | 21(3.3) a | 48(7.0) a | 4.8(0.31) ab | 78(4.3) a |
| Cd5.0 | 22(0.77) b | 4.0(0.89) b | 22(3.20) b | 3.9(0.20) a | 28(0.90) b | 5.0(0.08) a | 20(3.1) a | 47(6.5) a | 4.4(0.33) b | 71(4.7) b |
| NaCl0 | 19(0.32) a | 0.10(0.001) a | 2.9(0.30) a | 4.4(0.36) a | 23(0.91) a | 4.9(0.10) ab | 1.9(0.06) a | 6.4(0.23) a | 3.2(0.13) a | 53(1.7) a |
| NaCl20 | 21(0.33) ab | 2.4(0.14) b | 23(0.82) b | 4.2(0.32) a | 32(1.1) b | 4.7(0.10) b | 18(0.27) b | 46(0.89) b | 4.0(0.11) b | 71(1.8) b |
| NaCl40 | 22(0.47) b | 4.7(0.30) c | 30(1.10) c | 4.3(0.28) a | 34(1.5) b | 4.9(0.05) ab | 27(0.40) c | 61(2.4) c | 5.5(0.22) c | 83(1.4) c |
| NaCl60 | 27(0.77) c | 10(0.46) d | 39(1.05) d | 4.6(0.27) a | 34(2.1) b | 5.1(0.06) b | 35(0.70) d | 76(1.9) d | 6.0(1.14) d | 95(2.4) d |
| CdxNaCl | ns | * | ns | ns | ns | ns | ns | ns | ns | ns |
| Treatment | Strawberry Fruits | Lettuce Leaves 30 DASAL | ||||||||
| DM % | Na | Cl | Cu | Zn | DM % | Na | Cl | Cu | Zn | |
| g/kg | mg/kg | g/kg | mg/kg | |||||||
| Cd0 | 7.9(0.20) a | 3.3(0.81) a | 13(2.2) a | 4.5(0.33) a | 24(1.4) a | 5.8(0.14) a | 34(5.5) a | 63(9.7) a | 6.2(0.46) a | 107(9.6) a |
| Cd2.5 | 8.1(0.14) a | 3.0(0.60) a | 11(1.5) ab | 3.9(0.25) b | 20(1.0) b | 5.6(0.13) a | 35(5.3) a | 62(9.4) a | 5.8(0.47) a | 96(7.0) ab |
| Cd5.0 | 8.2(0.22) a | 2.8(0.75) a | 10(1.7) b | 4.0(0.32) b | 20(1.0) b | 5.6(0.11) a | 33(5.0) a | 59(8.7) a | 4.9(0.46) b | 88(8.2) b |
| NaCl0 | 8.2(0.07) ab | 0.2(0.01) a | 3.2(0.30) a | 3.1(0.13) a | 16(0.77) a | 5.9(0.15) a | 2.0(0.03) a | 6.5(0.15) a | 3.5(0.20) a | 54(3.0) a |
| NaCl20 | 8.3(0.27) a | 2.0(0.30) b | 7.8(0.58) b | 3.5(0.15) ab | 21(1.0) b | 5.3(0.12) b | 33(0.80) b | 58(1.3) b | 4.6(0.20) b | 93(3.0) b |
| NaCl40 | 8.1(0.22) ab | 3.0(0.27) b | 12(0.92) c | 4.1(0.13) b | 22(1.0) b | 5.5(0.17) ab | 45(1.7) c | 79(2.9) c | 6.7(0.36) c | 105(2.5) b |
| NaCl60 | 7.6(0.19) b | 7.1(0.64) c | 21(1.3) d | 5.9(0.26) c | 27(1.0) c | 5.9(0.08) a | 56(1.8) d | 102(3.3) d | 7.7(0.30) d | 136(8.2) c |
| CdxNaCl | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Nutrient Solution | Concentration | Peat Soil |
|---|---|---|
| pH | 6.5(±0.1) | 5.23 (±0.2) |
| OM | 902 (±61) g/kg | |
| EC | 1.7(±0.1) dS/m | |
| NO3− | 10 mM | |
| H2PO4− | 1.5 mM | |
| K+ | 7.5 mM | |
| Ca2+ | 2.0 mM | |
| Mg2+ | 2.0 mM | |
| SO42− | 2.0 mM | |
| Fe | 15 µM | |
| Mn | 10 µM | |
| Total Cu | 10 µM | 12.9 (±0.06) mg/kg |
| B | 5 µM | |
| Total Zn | 0.7 µM | 14.7 (±0.04) mg/kg |
| Mo | 0.7 µM | |
| Total Cd | 0.33 (±0.01) mg/kg | |
| Salinity treatment | 0 (NaCl0), 20 (NaCl20), 40 (NaCl40) and 60 (NaCl60) mM NaCl | |
| Cd treatment | 0.33 (±0.01; Cd0), 2.5 (±0.15; Cd2.5) and 5.0 (±0.20; Cd5.0) mg/kg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ondrasek, G.; Rengel, Z.; Maurović, N.; Kondres, N.; Filipović, V.; Savić, R.; Blagojević, B.; Tanaskovik, V.; Gergichevich, C.M.; Romić, D. Growth and Element Uptake by Salt-Sensitive Crops under Combined NaCl and Cd Stresses. Plants 2021, 10, 1202. https://doi.org/10.3390/plants10061202
Ondrasek G, Rengel Z, Maurović N, Kondres N, Filipović V, Savić R, Blagojević B, Tanaskovik V, Gergichevich CM, Romić D. Growth and Element Uptake by Salt-Sensitive Crops under Combined NaCl and Cd Stresses. Plants. 2021; 10(6):1202. https://doi.org/10.3390/plants10061202
Chicago/Turabian StyleOndrasek, Gabrijel, Zed Rengel, Nada Maurović, Nada Kondres, Vilim Filipović, Radovan Savić, Boško Blagojević, Vjekoslav Tanaskovik, Cristian Meriño Gergichevich, and Davor Romić. 2021. "Growth and Element Uptake by Salt-Sensitive Crops under Combined NaCl and Cd Stresses" Plants 10, no. 6: 1202. https://doi.org/10.3390/plants10061202
APA StyleOndrasek, G., Rengel, Z., Maurović, N., Kondres, N., Filipović, V., Savić, R., Blagojević, B., Tanaskovik, V., Gergichevich, C. M., & Romić, D. (2021). Growth and Element Uptake by Salt-Sensitive Crops under Combined NaCl and Cd Stresses. Plants, 10(6), 1202. https://doi.org/10.3390/plants10061202









