Control of Plant Viral Diseases by CRISPR/Cas9: Resistance Mechanisms, Strategies and Challenges in Food Crops
Abstract
:1. Introduction
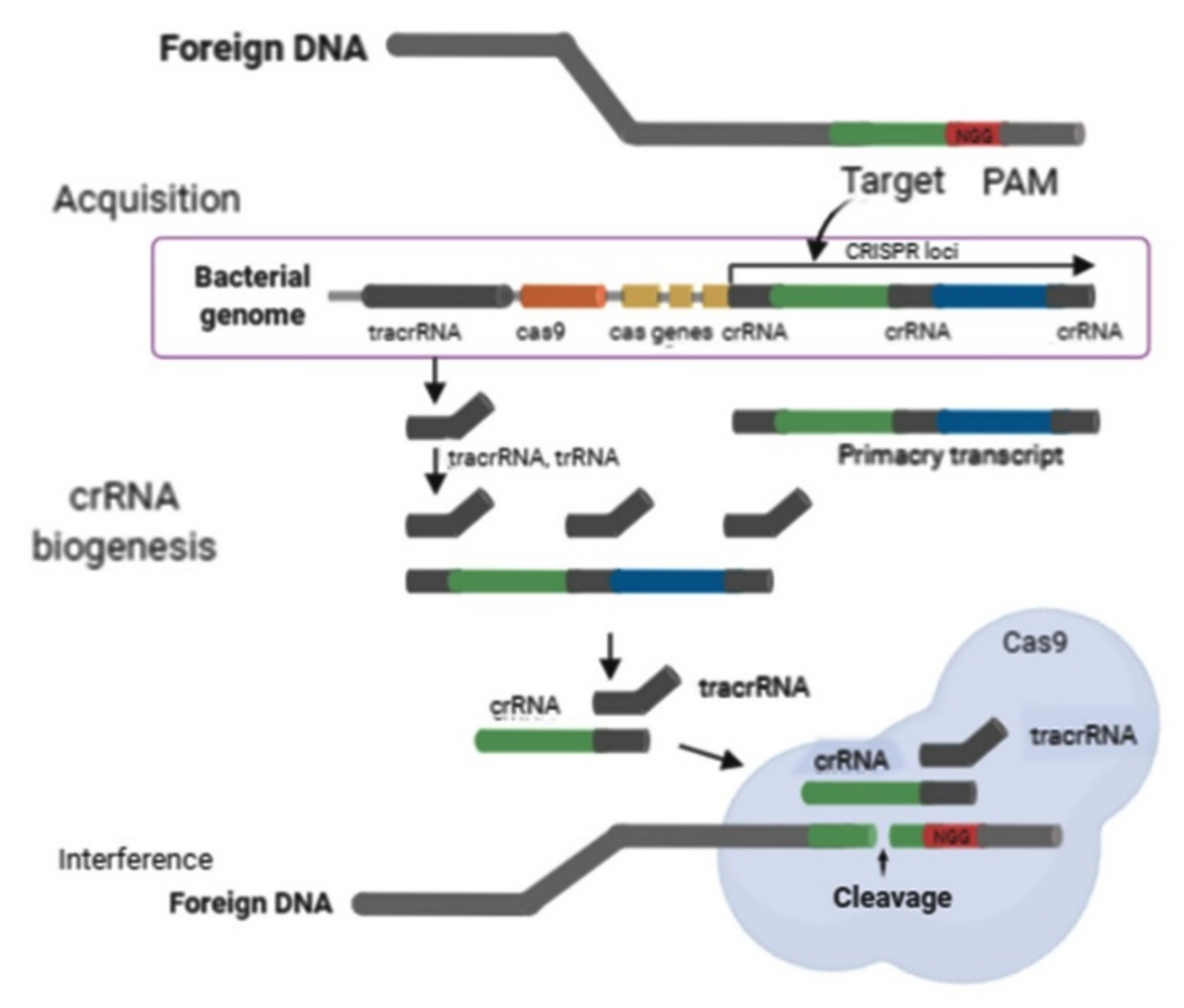
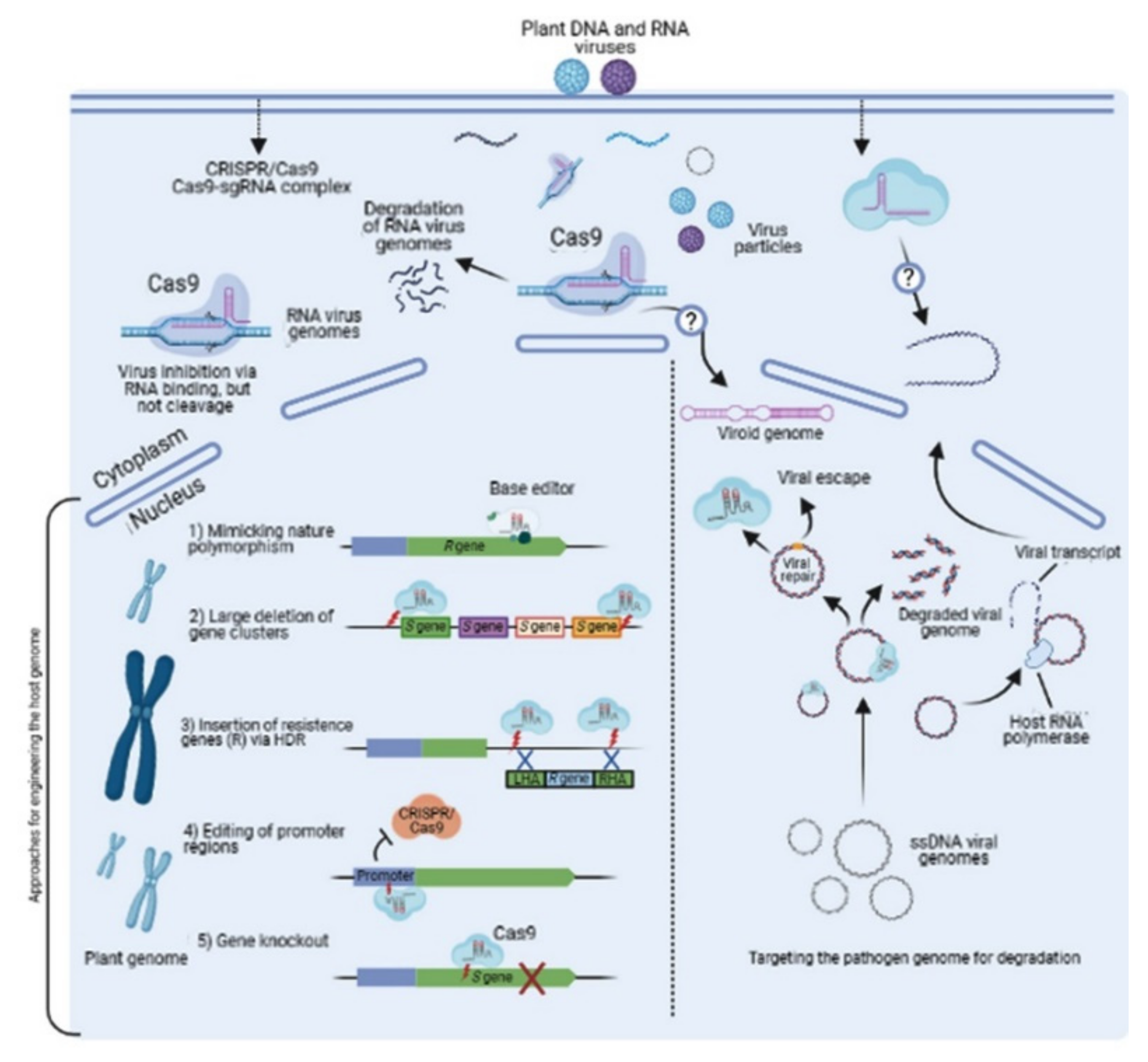
2. Virus-Resistance Mechanism of CRISPR/Cas9 in Crops
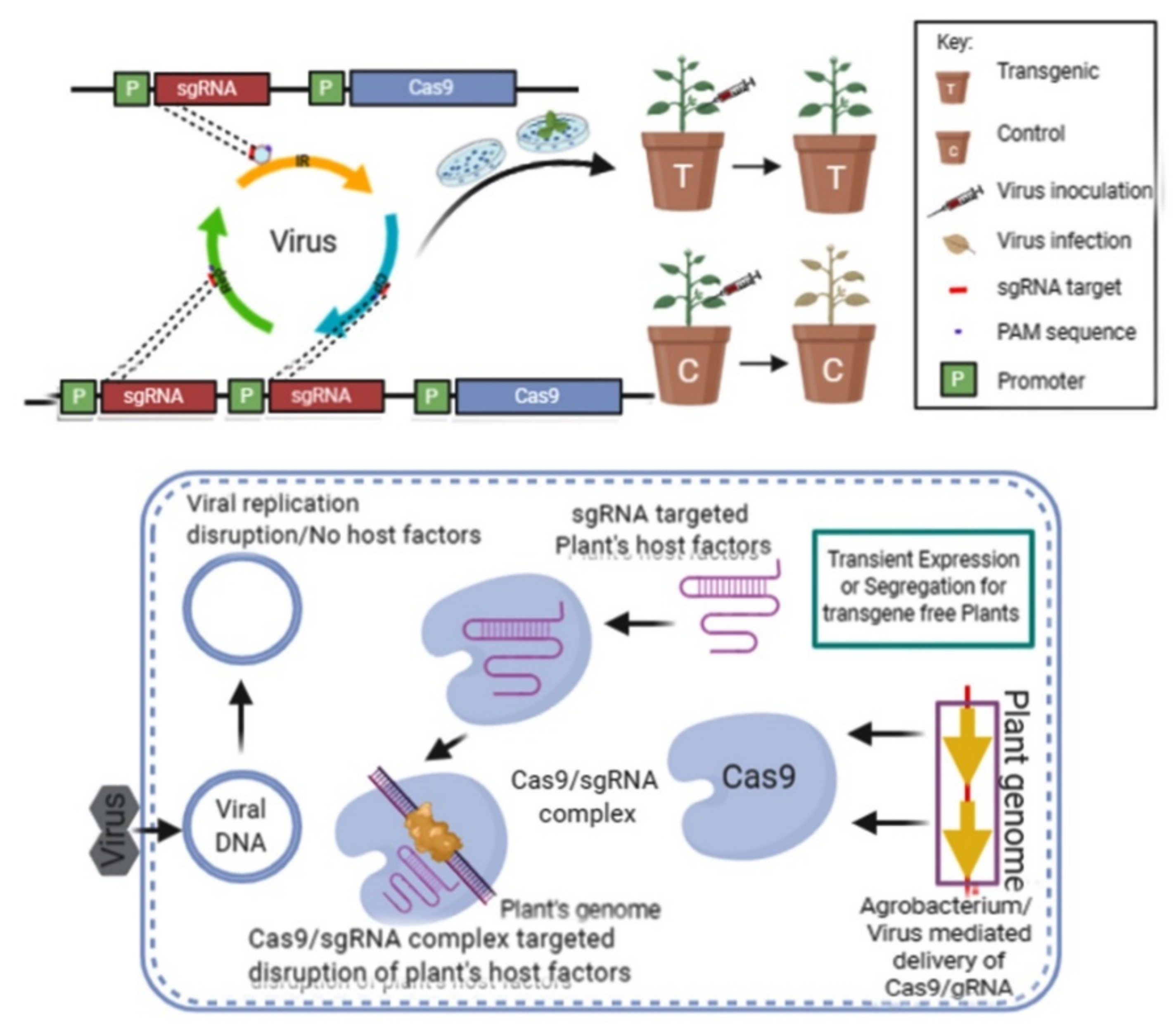
3. Plant Virus-Resistance Strategies Using Cas9 Endonuclease
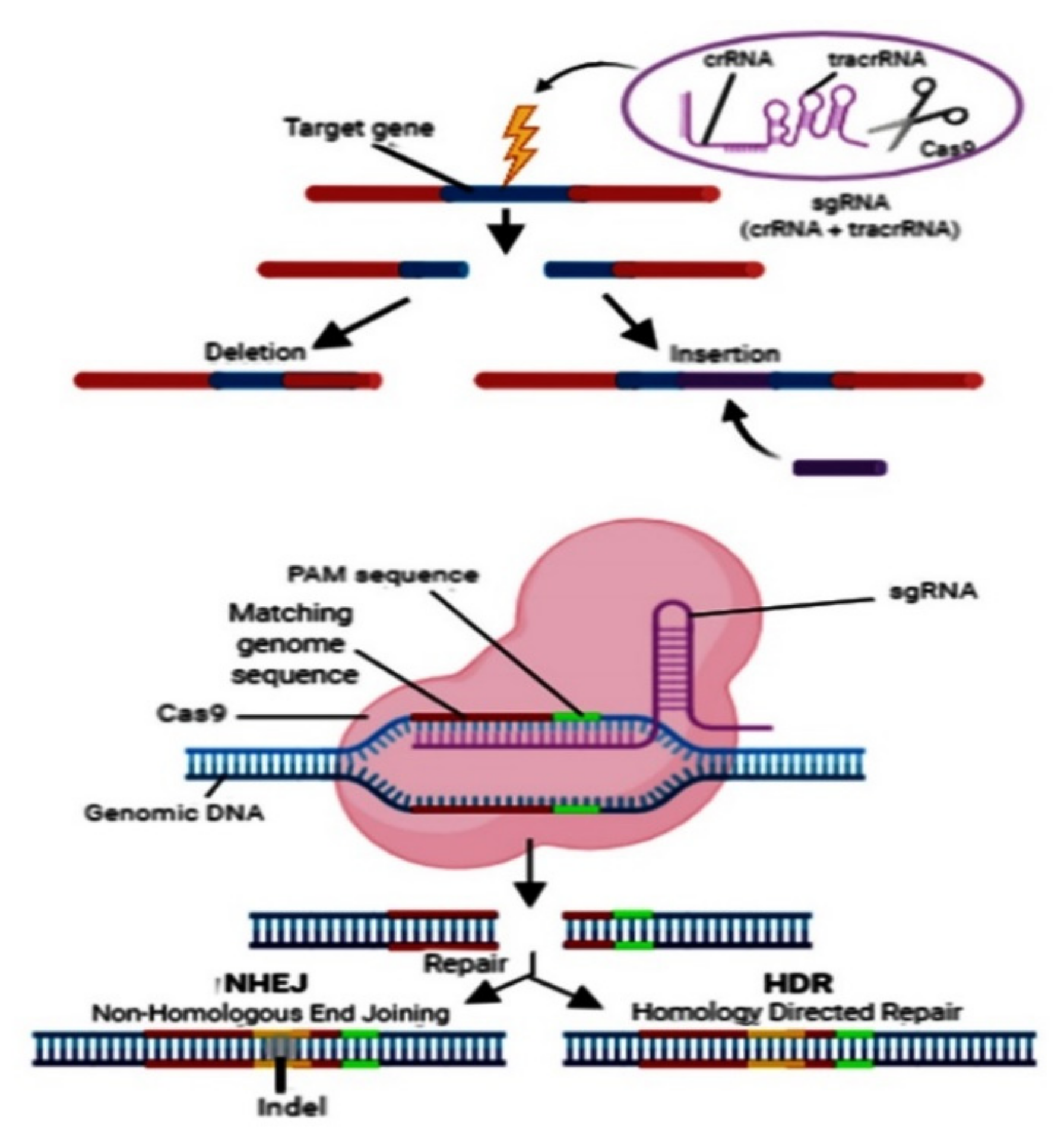
4. Deletion and Insertion of the Target Gene
5. Off-Target Mutations in CRISPR/Cas9
6. Overcoming Off-Target Effects
7. CRISPR/Cas9 Toolkit in Crop Improvement
8. Genetics of Plant Virus Resistance
9. Economic Importance of Plant Viral Diseases in Food Crops
10. Challenges
11. Research Opportunities
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velásquez, A.C.; Castroverde, C.D.; He, S.Y. Plant–Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef] [Green Version]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Esse, H.P.; Reuber, T.L.; Does, D. Genetic modification to improve disease resistance in crops. New Phytol. 2020, 225, 70–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, O.X.; Ronald, P.C. Genetic engineering for disease resistance in plants: Recent progress and future perspectives. Plant Physiol. 2019, 180, 26–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Bulk, R.W. Application of cell and tissue culture and in vitro selection for disease resistance breeding—A review. Euphytica 1991, 56, 269–285. [Google Scholar] [CrossRef]
- Nelson, R.; Wiesner-Hanks, T.; Wisser, R.; Balint-Kurti, P. Navigating complexity to breed disease-resistant crops. Nat. Rev. Genet. 2017, 19, 21–33. [Google Scholar] [CrossRef]
- Deshpande, K.; Vyas, A.; Balakrishnan, A.; Vyas, D. Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 Genetic Engineering: Robotic Genetic Surgery. Am. J. Robot. Surg. 2016, 2, 49–52. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Jain, M. The CRISPR-Cas system for plant genome editing: Advances and opportunities. J. Exp. Bot. 2015, 66, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Barakate, A.; Stephens, J. An overview of crispr-based tools and their improvements: New opportunities in understanding plant-pathogen interactions for better crop protection. Front. Plant Sci. 2016, 7, 765. [Google Scholar] [CrossRef] [Green Version]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef] [Green Version]
- Larson, M.H.; Gilbert, L.A.; Wang, X.; Lim, W.A.; Weissman, J.S.; Qi, L.S. CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat. Protoc. 2013, 8, 2180–2196. [Google Scholar] [CrossRef] [Green Version]
- Marraffini, L.A.; Sontheimer, E.J. CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. Nat. Rev. Genet. 2010, 11, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Shan-e-Ali Zaidi, S.; Mansoor, S.; Ali, Z.; Tashkandi, M.; Mahfouz, M.M. Engineering Plants for Geminivirus Resistance with CRISPR/Cas9 System. Trends Plant Sci. 2016, 21, 279–281. [Google Scholar] [CrossRef]
- Sternberg, S.H.; Redding, S.; Jinek, M.; Greene, E.C.; Doudna, J.A. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature 2014, 507, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Arora, L.; Narula, A. Gene editing and crop improvement using CRISPR-cas9 system. Front. Plant Sci. 2017, 8, 1932. [Google Scholar] [CrossRef] [Green Version]
- Deltcheva, E.; Chylinski, K.; Sharma, C.M.; Gonzales, K.; Chao, Y.; Pirzada, Z.A.; Eckert, M.R.; Vogel, J.; Charpentier, E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 2011, 471, 602–607. [Google Scholar] [CrossRef] [Green Version]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; Van Der Oost, J.; Regev, A.; et al. Cpf1 Is a Single RNA-Guided Endonuclease of a Class 2 CRISPR-Cas System. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef] [Green Version]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhou, H.; Bi, H.; Fromm, M.; Yang, B.; Weeks, D.P. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 2013, 41. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.S.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-γuided platform for sequence-specific control of gene expression. Cell 2013, 152, 1173–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef] [Green Version]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, S.S.; Mahas, A.; Vanderschuren, H.; Mahfouz, M.M. Engineering crops of the future: CRISPR approaches to develop climate-resilient and disease-resistant plants. Genome Biol. 2020, 21, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.; Mukhtar, S.; Sakina, A.; Dar, A.A.; Bhat, R.; Deshmukh, R.; Molla, K.; Kundoo, A.A.; Dar, M.S. Tweaking genome-editing approaches for virus interference in crop plants. Plant Physiol. Biochem. 2020, 147, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhou, H.; Zhou, X.; Li, F. Control of Plant Viruses by CRISPR/Cas System-Mediated Adaptive Immunity. Front. Microbiol. 2020, 11, 593700. [Google Scholar] [CrossRef]
- Pramanik, D.; Shelake, R.M.; Park, J.; Kim, M.J.; Hwang, I.; Park, Y.; Kim, J.Y. CRISPR/Cas9-mediated generation of pathogen-resistant tomato against tomato yellow leaf curl virus and powdery mildew. Int. J. Mol. Sci. 2021, 22, 1878. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Venkataramani, P.; Nandi, S.; Bhattacharjee, S. CRISPR-Cas9 a boon or bane: The bumpy road ahead to cancer therapeutics 06 Biological Sciences 0604 Genetics. Cancer Cell Int. 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, L.; Sun, S.; Wu, C.; Yao, W.; Jiang, B.; Han, T.; Hou, W. CRISPR/Cas9-mediated deletion of large genomic fragments in Soybean. Int. J. Mol. Sci. 2018, 19, 3835. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Liu, B.; Weeks, D.P.; Spalding, M.H.; Yang, B. Large chromosomal deletions and heritable small genetic changes induced by CRISPR/Cas9 in rice. Nucleic Acids Res. 2014, 42, 10903–10914. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Rehman, S.; Tang, X.; Gu, K.; Fan, Q.; Chen, D.; Ma, W. Methodologies for improving HDR efficiency. Front. Genet. 2019, 10, 691. [Google Scholar] [CrossRef]
- Van Vu, T.; Sung, Y.W.; Kim, J.; Doan, D.T.; Tran, M.T.; Kim, J.Y. Challenges and Perspectives in Homology-Directed Gene Targeting in Monocot Plants. Rice 2019, 12, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ge, X.; Yang, F.; Zhang, L.; Zheng, J.; Tan, X.; Jin, Z.B.; Qu, J.; Gu, F. Comparison of non-canonical PAMs for CRISPR/Cas9-mediated DNA cleavage in human cells. Sci. Rep. 2014, 4, 5405. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, N.O.; Khromov, A.; Love, A.J.; Taliansky, M.E. CRISPR Applications in Plant Virology: Virus Resistance and Beyond. Phytopathology 2020, 110, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Manghwar, H.; Li, B.; Ding, X.; Hussain, A.; Lindsey, K.; Zhang, X.; Jin, S. CRISPR/Cas Systems in Genome Editing: Methodologies and Tools for sgRNA Design, Off-Target Evaluation, and Strategies to Mitigate Off-Target Effects. Adv. Sci. 2020, 7, 1902312. [Google Scholar] [CrossRef]
- Zhang, Y.; Massel, K.; Godwin, I.D.; Gao, C. Applications and potential of genome editing in crop improvement 06 Biological Sciences 0604 Genetics 06 Biological Sciences 0607 Plant Biology 07 Agricultural and Veterinary Sciences 0703 Crop and Pasture Production. Genome Biol. 2018, 19, 210. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, Q.; Chen, Y.; Liu, Y.G. CRISPR/Cas9 Platforms for Genome Editing in Plants: Developments and Applications. Mol. Plant 2016, 9, 961–974. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Geng, L.; Yuan, M.; Wei, J.; Jin, C.; Li, M.; Yu, K.; Zhang, Y.; Jin, H.; Wang, E.; et al. Deletion of a target gene in Indica rice via CRISPR/Cas9. Plant Cell Rep. 2017, 36, 1333–1343. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, L.; Liu, X.; Guo, C.; Sun, S.; Wu, C.; Jiang, B.; Han, T.; Hou, W. CRISPR/Cas9-mediated targeted mutagenesis of GmFT2a delays flowering time in soya bean. Plant Biotechnol. J. 2018, 16, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Soyk, S.; Müller, N.A.; Park, S.J.; Schmalenbach, I.; Jiang, K.; Hayama, R.; Zhang, L.; Van Eck, J.; Jiménez-Gómez, J.M.; Lippman, Z.B. Variation in the flowering gene SELF PRUNING 5G promotes day-neutrality and early yield in tomato. Nat. Genet. 2017, 49, 162–168. [Google Scholar] [CrossRef]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol. J. 2017, 15, 1509–1519. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.C.; Yeam, I.; Jahn, M.M. Genetics of plant virus resistance. Annu. Rev. Phytopathol. 2005, 43, 581–621. [Google Scholar] [CrossRef] [Green Version]
- Salgotra, R.K.; Neal Stewart, C. Functional markers for precision plant breeding. Int. J. Mol. Sci. 2020, 21, 4792. [Google Scholar] [CrossRef] [PubMed]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier Academic Press: Burlington, MA, USA, 2005. [Google Scholar]
- Tripathi, J.N.; Ntui, V.O.; Ron, M.; Muiruri, S.K.; Britt, A.; Tripathi, L. CRISPR/Cas9 editing of endogenous banana streak virus in the B genome of Musa spp. overcomes a major challenge in banana breeding. Commun. Biol. 2019, 2, 46. [Google Scholar] [CrossRef] [Green Version]
- Baltes, N.J.; Hummel, A.W.; Konecna, E.; Cegan, R.; Bruns, A.N.; Bisaro, D.M.; Voytas, D.F. Conferring resistance to geminiviruses with the CRISPR-Cas prokaryotic immune system. Nat. Plants 2015, 1, 15145. [Google Scholar] [CrossRef]
- Ji, X.; Si, X.; Zhang, Y.; Zhang, H.; Zhang, F.; Gao, C. Conferring DNA virus resistance with high specificity in plants using virus-inducible genome-editing system. Genome Biol. 2018, 19, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez, M.A.; Lin, Z.D.; Moll, T.; Chauhan, R.D.; Renninger, K.; Beyene, G.; Taylor, N.J.; Carrington, J.; Staskawicz, B.; Bart, R. Simultaneous CRISPR/Cas9-mediated editing of cassava eIF4E isoforms nCBP-1 and nCBP-2 reduces cassava brown streak disease symptom severity and incidence. bioRxiv 2017, 209874. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Soyars, C.L.; Li, J.; Fei, Q.; He, G.; Peterson, B.A.; Meyers, B.C.; Nimchuk, Z.L.; Wang, X. CRISPR/Cas9-mediated resistance to cauliflower mosaic virus. Plant Direct 2018, 2, e00047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zheng, Q.; Yi, X.; An, H.; Zhao, Y.; Ma, S.; Zhou, G. Establishing RNA virus resistance in plants by harnessing CRISPR immune system. Plant Biotechnol. J. 2018, 16, 1415–1423. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekaran, J.; Brumin, M.; Wolf, D.; Leibman, D.; Klap, C.; Pearlsman, M.; Sherman, A.; Arazi, T.; Gal-On, A. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant Pathol. 2016, 17, 1140–1153. [Google Scholar] [CrossRef] [Green Version]
- Macovei, A.; Sevilla, N.R.; Cantos, C.; Jonson, G.B.; Slamet-Loedin, I.; Čermák, T.; Voytas, D.F.; Choi, I.R.; Chadha-Mohanty, P. Novel alleles of rice eIF4G generated by CRISPR/Cas9-targeted mutagenesis confer resistance to Rice tungro spherical virus. Plant Biotechnol. J. 2018, 16, 1918–1927. [Google Scholar] [CrossRef] [Green Version]
- Pyott, D.E.; Sheehan, E.; Molnar, A. Engineering of CRISPR/Cas9-mediated potyvirus resistance in transgene-free Arabidopsis plants. Mol. Plant Pathol. 2016, 17, 1276–1288. [Google Scholar] [CrossRef] [Green Version]
- Ali, Z.; Ali, S.; Tashkandi, M.; Zaidi, S.S.; Mahfouz, M.M. CRISPR/Cas9-Mediated Immunity to Geminiviruses: Differential Interference and Evasion. Sci. Rep. 2016, 6, 26912. [Google Scholar] [CrossRef] [Green Version]
- Tashkandi, M.; Ali, Z.; Aljedaani, F.; Shami, A.; Mahfouz, M.M. Engineering resistance against Tomato yellow leaf curl virus via the CRISPR/Cas9 system in tomato. Plant Signal. Behav. 2018, 13, e1525996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, Z.; Abulfaraj, A.; Idris, A.; Ali, S.; Tashkandi, M.; Mahfouz, M.M. CRISPR/Cas9-mediated viral interference in plants. Genome Biol. 2015, 16, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kis, A.; Hamar, É.; Tholt, G.; Bán, R.; Havelda, Z. Creating highly efficient resistance against wheat dwarf virus in barley by employing CRISPR/Cas9 system. Plant Biotechnol. J. 2019, 17, 1004–1006. [Google Scholar] [CrossRef]
- Reddy, D.V.; Sudarshana, M.R.; Fuchs, M.; Rao, N.C.; Thottappilly, G. Genetically engineered virus-resistant plants in developing countries: Current status and future prospects. Adv. Virus Res. 2009, 75, 185–220. [Google Scholar] [CrossRef] [PubMed]
- Gosavi, G.; Yan, F.; Ren, B.; Kuang, Y.; Yan, D.; Zhou, X.; Zhou, H. Applications of CRISPR technology in studying plant-pathogen interactions: Overview and perspective. Phytopathol. Res. 2020, 2, 1–9. [Google Scholar] [CrossRef]
- Fondong, V.N. The search for resistance to cassava mosaic geminiviruses: How much we have accomplished, and what lies ahead. Front. Plant Sci. 2017, 8, 408. [Google Scholar] [CrossRef] [Green Version]
- Houngue, J.A.; Zandjanakou-Tachin, M.; Ngalle, H.B.; Pita, J.S.; Cacaï, G.H.; Ngatat, S.E.; Bell, J.M.; Ahanhanzo, C. Evaluation of resistance to cassava mosaic disease in selected African cassava cultivars using combined molecular and greenhouse grafting tools. Physiol. Mol. Plant Pathol. 2019, 105, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Tsao, T.T. Towards the Development of Transgenic Banana Bunchy Top Virus (BBTV)-Resistant Banana Plants: Interference with Replication. Ph.D. Thesis, Queensland University of Technology, Brisbane, Australia, 2008. [Google Scholar]
- Jarošová, J.; Singh, K.; Chrpová, J.; Kundu, J.K. Analysis of Small RNAs of Barley Genotypes Associated with Resistance to Barley Yellow Dwarf Virus. Plants 2020, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, S.; Larkin, P.; Xu, R.; Hayden, M.; Forrest, K.; Meinke, H.; Hu, H.; Zhou, M.; Fan, Y. Genome wide association study reveals novel QTL for barley yellow dwarf virus resistance in wheat. BMC Genom. 2019, 20, 89. [Google Scholar] [CrossRef] [Green Version]
- Pasev, G.; Kostova, D.; Sofkova, S. Identification of Genes for Resistance to Bean Common Mosaic Virus and Bean Common Mosaic Necrosis Virus in Snap Bean ( Phaseolus vulgaris L.) Breeding Lines Using Conventional and Molecular Methods. J. Phytopathol. 2014, 162, 19–25. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Zhou, G.; Zhang, T. Engineering plant virus resistance: From RNA silencing to genome editing strategies. Plant Biotechnol. J. 2020, 18, 328–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velez, J.J.; Bassett, M.J.; Beaver, J.S.; Molina, A. Inheritance of resistance to bean golden mosaic virus in common bean. J. Am. Soc. Hortic. Sci. 1998, 123, 628–631. [Google Scholar] [CrossRef] [Green Version]
- Blair, M.W.; Rodriguez, L.M.; Pedraza, F.; Morales, F.; Beebe, S. Genetic mapping of the bean golden yellow mosaic geminivirus resistance gene bgm-1 and linkage with potyvirus resistance in common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2007, 114, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Grimmer, M.K.; Bean, K.M.; Qi, A.; Stevens, M.; Asher, M.J. The action of three Beet yellows virus resistance QTLs depends on alleles at a novel genetic locus that controls symptom development. Plant Breed. 2008, 127, 391–397. [Google Scholar] [CrossRef]
- Vigne, E.; Komar, V.; Fuchs, M. Field safety assessment of recombination in transgenic grapevines expressing the coat protein gene of Grapevine fanleaf virus. Transgenic Res. 2004, 13, 165–179. [Google Scholar] [CrossRef]
- German-Retana, S.; Walter, J.; Le Gall, O. Lettuce mosaic virus: From pathogen diversity to host interactors. Mol. Plant Pathol. 2008, 9, 127–136. [Google Scholar] [CrossRef]
- Jones, M.W.; Redinbaugh, M.G.; Louie, R. The Mdm1 locus and maize resistance to Maize dwarf mosaic virus. Plant Dis. 2007, 91, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Nair, S.K.; Babu, R.; Magorokosho, C.; Mahuku, G.; Semagn, K.; Beyene, Y.; Das, B.; Makumbi, D.; Lava Kumar, P.; Olsen, M.; et al. Fine mapping of Msv1, a major QTL for resistance to Maize Streak Virus leads to development of production markers for breeding pipelines. Theor. Appl. Genet. 2015, 128, 1839–1854. [Google Scholar] [CrossRef] [PubMed]
- Zuriaga, E.; Romero, C.; Blanca, J.M.; Badenes, M.L. Resistance to Plum Pox Virus (PPV) in apricot (Prunus armeniaca L.) is associated with down-regulation of two MATHd genes. BMC Plant Biol. 2018, 18, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, O.L.; Ribeiro, S.R.; Moreira, C.M.; Guedes, M.L.; Lyra, D.H.; Pinto, C.A. Introgression of the Rladg allele of resistance to potato leafroll virus in Solanum tuberosum L. Crop. Breed. Appl. Biotechnol. 2017, 17, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Bendahmane, A.; Köhm, B.A.; Dedi, C.; Baulcombe, D.C. The coat protein of potato virus X is a strain-specific elicitor of Rx1-mediated virus resistance in potato. Plant J. 1995, 8, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Torrance, L.; Cowan, G.H.; McLean, K.; MacFarlane, S.; Al-Abedy, A.N.; Armstrong, M.; Lim, T.Y.; Hein, I.; Bryan, G.J. Natural resistance to Potato virus Y in Solanum tuberosum Group Phureja. Theor. Appl. Genet. 2020, 133, 967–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesan, U.; Suri, S.S.; Rajasubramaniam, S.; Rajam, M.V.; Dasgupta, I. Transgenic expression of coat protein gene of Rice tungro bacilliform virus in rice reduces the accumulation of viral DNA in inoculated plants. Virus Genes 2009, 39, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Falk, B.W.; Nouri, S. Special issue: “Plant virus pathogenesis and disease control”. Viruses 2020, 12, 1049. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, T.J.; de Leon, N.; Kaeppler, S.M.; Tracy, W.F. Genetic analysis of sugarcane mosaic virus resistance in the Wisconsin diversity panel of maize. Crop. Sci. 2018, 58, 1853–1865. [Google Scholar] [CrossRef]
- Widyasari, K.; Alazem, M.; Kim, K.H. Soybean resistance to soybean mosaic virus. Plants 2020, 9, 219. [Google Scholar] [CrossRef] [Green Version]
- Pérez-de-Castro, A.; Esteras, C.; Alfaro-Fernández, A.; Daròs, J.A.; Monforte, A.J.; Picó, B.; Gómez-Guillamón, M.L. Fine mapping of wmv 1551, a resistance gene to Watermelon mosaic virus in melon. Mol. Breed. 2019, 39, 1–15. [Google Scholar] [CrossRef]
| Virus | Virus Family | Damaging Food Crop | Distribution | Vector | Crop Yield Loss |
|---|---|---|---|---|---|
| African cassava mosaic virus (ACMV) | Geminiviridae | Cassava | Africa and all cassava growing countries | Whitefly | 20–90% |
| Banana bunchy top virus (BBTV) | Nanoviridae | Banana | All banana growing countries | Aphid | Up to 100% |
| Banana streak virus (BSV) | Caulimoviridae | Banana | All banana growing countries | Mealybug | 6–15% |
| Barley yellow dwarf virus (BYDV) | Luteoviridae | Barley, oats, rye and wheat | Global | Aphid | 30–50%, |
| Bean yellow dwarf virus (BeYDV) | Potyviridae | Bean, peas and other legumes and non-legumes | Global | Aphid and mechanical infection | 30–70% |
| Bean common mosaic virus (BCMV) and bean yellow mosaic virus (BYMV) | Potyviridae | BCMV infects the only bean, BYMV also infects peas and yellow summer squash | Global | Aphid and seeds from infected plants | Up to 100% |
| Bean golden mosaic virus (BGMV) | Geminviridae | Bean, other legume crops and Malvaceous weeds | Global | Whitefly | Up to 100% |
| Beet severe curly top virus (BSCTV) | Geminiviridae | Sugar beet, bean, melons, spinach, sugar beet and tomato | Global | Leafhopper | Up to 100% |
| Beet yellows virus (BYV) | Closteroviridae | Sugar beets, spinach and table beets | All sugar beet growing countries | Aphid | Up to 50% |
| Cassava brown streak virus (CBSV) | Potyviridae | Cassava | East Africa including Kenya, Mozambique and Tanzania | Whitefly | Up to 30%, reduced market price up to 90% |
| Cauliflower mosaic virus (CaMV) | Caulimoviridae | Vegetables | Many parts of the world | Aphid | 20–50% |
| Cotton leaf curl kokhran virus (CLCuKoV) | Geminiviridae | Okra | China, India, Pakistan and Philippines | Whitefly | Up to 55% |
| Cucumber mosaic virus (CMV) | Bromoviridae | Bananas, beans, beets, celery, crucifers, cucumbers, melons, peppers, spinach, squash, tomatoes and vegetables | Global | Aphid | 80% and above |
| Cucumber vein yellowing virus (CVYV) | Potyviridae | Cucumber, melon or watermelon and other crops of Cucurbitaceae family | Global | Whitefly | Up to 70% |
| Grapevine fan leaf virus (GFLV) | Secoviridae | Grape | Global | Nematodes | Up to 80% |
| Lettuce infectious yellows virus (LIYV) | Closteroviridae | Lettuce, cantaloupe, carrot, melon, squash, sugar beet, squash | Global | Whitefly | 30–100% |
| Lettuce mosaic virus (LMV) | Potyviridae | Lettuce, marigold, pea and sweet pea | Global | Aphid and infected seeds | 55–85% |
| Maize dwarf mosaic virus (MDMV) | Potyviridae | Maize | Global | Aphid | Up to 70% |
| Maize streak virus (MSV) | Geminiviridae | Maize, rice, wheat, millet and sugarcane | India and southern part of Africa | Leafhopper | Up to 100% |
| Merremia mosaic virus (MeMV) | Geminiviridae | Hot pepper, sweet pepper and so on. | Where irrigated crop production practiced worldwide | Whitefly | 70–80% |
| Okra yellow vein mosaic virus (OYVMV) | Geminiviridae | Okra | Global | Whitefly | More than 90% |
| Papaya ring spot virus-W (PRSV-W) | Potyviridae | Papaya and cucurbits | Global | Aphid | Up to 100% |
| Plum pox virus (PPV) | Potyviridae | Apricot, nectarine, peach and plum | Global including Asia, Europe and North America | Aphid, budding and grafting | 80–100% |
| Potato leafroll virus (PLRV) | Luteoviridae | Potato | Global | Aphid | Up to 90% |
| Potato virus X (PVX) | Alphaflexiviridae | Potato, pepper, tobacco and tomato | Global | Handling of the plant materials | 30–40% |
| Potato virus Y (PVY) | Potyviridae | Potato, pepper, tobacco and tomato | Global | Aphid | Up to 70% |
| Rice tungro bacilliform virus (RTBV) and Rice tungro spherical virus (RTSV) | Caulimoviridae (RTBV), Secoviridae (RTSV) | Rice | South and Southeast Asia | Leafhopper | Up to 100% |
| Soybean mosaic virus (SMV) | Potyviridae | Soybean and various crops of Fabaceae and Leguminosae family | Global | Aphid | 35–90% |
| Squash leaf curl virus (SLCV) | Geminiviridae | All cucurbits such as squash, cucumber, watermelon and cantaloupe | Southern region of California | Whitefly | 70–80% |
| Sugarcane mosaic virus (SCMV) | Potyviridae | Corn, sorghum, sugarcane and other crops of Gramineae family | Global | Aphid | Up to 40% |
| Tomato mosaic virus (ToMV) | Virgaviridae | Tomato and other Solanaceous crops | Global | Infected seeds | 20–90% |
| Tomato mottle virus (TMoV) | Geminiviridae | Common bean, tobacco and tomato | Global | Whitefly | Up to 95% |
| Tomato ring spot virus (TomRSV) | Secoviridae | Tomato, strawberries, raspberries, grapes and apple | Global | Nematodes | 50–80% |
| Tomato spotted wilt virus (TSWV) | Bunyaviridae | Lettuce, papaya, peanut, pineapple, tomato and various fruits and vegetables | Tropics and subtropics and a few temperate regions of the world | Thrip | 50–90% |
| Tomato yellow leaf curl Sardinia virus (TYLCSV) | Geminiviridae | Watermelon, tomato, squash, potato, pepper, melon, cotton, cassava and bean | Global particularly prevalent in tropics and subtropics regions | Whitefly | Up to 100% |
| Tomato yellow leaf curl virus (TYLCV) | Geminiviridae | Tomato and many food crops | Global | Whitefly | Up to 100% |
| Turnip mosaic virus (TuMV) | Potyviridae | Brussels, sprouts, cabbage and cauliflower | Global | Aphid | Up to 70% |
| Watermelon mosaic virus (WMV) | Potyviridae | Peas and Leguminous crops | Global | Aphid | Up to 100% |
| Wheat dwarf virus (WDV) | Geminiviridae | Wheat and barley | Throughout Europe | Leafhopper | Up to 75% |
| Zucchini yellow mosaic virus (ZYMV) | Potyviridae | Cucumber, muskmelon, watermelon, zucchini squash. | Global | Aphid | Up to 70% |
| Virus in Food Crops | Experimental/Model Host | Targeted Gene Region(s) | Key Strategies of CRISPR/Cas9 | Reference |
|---|---|---|---|---|
| Banana streak virus (BSV) | Arabidopsis thaliana | BSOLV and eBSOLV | The gRNAs were designed to target the sequences of BSOLV and eBSOLV via the CRISPR/Cas9 technology. Three gRNAs based on their specificity to their target site (targeting sequence S1, S2 and S3 from ORF1, ORF2 and ORF3, respectively) and minimal potential off-targets were introduced into the triploid Musa genome (Gonja Manjaya, AAB). The gRNA cassette, containing OsU6 promoter followed by two BbsI restriction sites and tracer RNA scaffold, was amplified from pZKOsU6-gRNA plasmid and cloned into pENTR-D/Topo. One gRNA from each ORF of the BSV genomic sequence was synthesized and cloned into pMR185 to generate the gRNA modules. The Cas9 endonuclease was employed in the plasmid of Arabidopsis codon-optimized and regulated by parsley ubiquitin promoter (PcUbi). | [46] |
| Bean yellow dwarf virus (BeYDV) | A. thaliana; Nicotiana benthamiana | IR, CP, and Rep protein | CRISPR/Cas9 technology was successfully utilized in engineering resistance to the bean yellow dwarf virus (BeYDF). Baltes et al. [46] established a transient assay to detect the activity of Cas9 and sgRNA in N. benthamiana using BeYDV. They used double 35S promoter and AtU6/At7SL RNA polymerase III promoter to express Cas9 and sgRNAs, respectively. The enhanced green fluorescent protein (eGFP) gene replaced the coat protein genes and movement protein for Cas9 and sgRNAs activity assessment against BeYDV in N. benthamiana. | [47] |
| Beet severe curly top virus (BSCTV) | N. bethamiana | CP and Rep protein | The CRISPR/Cas9 tool constructed two vectors (pV86-401 and pC86-401) in which the Cas9 protein is driven by one or two BSCTV promoters and sgRNA complex is driven by AtU6 promoter. The expression of Cas9 under both pV86 and pC86 promoters was significantly induced after BSCTV accumulation in N. benthamiana and pC86 promoter appeared to enable higher-level induction than the pV86 promoter. Previously employed pV86-sgRNA and pC86-sgRNA vectors using four highly active sgRNAs were then constructed to determine the system efficiency against BSCTV. | [48] |
| Cassava brown streak virus (CBSV) | Morchella esculenta | eIF4E, nCBP-1 and nCBP-2 | Cassava brown streak virus (CBSV) is a significant threat to cassava production in Africa. For the disease development in the host, CBSV requires the interaction between “viral genome-linked protein (VPg)” and “eukaryotic translation initiation factor 4E (eIF4E) isoforms” of the host. The nCBP clade was consistently associated with VPg protein and given priority due to its functional characterization. The CRISPR/Cas9 system was employed to produce mutant alleles of nCBP isoforms in cassava. Five constructs were combined, simultaneously targeting the different locations within nCBP-1 and nCBP-2 clades. | [49] |
| Cauliflower mosaic virus (CaMV) | A. thaliana | CP | The target sites in the CaMV CP gene were selected using standard Cas9 protein. Linear arrays of Arabidopsis U6 promoter: sgRNA units were designed and subsequently synthesized. When controlling viruses using the CRISPR-Cas9 system, both Cas9 and sgRNAs are consistently expressed in the cells. Recruiting Cas9 to viral DNA depends on the presence and abundance of sgRNAs. However, due to the existence of folded dsRNA domains in sgRNAs, siRNAs can be formed to contain the alien RNAs. | [50] |
| Cucumber mosaic virus (CMV) | N. benthamiana | PAMs | CMV was artificially injected into A. thaliana and N. benthamiana through vector pCR01. The pCR01 vector contained F. novicida Cas9 (FnCas9) a codon-optimized protein that is driven by an enhanced 35S promoter and a short-range RNA (sgRNA) guide that is driven by an AtU6 promoter. Complementary oligonucleotides were synthesized based on target gene sequences and were inserted inside the pCR01 vector efficiently to construct 23 corresponding vectors of the pCR01-sgRNA complex. | [51] |
| Cucumber Vein Yellowing Virus (CVYV), Papaya ring spot virus-W (PRSV-W) and Zucchini yellow mosaic virus (ZYMV) | Crocus sativus | eIF4E | CMV was artificially injected into A. thaliana and N. benthamiana through vector pCR01. The Cas9/sgRNAs complex was constructed to target the eIF4E gene in cucumber plants. The sgRNA1 sequence was expected to destroy the intact eIF4E gene, and the sgRNA2 sequence to allow the translation of two-thirds portions of the total protein products. One diploid genome with another single eIF4E gene and homozygous mutant plants were propagated to knock out the expression of the eIF4E gene. The Cas9/sgRNA complex was employed to disrupt the function of the recessive eIF4E gene and thus enhance virus resistance in cucumber. | [52] |
| Rice tungro spherical virus (RTSV) | Oryza sativa | eIF4G | Natural resistance to RTSV is a recessive trait that is controlled by a gene, namely, translation initiation factor 4 gamma gene (eIF4G). Mutations that occurred within eIF4G genes were generated utilizing the CRISPR/Cas9 technology to develop new resistance sources in the RTSV-susceptible variety IR64. The final products containing RTSV resistance were found to no longer have the Cas9 sequence under greenhouse conditions. | [53] |
| Turnip mosaic virus (TuMV) | A. thaliana | eIF4E and eIF(iso) 4E | The CRISPR/Cas9 technology was applied to generate the significant genetic resistance against TuMV in A. thaliana plants by deletion of a known host factor (eIF(iso)4E), which was strictly needed for viral existence. Transgenic delivery of the Cas9-sgRNA complex through CRISPR/Cas9 technology has proved its feasibility for the segregation of the transgene originated by induced mutation at the targeted eIF(iso)4E location, initially to generate stable and heritable mutations except for any persistent transgene. This approach is hypothesized as the reason why the recessive gene allele eIF(iso)4E exhibits more durable resistance against TuMV infection; the possible presence of VPg polymorphisms performing via eIF(iso)4E is an independent pathway. | [54] |
| Tomato yellow leaf curl Sardinia virus (TYLCSV) | N. benthamiana | CP and IR | The virus highly conserved a non-nucleotide sequence, which forms a stem-loop-like structure inside the IR sites. This conserved structure was directly involved in the Rep binding site in virus replication and contained some illegal bidirectional gene promoters. The IRs were also strain-specific and associated only with targeted Rep proteins. Authentic TYLCSV-IR-sgRNA was utilized to target the virus TYLCSV. Infectious clones of TYLCSV were injected in N. benthamiana plants via agro-infection overexpressing the CRISPR/Cas9 method. The IR target sequences were examined by loss assay assessment of the SspI enzyme, which confirmed the complete absence of recognized indels. | [55] |
| Tomato yellow leaf curl virus (TYLCV) | N. benthamiana; Solanum lycopersicum | CP, IR and Rep sequences | Agrobacterium-mediated transfer DNA (T-DNA) modification was used to express the sgRNAs cassettes by U6-26s promoter and Cas9 protein by CaMV-35S promoter in N. benthamiana plants. The U6-sgRNA cassette and Cas9 protein were cloned within a binary vector controlling by the CaMV-35S promoter and transferred in the N. benthamiana leaf discs utilizing Agrobacterium tumefaciens. The results proved the ability of CRISPR/Cas9 technology to target the infectious strains of TYLCV in the CP, IR and Rep sequences in transgenic N. benthamiana plants. | [27,55,56,57] |
| Wheat dwarf virus (WDV) | Hordeum vulgare | CP, MP, LIR and Rep | To identify the multiple target regions, the WDV genome was mapped for efficient CRISPR/Cas9 target sequences encircling the PAMs motif. Four different target sites were selected that showed no off-target effects and were capable of attacking several viral DNA sequences. The sgRNA WDV1 exhibits the complementarity overlapping in the CP and MP coding regions, sgRNA WDV2 targets the Rep/Rep A coding regions remaining in the N-terminus of the proteins, sgRNA WDV3 targets the LIR region and sgRNA WDV4 targets the Rep protein region and encodes the C-terminus of the protein. | [58] |
| SL. No. | Virus in Food Crop | Identified Virus Resistant Gene | Reference |
|---|---|---|---|
| 01 | African cassava mosaic virus (ACMV) | CMD1 (recessive resistance gene), CMD2 (major dominant gene) and CMD3 (conferring resistance) | [61,62] |
| 02 | Banana bunchy top virus (BBTV) | BBTV DNA-R and BBTV DNA-S1 | [63] |
| 03 | Barley yellow dwarf virus (BYDV) | Bdv1, Bdv2, Bdv3, Bdv4 and Ryd2 | [64,65] |
| 04 | Bean common mosaic virus (BCMV) | I (dominant resistance gene), bc-u, bc-1, bc-12, bc-2, bc-22, and bc-3 (recessive resistance gene) | [66] |
| 05 | Bean yellow mosaic virus (BYMV) | rym4/5 | [67] |
| 06 | Bean golden mosaic virus (BGMV) | bgm-1 and bgm-2 | [68,69] |
| 07 | Beet yellows virus (BYV) | III, V and VI QTLs | [70] |
| 08 | Grapevine fanleaf virus (GFLV) | F13, EcoRI and StyI | [71] |
| 09 | Lettuce mosaic virus (LMV) | eIF(iso)4G1,mo11 and mo12 (recessive resistance gene), Mo2 (dominant resistance gene) | [72] |
| 10 | Maize dwarf mosaic virus (MDMV) | Mdm1 | [73] |
| 11 | Maize streak virus (MSV) | msv1 | [74] |
| 12 | Plum pox virus (PPV) | eIF(iso)4G1, eIF(iso)4E,eIFiso4G11, PpDDXL ParP-1 to Par-P-6, ParPMC1 and ParPMC2 | [75] |
| 13 | Potato leafroll virus (PLRV) | Rladg | [76] |
| 14 | Potato virus X (PVX) | Rx1 and Rx2 | [77] |
| 15 | Potato virus Y (PVY) | Ryadg, Rysto, Y-1, pvr1, pvr21, pvr22 + pvr6, pot-1 | [78] |
| 16 | Rice tungro bacilliform virus (RTBV) | RTBV ORF IV and RTBV-CP | [79] |
| 17 | Squash leaf curl virus (SLCV) | slc-2 | [80] |
| 19 | Sugarcane mosaic virus (SCMV) | Scmv1 and Scmv2 | [81] |
| 19 | Soybean mosaic virus (SMV) | Rsv1, Rsv3, Rsv4, Rsv5, Rsv7, Rsv8, Rsv15 and Rsv20 | [82] |
| 20 | Watermelon mosaic virus (WMV) | Wmv1551 | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahriar, S.A.; Islam, M.N.; Chun, C.N.W.; Rahim, M.A.; Paul, N.C.; Uddain, J.; Siddiquee, S. Control of Plant Viral Diseases by CRISPR/Cas9: Resistance Mechanisms, Strategies and Challenges in Food Crops. Plants 2021, 10, 1264. https://doi.org/10.3390/plants10071264
Shahriar SA, Islam MN, Chun CNW, Rahim MA, Paul NC, Uddain J, Siddiquee S. Control of Plant Viral Diseases by CRISPR/Cas9: Resistance Mechanisms, Strategies and Challenges in Food Crops. Plants. 2021; 10(7):1264. https://doi.org/10.3390/plants10071264
Chicago/Turabian StyleShahriar, Saleh Ahmed, M. Nazrul Islam, Charles Ng Wai Chun, Md. Abdur Rahim, Narayan Chandra Paul, Jasim Uddain, and Shafiquzzaman Siddiquee. 2021. "Control of Plant Viral Diseases by CRISPR/Cas9: Resistance Mechanisms, Strategies and Challenges in Food Crops" Plants 10, no. 7: 1264. https://doi.org/10.3390/plants10071264
APA StyleShahriar, S. A., Islam, M. N., Chun, C. N. W., Rahim, M. A., Paul, N. C., Uddain, J., & Siddiquee, S. (2021). Control of Plant Viral Diseases by CRISPR/Cas9: Resistance Mechanisms, Strategies and Challenges in Food Crops. Plants, 10(7), 1264. https://doi.org/10.3390/plants10071264









