Baicalein 5,6-Dimethyl Ether Prevents Memory Deficits in the Scopolamine Zebrafish Model by Regulating Cholinergic and Antioxidant Systems
Abstract
:1. Introduction
2. Results
2.1. Isolation and Structure Elucidation of the Isolated Compound
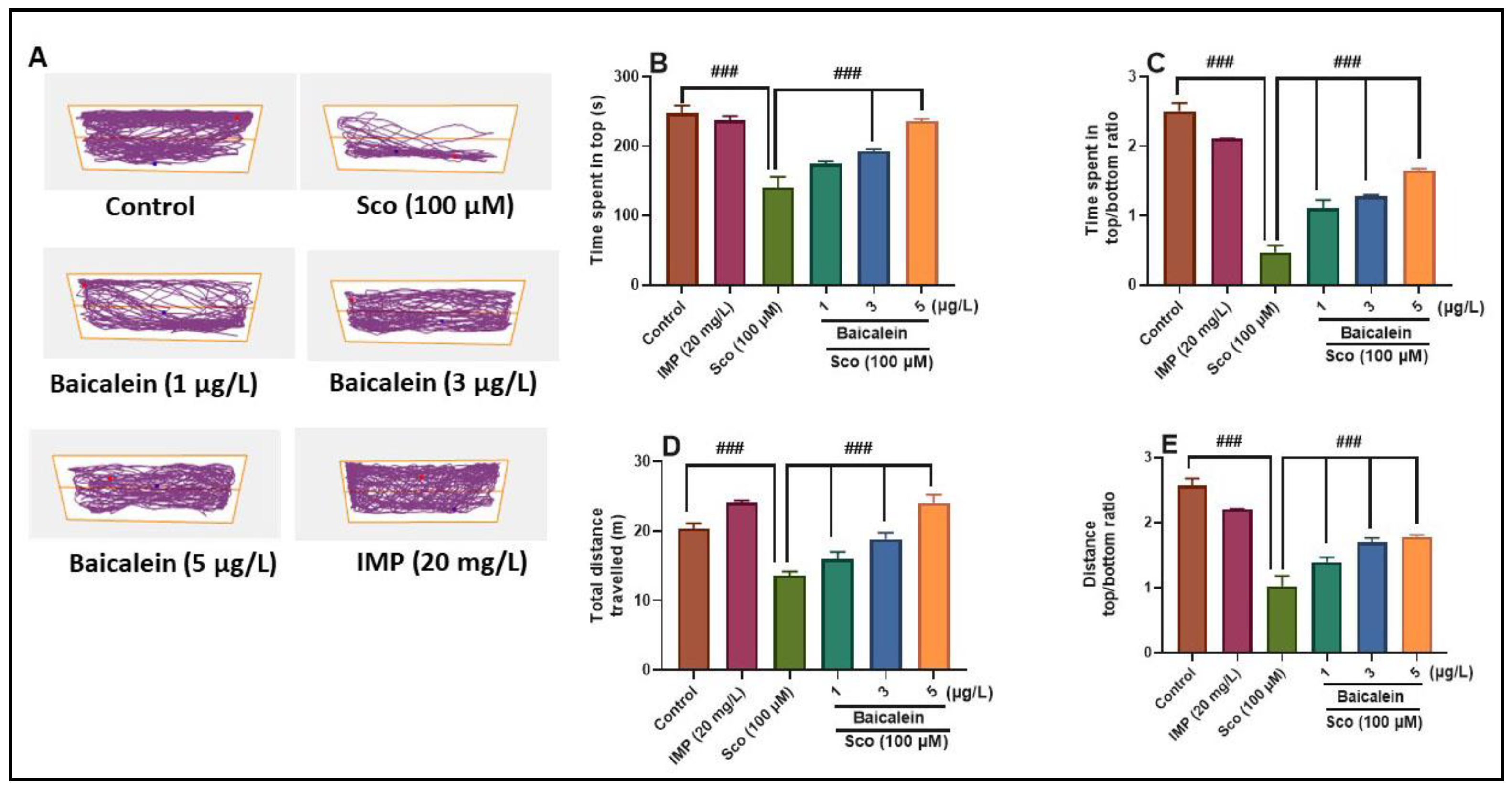
2.2. The Effect of Baicalein 5,6-Dimethyl Ether on Scopolamine-Induced Anxiety in Zebrafish
2.3. The Effect of Baicalein 5,6-Dimethyl Ether on Scopolamine-Induced Deficits of Exploratory Behavior and Recognition Memory in Zebrafish
2.4. In Vivo Inhibitory Activity of Baicalein 5,6-Dimethyl Ether Against Acetylcholinesterase Activity
2.5. In Vivo Activity of Baicalein 5,6-Dimethyl Ether on the Antioxidant Defense System
2.6. Correlation between Behavioral Scores, Enzymatic Activities, and Lipid Peroxidation
3. Materials and Methods
3.1. Plant Material
3.2. Apparatus
3.3. Baicalein 5,6-Dimethyl Ether Extraction and Isolation
3.4. Baicalein 5,6-Dimethyl Ether (1)
3.5. Experimental Animals and Treatment
3.6. Novel Tank-Diving Test (NTT)
3.7. Y-Maze Task
3.8. Novel Object Recognition Test (NOR)
3.9. Tissue Preparation
3.10. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, X.; He, T.; Chang, Y.; Zhao, Y.; Chen, X.; Bai, S.; Wang, L.; Shen, M.; She, G. The Genus Alnus, A Comprehensive Outline of Its Chemical Constituents and Biological Activities. Molecules 2017, 22, 1383. [Google Scholar] [CrossRef] [Green Version]
- Sati, S.; Sati, O.; Sati, N. Bioactive constituents and medicinal importance of genus Alnus. Pharmacogn. Rev. 2011, 5, 174. [Google Scholar] [CrossRef] [Green Version]
- Huxley, A. New RHS Dictionary of Gardening 1: 203–205; Macmillan: New York, NY, USA, 1992; ISBN 0-333-47494-5. [Google Scholar]
- Rashed, K.; Ćirić, A.; Glamočlija, J.; Calhelha, R.C.; Ferreira, I.C.F.R.; Soković, M. Antimicrobial and cytotoxic activities of Alnus rugosa L. aerial parts and identification of the bioactive components. Ind. Crops Prod. 2014, 59, 189–196. [Google Scholar] [CrossRef]
- Rashed, K.N.Z.; Cardoso Sucupira, A.C.; Moita Neto, J.M.; Feitosa, C.M. Evaluation of Acetylcholinesterase inhibition by Alnus rugosa L. stems methanol extract and phytochemical content. Int. J. Biomed. Adv. Res. 2013, 4, 606. [Google Scholar] [CrossRef] [Green Version]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Van Leyen, K.; Kim, H.Y.; Lee, S.R.; Jin, G.; Arai, K.; Lo, E.H. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke 2006, 37, 3014–3018. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef]
- Hsieh, Y.C.S.; Hsieh, S.J.; Chang, Y.S.; Hsueh, C.M.; Hsu, S.L. The lipoxygenase inhibitor, baicalein, modulates cell adhesion and migration by up-regulation of integrins and vinculin in rat heart endothelial cells. Br. J. Pharmacol. 2007, 151, 1235–1245. [Google Scholar] [CrossRef] [Green Version]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. Baicalein as a potent neuroprotective agent: A review. Biomed. Pharmacother. 2017, 95, 1021–1032. [Google Scholar] [CrossRef]
- Mu, X.; He, G.; Cheng, Y.; Li, X.; Xu, B.; Du, G. Baicalein exerts neuroprotective effects in 6-hydroxydopamine-induced experimental parkinsonism in vivo and in vitro. Pharmacol. Biochem. Behav. 2009, 92, 642–648. [Google Scholar] [CrossRef]
- Wang, S.Y.; Wang, H.H.; Chi, C.W.; Chen, C.F.; Liao, J.F. Effects of baicalein on β-amyloid peptide-(25-35)-induced amnesia in mice. Eur. J. Pharmacol. 2004, 506, 55–61. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Hölscher, C. Therapeutic Potential of Baicalein in Alzheimer’s Disease and Parkinson’s Disease. CNS Drugs 2017, 31, 639–652. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Huang, X.; Chen, W. The effects of Baicalin and Baicalein on cerebral ischemia: A review. Aging Dis. 2017, 8, 850–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Men, W.; Shan, X.; Zhai, H.; Qiao, X.; Geng, L.; Li, C. Baicalein exerts neuroprotective effect against ischaemic/reperfusion injury via alteration of NF-kB and LOX and AMPK/Nrf2 pathway. Inflammopharmacology 2020, 28, 1327–1341. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.Q.; Jia, J.N.; Sun, Q.Y.; Zhou, H.H.; Jin, W.L.; Mao, X.Y. Baicalein exerts neuroprotective effects in FeCl3-induced posttraumatic epileptic seizures via suppressing ferroptosis. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.A.; Attele, A.S.; Zhang, L.; Yuan, C.S. Anti-HIV activity of medicinal herbs: Usage and potential development. Am. J. Chin. Med. 2001, 29, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chan, K.H.; Jiang, Y.; Kao, R.Y.T.; Lu, H.T.; Fan, K.W.; Cheng, V.C.C.; Tsui, W.H.W.; Hung, I.F.N.; Lee, T.S.W.; et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 2004, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, E.; Teoh, B.T.; Sam, S.S.; Lani, R.; Hassandarvish, P.; Chik, Z.; Yueh, A.; Abubakar, S.; Zandi, K. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [Green Version]
- Oo, A.; Rausalu, K.; Merits, A.; Higgs, S.; Vanlandingham, D.; Bakar, S.A.; Zandi, K. Deciphering the potential of baicalin as an antiviral agent for Chikungunya virus infection. Antiviral Res. 2018, 150, 101–111. [Google Scholar] [CrossRef]
- Jin, J.; Chen, Y.; Wang, D.; Ma, L.; Guo, M.; Zhou, C.; Dou, J. The inhibitory effect of sodium baicalin on oseltamivir-resistant influenza A virus via reduction of neuraminidase activity. Arch. Pharm. Res. 2018, 41, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Oo, A.; Teoh, B.T.; Sam, S.S.; Bakar, S.A.; Zandi, K. Baicalein and baicalin as Zika virus inhibitors. Arch. Virol. 2019, 164, 585–593. [Google Scholar] [CrossRef]
- Luo, Z.; Kuang, X.P.; Zhou, Q.Q.; Yan, C.Y.; Li, W.; Gong, H.B.; Kurihara, H.; Li, W.X.; Li, Y.F.; He, R.R. Inhibitory effects of baicalein against herpes simplex virus type 1. Acta Pharm. Sin. B 2020, 10, 2323–2338. [Google Scholar] [CrossRef] [PubMed]
- Yendapalli, P.R.; David, D.C.; Balasundaram, A. Evaluating the Combined Cognitive Enhancement Effect of Brassica Juncea and Cynadon Dactylon Extract in Scopolamine Induced Amnesia Zebrafish Model. Toxicol. Environ. Health Sci. 2019, 11, 190–196. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.J.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todd, K.J.; Slatter, C.A.B.; Ali, D.W. Activation of Ionotropic Glutamate Receptors on Peripheral Axons of Primary Motoneurons Mediates Transmitter Release at the Zebrafish NMJ. J. Neurophysiol. 2004, 91, 828–840. [Google Scholar] [CrossRef]
- Clemente, D.; Porteros, Á.; Weruaga, E.; Alonso, J.R.; Arenzana, F.J.; Aijón, J.; Arévalo, R. Cholinergic elements in the zebrafish central nervous system: Histochemical and immunohistochemical analysis. J. Comp. Neurol. 2004, 474, 75–107. [Google Scholar] [CrossRef]
- De Cognato, G.P.; Bortolotto, J.W.; Blazina, A.R.; Christoff, R.R.; Lara, D.R.; Vianna, M.R.; Bonan, C.D. Y-Maze memory task in zebrafish (Danio rerio): The role of glutamatergic and cholinergic systems on the acquisition and consolidation periods. Neurobiol. Learn. Mem. 2012, 98, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Richetti, S.K.; Blank, M.; Capiotti, K.M.; Piato, A.L.; Bogo, M.R.; Vianna, M.R.; Bonan, C.D. Quercetin and rutin prevent scopolamine-induced memory impairment in zebrafish. Behav. Brain Res. 2011, 217, 10–15. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Lee, Y.; Kim, D.; Jung, M.W.; Lee, C.-J. Scopolamine-induced learning impairment reversed by physostigmine in zebrafish. Neurosci. Res. 2010, 67, 156–161. [Google Scholar] [CrossRef]
- Buschi, C.; Pomilio, A. Terpenoids, flavonoids and protoalkaloids from Gomphrena boliviana. An. la Asoc. Química Argentina 1982, 70, 855–861. [Google Scholar]
- Markham, K.; Geiger, H.; Harborne, J. The Flavonoids: Advances in Research Since 1986; Chapman & Hall: New York, NY, USA, 1994. [Google Scholar]
- Ayoub, I.M.; Korinek, M.; Hwang, T.-L.; Chen, B.-H.; Chang, F.-R.; El-Shazly, M.; Singab, A.N.B. Probing the antiallergic and anti-inflammatory activity of biflavonoids and dihydroflavonols from Dietes bicolor. J. Nat. Prod. 2018, 81, 243–253. [Google Scholar] [CrossRef]
- Biekofsky, R.R.; Buschi, C.A.; Pomilio, A.B. Conformational analysis of 5,6,7-trisubstituted flavones: 13C NMR and molecular mechanics study. Magn. Reson. Chem. 1991, 29, 569–575. [Google Scholar] [CrossRef]
- Du, H.X.; Chen, X.G.; Zhang, L.; Liu, Y.; Zhan, C.S.; Chen, J.; Zhang, Y.; Yu, Z.Q.; Zhang, J.; Yang, H.Y.; et al. Microglial activation and neurobiological alterations in experimental autoimmune prostatitis-induced depressive-like behavior in mice. Neuropsychiatr. Dis. Treat. 2019, 15, 2231–2245. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, R.S.M.; Duarte, F.S.; De Lima, T.C.M. Involvement of GABAergic non-benzodiazepine sites in the anxiolytic-like and sedative effects of the flavonoid baicalein in mice. Behav. Brain Res. 2011, 221, 75–82. [Google Scholar] [CrossRef]
- Liao, J.F.; Hung, W.Y.; Chen, C.F. Anxiolytic-like effects of baicalein and baicalin in the Vogel conflict test in mice. Eur. J. Pharmacol. 2003, 464, 141–146. [Google Scholar] [CrossRef]
- Hamilton, T.J.; Morrill, A.; Lucas, K.; Gallup, J.; Harris, M.; Healey, M.; Pitman, T.; Schalomon, M.; Digweed, S.; Tresguerres, M. Establishing zebrafish as a model to study the anxiolytic effects of scopolamine. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.; Lee, C.J.; Choi, J.; Hwang, J.; Lee, Y. Anxiolytic effects of an acetylcholinesterase inhibitor, physostigmine, in the adult zebrafish. Anim. Cells Syst. 2012, 16, 198–206. [Google Scholar] [CrossRef]
- Hughes, R.N.; Otto, M.T. Anxiolytic effects of environmental enrichment attenuate sex-related anxiogenic effects of scopolamine in rats. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2013, 40, 252–259. [Google Scholar] [CrossRef]
- Smythe, J.W.; Bhatnagar, S.; Murphy, D.; Timothy, C.; Costall, B. The effects of intrahippocampal scopolamine infusions on anxiety in rats as measured by the black-white box test. Brain Res. Bull. 1998, 45, 89–93. [Google Scholar] [CrossRef]
- Bruzzone, M.; Gatto, E.; Xiccato, T.L.; Valle, L.D.; Fontana, C.M.; Meneghetti, G.; Bisazza, A. Measuring recognition memory in zebrafish larvae: Issues and limitations. PeerJ 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Aghajanzadeh, M.; Andalib, S.; Danafar, H.; Rostamizadeh, K.; Sharafi, A. The effect of baicalein-loaded Y-shaped miktoarm copolymer on spatial memory and hippocampal expression of DHCR24, SELADIN and SIRT6 genes in rat model of Alzheimer. Int. J. Pharm. 2020, 586. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Du, G.; Qin, X.; Gao, L. Integration of transcriptomics and network analysis deciphers the mechanisms of baicalein in improving learning and memory impairment in senescence-accelerated mouse prone 8 (SAMP8). Eur. J. Pharmacol. 2019, 865. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Q.; Ran, D.; Wang, H.; Du, W.; Luo, Y.; Jiang, W.; Yang, Y.; Yang, J. Changes in the levels of 12/15-lipoxygenase, apoptosis-related proteins and inflammatory factors in the cortex of diabetic rats and the neuroprotection of baicalein. Free Radic. Biol. Med. 2019, 134, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, Y. Baicalein inhibits neuroapoptosis via pathways in sevoflurane induced rats. Transl. Neurosci. 2018, 9, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Li, J.; Zhou, Y.; Huang, X.; Qin, X.; Du, G. Effects of Baicalein on Cortical Proinflammatory Cytokines and the Intestinal Microbiome in Senescence Accelerated Mouse Prone 8. ACS Chem. Neurosci. 2018, 9, 1714–1724. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.D.; Wang, K.X.; Zhou, Y.Z.; Qin, X.M.; Gao, L.; Du, G.H. Baicalein Exerts Beneficial Effects in d-Galactose-Induced Aging Rats Through Attenuation of Inflammation and Metabolic Dysfunction. Rejuvenation Res. 2017, 20, 506–516. [Google Scholar] [CrossRef]
- Janjušević, L.; Karaman, M.; Šibul, F.; Tommonaro, G.; Iodice, C.; Jakovljević, D.; Pejin, B. The lignicolous fungus Trametes versicolor (L.) Lloyd (1920): A promising natural source of antiradical and AChE inhibitory agents. J. Enzym. Inhib. Med. Chem. 2017, 32, 355–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Tan, S.; Shan, Y.L.; Wang, Y.G.; Cai, W.; Huang, X.H.; Liao, X.Y.; Li, H.Y.; Zhang, L.; Zhang, B.J.; et al. Baicalein improves behavioral dysfunction induced by Alzheimer’s disease in rats. Neuropsychiatr. Dis. Treat. 2016, 12, 3145–3152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, X.; Wang, Z.R.; Zheng, J.J.; Ding, J.Q.; Zhong, J.G.; Zhang, T.Y.; Li, W.; Zhang, M. Baicalein improves cognitive deficits and hippocampus impairments in temporal lobe epilepsy rats. Brain Res. 2019, 1714, 111–118. [Google Scholar] [CrossRef]
- Wei, N.; Wei, Y.; Li, B.; Pang, L. Baicalein Promotes Neuronal and Behavioral Recovery After Intracerebral Hemorrhage Via Suppressing Apoptosis, Oxidative Stress and Neuroinflammation. Neurochem. Res. 2017, 42, 1345–1353. [Google Scholar] [CrossRef]
- Kim, K.C.; Lee, I.K.; Kang, K.A.; Kim, H.S.; Kang, S.S.; Hyun, J.W. Baicalein (5,6,7-trihydroxyflavone) reduces oxidative stress-induced DNA damage by upregulating the DNA repair system. Cell Biol. Toxicol. 2012, 28, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Brinza, I.; Abd-Alkhalek, A.M.; El-Raey, M.A.; Boiangiu, R.S.; Eldahshan, O.A.; Hritcu, L. Ameliorative Effects of Rhoifolin in Scopolamine-Induced Amnesic Zebrafish (Danio rerio) Model. Antioxidants 2020, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Capatina, L.; Todirascu-Ciornea, E.; Napoli, E.M.; Ruberto, G.; Hritcu, L.; Dumitru, G. Thymus vulgaris Essential Oil Protects Zebrafish against Cognitive Dysfunction by Regulating Cholinergic and Antioxidants Systems. Antioxidants 2020, 9, 1083. [Google Scholar] [CrossRef]
- Capatina, L.; Boiangiu, R.S.; Dumitru, G.; Napoli, E.M.; Ruberto, G.; Hritcu, L.; Todirascu-Ciornea, E. Rosmarinus officinalis Essential Oil Improves Scopolamine-Induced Neurobehavioral Changes via Restoration of Cholinergic Function and Brain Antioxidant Status in Zebrafish (Danio rerio). Antioxidants 2020, 9, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boiangiu, R.S.; Mihasan, M.; Gorgan, D.L.; Stache, B.A.; Hritcu, L. Anxiolytic, Promnesic, Anti-Acetylcholinesterase and Antioxidant Effects of Cotinine and 6-Hydroxy-L-Nicotine in Scopolamine-Induced Zebrafish (Danio rerio) Model of Alzheimer’s Disease. Antioxidants 2021, 10, 212. [Google Scholar] [CrossRef]
- Levin, E.D.; Bencan, Z.; Cerutti, D.T. Anxiolytic effects of nicotine in zebrafish. Physiol. Behav. 2007, 90, 54–58. [Google Scholar] [CrossRef]
- Stefanello, F.V.; Fontana, B.D.; Ziani, P.R.; Müller, T.E.; Mezzomo, N.J.; Rosemberg, D.B. Exploring Object Discrimination in Zebrafish: Behavioral Performance and Scopolamine-Induced Cognitive Deficits at Different Retention Intervals. Zebrafish 2019, 16, 370–378. [Google Scholar] [CrossRef]
- Gaspary, K.V.; Reolon, G.K.; Gusso, D.; Bonan, C.D. Novel object recognition and object location tasks in zebrafish: Influence of habituation and NMDA receptor antagonism. Neurobiol. Learn. Mem. 2018, 155, 249–260. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.J.; Featherstone, R.M.; Feather-Stone, R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Winterbourn, C.; Hawkins, R.; Brian, M.; Carrell, R. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975, 85, 337. [Google Scholar] [PubMed]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Tokumura, A. Glutathione peroxidase activity in tissues of vitamin E-deficient mice. J. Nutr. Sci. Vitaminol. 1976, 22, 405–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, C.N.; Ahn, B.W.; Moerman, E.J.; Goldstein, S.; Stadtman, E.R. Age-related changes in oxidized proteins. J. Biol. Chem. 1987, 262, 5488–5491. [Google Scholar] [CrossRef]
- Luo, S.; Wehr, N.B. Protein carbonylation: Avoiding pitfalls in the 2,4-dinitrophenylhydrazine assay. Redox. Rep. 2009, 14, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brinza, I.; Ayoub, I.M.; Eldahshan, O.A.; Hritcu, L. Baicalein 5,6-Dimethyl Ether Prevents Memory Deficits in the Scopolamine Zebrafish Model by Regulating Cholinergic and Antioxidant Systems. Plants 2021, 10, 1245. https://doi.org/10.3390/plants10061245
Brinza I, Ayoub IM, Eldahshan OA, Hritcu L. Baicalein 5,6-Dimethyl Ether Prevents Memory Deficits in the Scopolamine Zebrafish Model by Regulating Cholinergic and Antioxidant Systems. Plants. 2021; 10(6):1245. https://doi.org/10.3390/plants10061245
Chicago/Turabian StyleBrinza, Ion, Iriny M. Ayoub, Omayma A. Eldahshan, and Lucian Hritcu. 2021. "Baicalein 5,6-Dimethyl Ether Prevents Memory Deficits in the Scopolamine Zebrafish Model by Regulating Cholinergic and Antioxidant Systems" Plants 10, no. 6: 1245. https://doi.org/10.3390/plants10061245
APA StyleBrinza, I., Ayoub, I. M., Eldahshan, O. A., & Hritcu, L. (2021). Baicalein 5,6-Dimethyl Ether Prevents Memory Deficits in the Scopolamine Zebrafish Model by Regulating Cholinergic and Antioxidant Systems. Plants, 10(6), 1245. https://doi.org/10.3390/plants10061245








