Continuous Lighting and High Daily Light Integral Enhance Yield and Quality of Mass-Produced Nasturtium (Tropaeolum majus L.) in Plant Factories
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatments and Growth Conditions
2.2. Measurement
2.2.1. Growth Parameters
2.2.2. Chlorophyll Fluorescence Characteristics
2.2.3. Antioxidant Activity (1,1-Diphenyl-2-picrylhydrazyl Assay)
2.2.4. Total Phenolic Content
2.2.5. Light Use Efficiency
2.2.6. Statistical Analyses
3. Results
3.1. Plant Growth in Experiment 1
3.2. Light Stress Indicator, Antioxidant Capacity, and Total Phenolic Content in the Leaves of Nasturtium in Experiment 1
3.3. Plant Growth in Experiment 2
3.4. Antioxidant Capacity and Total Phenolic Content in the Leaves of Nasturtium in Experiment 2
3.5. Chlorophyll Fluorescence Characteristics of Nasturtium in Experiment 2
3.6. Relationship between RGR and LAI in Experiment 1 and Experiment 2
4. Discussion
4.1. Effects of CL on the Growth and LUE of Hydroponic Nasturtium under the Same DLI
4.2. Effects of CL on the Growth and LUE of Hydroponic Nasturtium under Different DLIs
4.3. Effects of CL on the Tolerance of Hydroponic Nasturtium under the Same and Different DLIs
4.4. Effects of DLI and CL on Antioxidants of Hydroponic Nasturtium
4.5. Plant Spacing and Effects of LAI on the RGR and LUE of Hydroponic Nasturtium
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, W.; Lu, N.; Kikuchi, M.; Takagaki, M. Effects of Node Position and Electric Conductivity of Nutrient Solution on Adventitious Rooting of Nasturtium (Tropaeolum majus L.) Cuttings. Agronomy 2021, 11, 363. [Google Scholar] [CrossRef]
- Zhang, X.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of environment lighting on the growth, photosynthesis, and quality of hydroponic lettuce in a plant factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Gao, W.; He, D.; Ji, F.; Zhang, S.; Zheng, J. Effects of daily light integral and LED spectrum on growth and nutritional quality of hydroponic spinach. Agronomy 2020, 10, 1082. [Google Scholar] [CrossRef]
- Yan, Z.; He, D.; Niu, G.; Zhou, Q.; Qu, Y. Growth, nutritional quality, and energy use efficiency of hydroponic lettuce as influenced by daily light integrals exposed to white versus white plus red light-emitting diodes. HortScience 2019, 54, 1737–1744. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.P.; Lu, N.; Kagawa, N.; Takagaki, M. Optimization of photosynthetic photon flux density and root-zone temperature for enhancing secondary metabolite accumulation and production of coriander in plant factory. Agronomy 2019, 9, 224. [Google Scholar] [CrossRef]
- Sysoeva, M.; Markovskaya, E.; Shibaeva, T. Plants under Continuous Light: A Review. Plant Stress 2010, 4, 5–17. [Google Scholar]
- Mao, H.; Hang, T.; Zhang, X.; Lu, N. Both Multi-Segment Light Intensity and Extended Photoperiod Lighting Strategies, with the Same Daily Light Integral, Promoted Lactuca sativa L. Growth and photosynthesis. Agronomy 2019, 9, 857. [Google Scholar] [CrossRef]
- Weaver, G.; van Iersel, M.W. Longer photoperiods with adaptive lighting control can improve growth of greenhouse-grown ‘Little Gem’ lettuce (Lactuca sativa). HortScience 2020, 55, 573–580. [Google Scholar] [CrossRef]
- Palmer, S.; van Iersel, M.W. Increasing growth of lettuce and mizuna under sole-source LED lighting using longer photoperiods with the same daily light integral. Agronomy 2020, 10, 1695. [Google Scholar] [CrossRef]
- Kitaya, Y.; Niu, G.; Kozai, T.; Ohashi, M. Photosynthetic photon flux, photoperiod, and CO2 concentration affect growth and morphology of lettuce plug transplants. HortScience 1998, 33, 988–991. [Google Scholar] [CrossRef]
- Ohyama, K.; Manabe, K.; Omura, Y. Potential Use of a 24-Hour Photoperiod (Continuous Light) with Alternating Air Temperature for Production of Tomato Plug Transplants in a Closed System. HortScience 2005, 40, 374–377. [Google Scholar] [CrossRef]
- Ohyama, K.; Omura, Y.; Kozai, T. Effects of air temperature regimes on physiological disorders and floral development of tomato seedlings grown under continuous light. HortScience 2005, 40, 1304–1306. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; Van Ieperen, W.; Vreugdenhil, D.; Millenaar, F.F. Plants under continuous light. Trends Plant Sci. 2011, 16, 310–318. [Google Scholar] [CrossRef]
- Shimomura, M.; Yoshida, H.; Fujiuchi, N.; Ariizumi, T.; Ezura, H.; Fukuda, N. Continuous blue lighting and elevated carbon dioxide concentration rapidly increase chlorogenic acid content in young lettuce plants. Sci. Hortic. 2020, 272, 109550. [Google Scholar] [CrossRef]
- Zha, L.; Zhang, Y.; Liu, W. Dynamic Responses of Ascorbate Pool and Metabolism in Lettuce to Long-term Continuous Light Provided by Red and Blue LEDs. Environ. Exp. Bot. 2019, 163, 15–23. [Google Scholar] [CrossRef]
- Avio, L.; Sbrana, C.; Giovannetti, M.; Frassinetti, S. Arbuscular mycorrhizal fungi affect total phenolics content and antioxidant activity in leaves of oak leaf lettuce varieties. Sci. Hortic. 2017, 224, 265–271. [Google Scholar] [CrossRef]
- Kitayama, M.; Nguyen, D.T.P.; Lu, N.; Takagaki, M. Effect of light quality on physiological disorder, growth, and secondary metabolite content of water spinach (Ipomoea aquatica forsk) cultivated in a closed-type plant production system. Hortic. Sci. Technol. 2019, 37, 206–218. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G. Role of the plant factory with artificial lighting (PFAL) in urban areas. In Plant Factory–An Indoor Vertical Farming System for Efficient Quality Food Production; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 7–33. [Google Scholar]
- Gauslaa, Y.; Solhaug, K.A. High-light-intensity damage to the foliose lichen Lobaria pulmonaria within a natural forest: The applicability of chlorophyll fluorescence methods. Lichenologist 2000, 32, 271–289. [Google Scholar] [CrossRef]
- Li, S.; Yang, W.; Yang, T.; Chen, Y.; Ni, W. Effects of Cadmium Stress on Leaf Chlorophyll Fluorescence and Photosynthesis of Elsholtzia argyi-A Cadmium Accumulating Plant. Int. J. Phytoremediation 2015, 17, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Camejo, D.; Nicolás, E.; Torres, W.; Alarcón, J.J. Differential heat-induced changes in the CO2 assimilation rate and electron transport in tomato (Lycopersicon esculentum Mill.). J. Hortic. Sci. Biotechnol. 2010, 85, 137–143. [Google Scholar] [CrossRef]
- Velez-ramirez, A.I.; Van Ieperen, W.; Vreugdenhil, D.; Van Poppel, P.M.J.A.; Heuvelink, E.; Millenaar, F.F. A single locus confers tolerance to continuous light and allows substantial yield increase in tomato. Nat. Commun. 2014, 5, 1–13. [Google Scholar] [CrossRef]
- Minh, D.; Chun, C. Growth and leaf injury in tomato plants under continuous light at di ff erent settings of constant and diurnally varied photosynthetic photon fl ux densities. Sci. Hortic. 2020, 269, 109347. [Google Scholar] [CrossRef]
- Kelly, N.; Choe, D.; Meng, Q.; Runkle, E.S. Promotion of lettuce growth under an increasing daily light integral depends on the combination of the photosynthetic photon flux density and photoperiod. Sci. Hortic. 2020, 272, 109565. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Responses of sweet basil to different daily light integrals in photosynthesis, morphology, yield, and nutritional quality. HortScience 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence-A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Liu, W.; Zhang, Y.; Zhou, C.; Shao, M. Morphological and Physiological Stress Responses of Lettuce to Different Intensities of Continuous Light. Front. Plant Sci. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.S.; Cavaco, T.; Carvalho, L.M.; Duque, P. Effect of photoperiod on flavonoid pathway activity in sweet potato (Ipomoea batatas (L.) Lam.) leaves. Food Chem. 2010, 118, 384–390. [Google Scholar] [CrossRef]
- Pennisi, G.; Pistillo, A.; Orsini, F.; Cellini, A.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Crepaldi, A.; Gianquinto, G.; Marcelis, L.F.M. Optimal light intensity for sustainable water and energy use in indoor cultivation of lettuce and basil under red and blue LEDs. Sci. Hortic. 2020, 272, 109508. [Google Scholar] [CrossRef]
- Ioslovich, I.; Gutman, P.O. Optimal control of crop spacing in a plant factory. Automatica 2000, 36, 1665–1668. [Google Scholar] [CrossRef]
- Lu, N.; Shimamura, S. Protocols, Issues and Potential Improvements of Current Cultivation Systems. Smart Plant Fact. 2018, 31–49. [Google Scholar] [CrossRef]
- Nunomura, O.; Kozai, T.; Shinozaki, K.; Oshio, T. Seeding, seedling production and transplanting. In Plant Factory–An Indoor Vertical Farming System for Efficient Quality Food Production; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 223–229. [Google Scholar]
- Kozai, T.; Sakaguchi, S.; Akiyama, T.; Yamada, K.; Ohshima, K. Design and Management of PFAL. In Plant Factory–An Indoor Vertical Farming System for Efficient Quality Food Production; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 295–312. [Google Scholar]
- Beccafichi, C.; Benincasa, P.; Guiducci, M.; Tei, F. Effect of crop density on growth and light interception in greenhouse lettuce. Acta Hortic. 2003, 614, 507–513. [Google Scholar] [CrossRef]
- Cho, Y.Y.; Lee, J.H.; Shin, J.H.; Son, J.E. Development of an expolinear growth model for pak-choi using the radiation integral and planting density. Hortic. Environ. Biotechnol. 2015, 56, 310–315. [Google Scholar] [CrossRef]
- Cho, Y.Y.; Son, J.E. Effects of planting density on growth and yield of hydroponically-grown pak-choi (Brassica campestris ssp. chinensis). Hortic. Environ. Biotechnol. 2005, 46, 291–294. [Google Scholar]
- Chapepa, B.; Mudada, N.; Mapuranga, R. The impact of plant density and spatial arrangement on light interception on cotton crop and seed cotton yield: An overview. J. Cott. Res. 2020, 3, 4–9. [Google Scholar] [CrossRef]
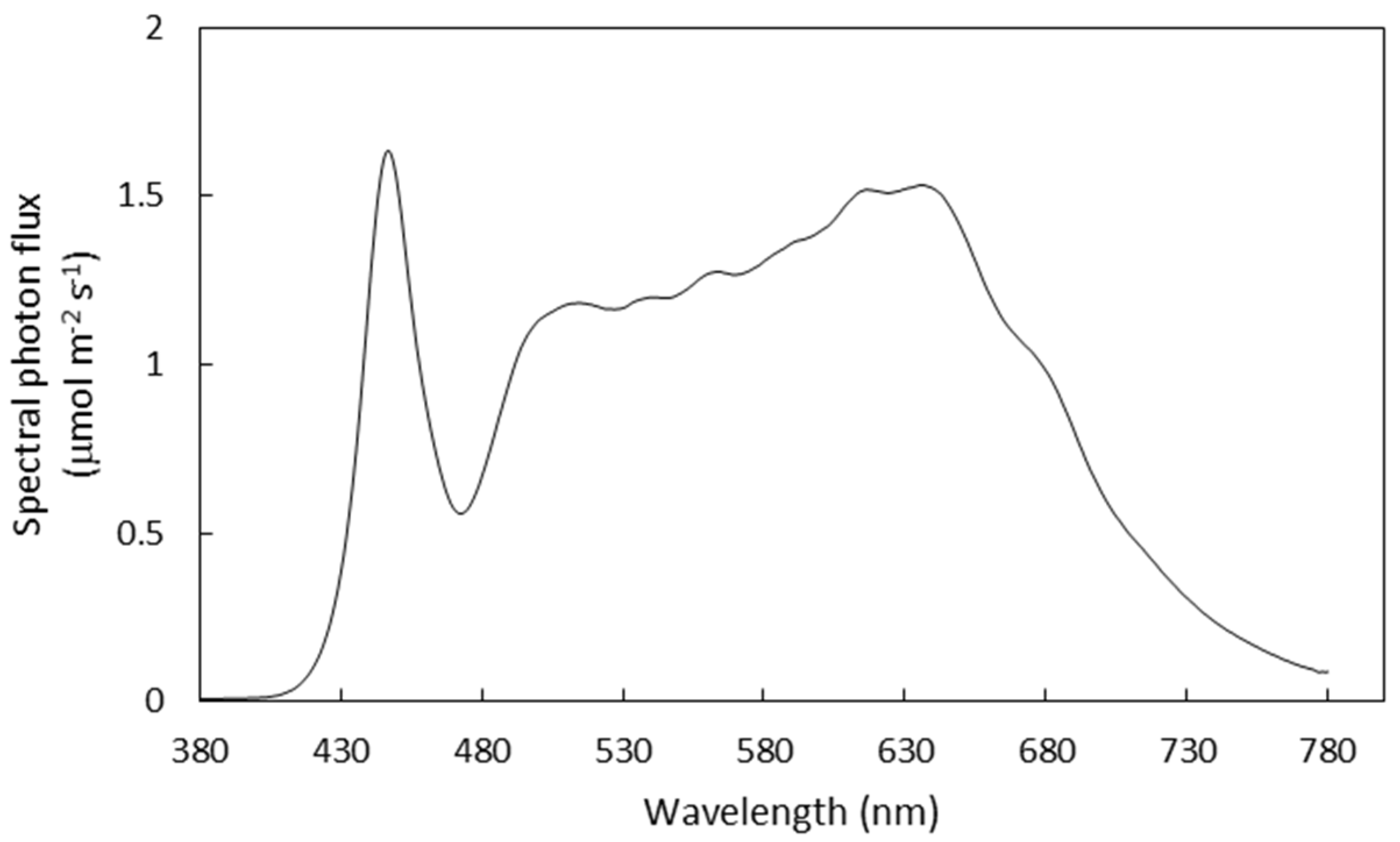






| Experiment | Treatment Symbols | Light Intensity (µmol m−2 s−1) | Light Period (h d−1) | DLI (mol m−2 d−1) |
|---|---|---|---|---|
| 1 | T16-300 | 300 | 16 | 17.3 |
| T24-200 | 200 | 24 | ||
| 2 | CL-200 | 200 | 24 | 17.3 |
| CL-300 | 300 | 24 | 25.9 | |
| CL-400 | 400 | 24 | 34.6 |
| Parameter | Unit | DAT | Treatment z | Difference (%) y | T-Test x p-Value | |||
|---|---|---|---|---|---|---|---|---|
| T16-300 | T24-200 | |||||||
| Fv/Fmax | (ratio) | 14 | 0.828 | a | 0.836 | a | 1.0 | 0.234 |
| 21 | 0.841 | a | 0.820 | a | −2.6 | 0.020 | ||
| Antioxidant capacity | (mg TE g−1 leaf FW) | 14 | 1.654 | a2* | 2.316 | a1* | 40 | 5 × 10−5 |
| 21 | 1.356 | b | 2.003 | b | 48 | 3 × 10−4 | ||
| Total phenolic content | (mg GAE g−1 leaf FW) | 14 | 2.041 | a | 2.251 | a | 10 | 0.040 |
| 21 | 2.081 | a | 2.396 | a | 15 | 0.056 | ||
| RGR | (g g−1 day) | 7 | 0.20 | a | 0.24 | a3* | 19 | 0.015 |
| 14 | 0.22 | a3* | 0.24 | a | 11 | 6 × 10−4 | ||
| 21 | 0.18 | b | 0.19 | b | 5 | 0.038 | ||
| LAI | (m2 m−2) | 7 | 0.75 | c | 1.16 | c | 55 | 0.014 |
| 14 | 4.64 | b | 5.83 | b | 26 | 0.007 | ||
| 21 | 6.47 | a9* | 6.93 | a9* | 7 | 0.422 | ||
| Parameter | Unit | DAT | Treatment z | Rate of Change (%) | ANOVA p-Value y | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CL-200 | CL-300 | CL-400 | CL300/ CL200 | CL400/ CL300 | |||||||
| Antioxidant capacity | (mg TE/ g FW) | 7 | 4.9 | a1* | 5.1 | a | 5.8 | a3* | 6 | 13 | 0.198 |
| 14 | 3.6 | ab | 3.8 | a | 3.6 | b | 6 | −5 | 0.952 | ||
| 21 | 3.2 | b | 3.8 | a | 2.9 | b | 19 | −24 | 0.137 | ||
| Total phenolic content | (mg GA/ g FW) | 7 | 3.1 | a | 3.2 | a | 3.5 | a3* | 2 | 9 | 0.256 |
| 14 | 2.7 | a | 2.6 | a | 2.5 | b | -3 | −4 | 0.840 | ||
| 21 | 2.5 | a | 2.8 | a | 2.5 | b | 12 | −13 | 0.252 | ||
| RGR of shoot DW | (g/g d) | 7 | 0.26 | a | 0.28 | a | 0.32 | a1* | 8 | 13 | 0.027 |
| 14 | 0.27 | a2* | 0.29 | a2* | 0.28 | ab | 8 | −1 | 0.035 | ||
| 21 | 0.24 | b | 0.25 | b | 0.26 | b | 4 | 5 | 6 × 10−6 | ||
| LAI | (m2 m−2) | 7 | 0.29 | c | 0.30 | c | 0.36 | c | 3 | 20 | 0.333 |
| 14 | 1.81 | b | 2.14 | b | 1.91 | b | 18 | −11 | 0.239 | ||
| 21 | 3.06 | a14* | 3.25 | a10* | 4.12 | a12* | 6 | 27 | 0.001 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Lu, N.; Kikuchi, M.; Takagaki, M. Continuous Lighting and High Daily Light Integral Enhance Yield and Quality of Mass-Produced Nasturtium (Tropaeolum majus L.) in Plant Factories. Plants 2021, 10, 1203. https://doi.org/10.3390/plants10061203
Xu W, Lu N, Kikuchi M, Takagaki M. Continuous Lighting and High Daily Light Integral Enhance Yield and Quality of Mass-Produced Nasturtium (Tropaeolum majus L.) in Plant Factories. Plants. 2021; 10(6):1203. https://doi.org/10.3390/plants10061203
Chicago/Turabian StyleXu, Wenshuo, Na Lu, Masao Kikuchi, and Michiko Takagaki. 2021. "Continuous Lighting and High Daily Light Integral Enhance Yield and Quality of Mass-Produced Nasturtium (Tropaeolum majus L.) in Plant Factories" Plants 10, no. 6: 1203. https://doi.org/10.3390/plants10061203
APA StyleXu, W., Lu, N., Kikuchi, M., & Takagaki, M. (2021). Continuous Lighting and High Daily Light Integral Enhance Yield and Quality of Mass-Produced Nasturtium (Tropaeolum majus L.) in Plant Factories. Plants, 10(6), 1203. https://doi.org/10.3390/plants10061203






