Abstract
Variation in cultivars can influence plant biological activities. This study aimed to identify superior cultivars while determining the variability in the phytochemical content, antioxidant, alpha-glucosidase inhibitory and antibacterial activities of cladode extracts from selected spineless Burbank cactus pear (Opuntia ficus-indica and Opuntia robusta) cultivars. Total phenolic and flavonoid contents were determined using the Folin-Ciocalteu and aluminum chloride spectrophotometric methods, respectively. Antioxidant activity was investigated using 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging and β-carotene linoleic acid assays. Alpha-glucosidase inhibition was determined using a spectrophotometric method and antibacterial activity using a non-polar (petroleum ether) and polar (50% methanol) extracts against two Gram-positive and two Gram-negative bacteria. Significant variation in phytochemical content, antioxidant, antidiabetic and antibacterial activities was observed amongst the cultivars. Alpha-glucosidase inhibitory activity varied widely with IC50 values ranging from 0.06 to 1.85 mg/mL. Radical scavenging activity of Polypoly cultivar was about seven fold higher than that recorded in other cultivars with low activity. Turpin and Berg x Mexican cultivars had the highest total phenolic and flavonoid contents, whilst the non-polar extract of Turpin also exhibited higher antibacterial activity against Bacillus subtilis and Escherichia coli. Sicilian Indian Fig was amongst the cultivars with a higher antioxidant activity, whilst also showing a strong inhibition against B. subtilis and E. coli. Polypoly cultivar demonstrated strong antioxidant and antidiabetic activities while its polar extract showed the highest total antibacterial activity against B. subtilis. The cultivar Malta was superior in terms of its antibacterial potency and efficacy against B. subtilis, Staphylococcus aureus and E. coli. The potential of using spineless cactus pear cladodes as a functional food with antioxidant, antidiabetic and antibacterial properties against pathogenic food spoilage bacteria in place of synthetic compounds was established. The significance of cultivar selection to increase this potential was highlighted.
1. Introduction
Lifestyle changes, poor nutrition and exposure to hazardous conditions, amongst others, have a negative impact on public health, as reflected in an increase in chronic diseases such as diabetes, cancer, obesity and hypertension [1]. According to the International Diabetes Federation (IDF) [2], diabetes is among the most common non-communicable diseases globally. Diabetes was rated as the fourth leading cause of death in most developed countries [3]. It is a lifetime progressive metabolic disease and is the most common endocrine disease that has affected an estimated 9.3% of the worldwide adult population [4]. The two common types of diabetes include Type 1, arising from the inability of the pancreatic β-cells to produce insulin, and Type 2, which is caused by insulin resistance and/or insufficient insulin production [1].
An increase in new and re-emerging pathogens with severe cases of antibiotic resistance remains a global concern. Resistance to current drugs, insufficient/incompatible therapies and negative side effects associated with some currently used drugs, amongst others, have rendered some management practices of diabetes and some infectious diseases almost ineffective [5,6]. All of these issues favor the growing interest in the use of herbal remedies for the treatment of diabetes and infectious diseases, as they are perceived to have a high economic value and fewer side effects when compared to synthetic agents [5,6]. Foodborne pathogenic bacteria such as Staphylococcus aureus, Bacillus subtilis and Escherichia coli have biofilm-forming abilities, causing food deterioration or spoilage, which is another global health dilemma [7,8]. There is an increase in research activities to find new alternative and strong antimicrobial agents, particularly from plants [9].
The use of Opuntia spp. (family Cactaceae), a climate-smart plant, as a remedy for diabetes and infectious diseases, has been documented from as early as the 1970′s, with Opuntia ficus-indica (L.) Mill. cladodes frequently used for the treatment of type-2 diabetes in Mexico [10]. Opuntia spp., commonly known as cactus or prickly pear, is well known for its multipurpose use, particularly as a food source and for medicinal purpose. Cactus pear cladodes contain beneficial and therapeutic phytochemicals that exhibit a vast variety of pharmacological activities including antioxidant, hypoglycemic and hypocholesterolemic activities, as well as protective effects against chronic diseases such as cancer, diabetes, and cardiovascular diseases [11,12]. Unlike the spiny cactus pear, which is highly invasive and is classified as a weed in South Africa, the spineless cultivars are relatively easy to manage and are cultivated as a climate-smart crop in South Africa, especially its cladodes, as animal fodder [13]. Different cactus pear cultivars contain several phytochemicals including antioxidants such as ascorbic acid, carotenoids, taurine, cysteine, reduced glutathione and flavonoids, such as kaempferol, quercetin and isorhamnetin [13,14]. The protective effect of cladode extracts against oxidative damage was mainly attributed to different antioxidants including vitamin E, ascorbic acid, carotenoids, flavonoids and phenolic acids [15,16]. Cactus pear cladode extracts also exhibited a potential growth inhibition of multi-drug-resistant food and human pathogens associated with skin infections, food contamination and nosocomial infections [17]. For example, Sánchez et al. [18] observed antimicrobial activities of Opuntia ficus-indica cladode methanol extracts from eight cultivars against Campylobacter jejuni, Vibrio cholera, and Clostridium perfringens [18]. Other studies indicated an antibacterial activity of cactus pear cladode methanolic extracts against Enterococcus faecium, E. coli, Salmonella spp., Pseudomonas aeruginosa, and S. aureus [19,20]. However, research has indicated that factors including cultivar type, plant age and environmental conditions can influence plant bioactive compound concentrations and biological activities [21,22]. For example, a comparative study indicated higher flavonol and phenolic contents in two South African cultivars compared to some Sicilian and Egyptian cultivars [22]. Another study showed variations in the antioxidant and antibacterial activities of extracts from eight cactus pear cultivars [18]. The aim of this study was to identify superior cultivars while determining the variability in the phytochemical content, antioxidant, alpha-glucosidase inhibitory and antibacterial activities of cladode extracts from selected spineless Burbank cactus pear (Opuntia ficus-indica and Opuntia robusta J.C. Wendl.) cultivars.
2. Results and Discussion
2.1. Cladode Extraction Yield
Two solvents (50% methanol and petroleum ether) with different polarities were used for extraction and the yields of the resulting crude extracts from both solvents are presented in Table 1. The yields varied amongst the cultivars in both solvent extractions. In general, extraction with 50% (v/v) methanol (MeOH) gave higher yields (ranging from 6.75 to 26.10% w/w) as compared to petroleum ether (ranging from 0.14 to 1.88% w/w). The higher yields recorded with 50% MeOH extracts may be due to the fact that polar solvents extract polar compounds, which are in a higher abundance than non-polar compounds that are extracted by a non-polar solvent (petroleum ether) [23]. The highest extract yields were recorded in cultivar Santa Rossa for methanol extracts and Muscatei for petroleum ether extracts, which were three-fold and thirteen-fold of the lowest yields for methanol and petroleum ether extracts, respectively.

Table 1.
Percentage yield of extracts from cladodes of 42 spineless cactus pear cultivars.
2.2. Total Phenolic and Flavonoid Contents
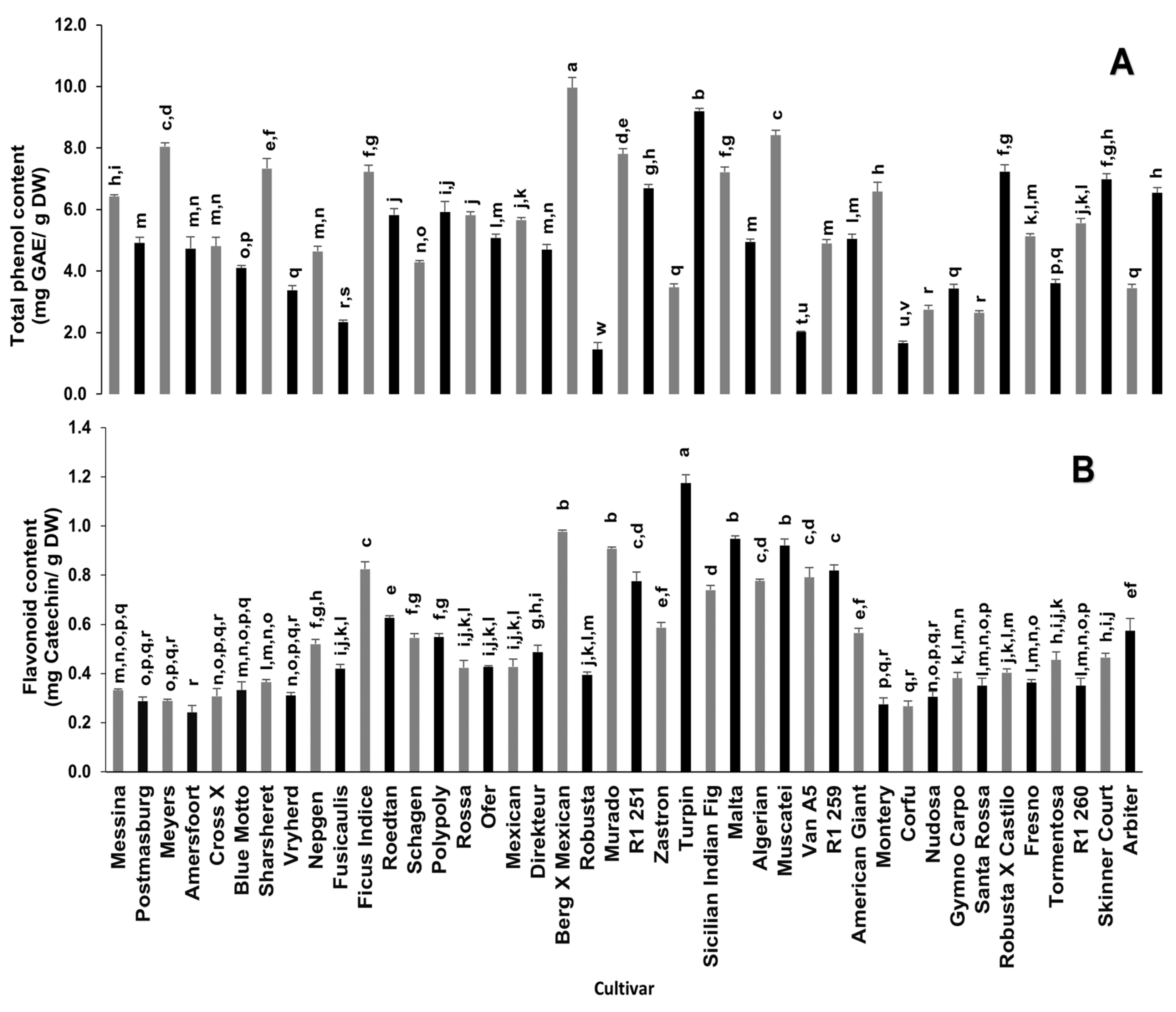
Phenolic compounds play a significant role in the antioxidant potential of various plants due to their redox properties, which allow them to act as reducing agents, hydrogen donors, singlet oxygen quenchers, and metal chelators [11]. Significant differences in total phenolic and flavonoid contents amongst the different cultivars were observed (Figure 1). Berg x Mexican and Turpin cultivars had significantly high total phenolic contents (9.96 mg gallic acid equivalent (GAE)/g dry weight (DW) and 9.19 mg GAE/g DW, respectively), which were five times higher when compared to the Robusta cultivar with the lowest total phenolic content (1.5 mg GAE/g DW). Similarly, the flavonoid content of Turpin (1.17 mg catechin equivalent (CE)/g DW) and Berg x Mexican (0.98 mg CE/g DW) cultivars were approximately four-fold of the cultivars with a low flavonoid content (Amersfoort, Postmasburg, Meyers, Cross X, Vryherd, Montery, Corfu and Nudosa), ranging from 0.24 to 0.31 mg CE/g DW.
Figure 1.
Total phenolic (A) and flavonoid (B) contents of cladodes from 42 spineless cactus pear cultivars. Bars bearing different letters in each graph are significantly different (p = 0.05) according to Duncan’s Multiple Range Test (DMRT). Values are mean ± standard errors (n = 3). GAE—Gallic Acid Equivalents.
A study by du Toit et al. [13] on the dried cladodes of five of the cultivars used in the current study reported a total phenolic content ranging from 0.18–0.27 mg/g, which was lower than those recorded in this study. Furthermore, the total phenolic content of the cultivars used in this study is higher than that of some Mexican cultivars [18] and some Brazilian cultivars [24]. Both the Mexican cultivars [18] and those used by du Toit et al. [13] were 6 months old and the Brazilian cultivars [24] were 3 years old as compared to the one-year-old cultivars used in this study. Conversely, the total phenolic content in the current study was low in comparison to that of some varieties (wild varieties of blanco, cristalino, morado and tempranillo) reported by Guevara-Figueroa et al. [25] Similarly, the flavonoid content in the current study, which ranged from 0.24 to 1.17 mg CE/g DW, was low when compared to the study of Sánchez et al. [18] who reported a flavonoid content ranging from 15.4 to 36.6 mg quercetin equivalent/g dry weight. Guevara-Figueroa et al. [25] also reported a high flavonoid content of 9.8 and 5.9 mg quercetin equivalent/g dry weight for the blanco and manso commercial varieties, respectively. Nonetheless, the total phenolic content in the current study was higher than the flavonoid content [25], in contrast to the report of Sanchez et al. [18]. Cladode maturity and/or environmental conditions can influence bioactive compound concentrations [25,26] and this fact may explain the variance observed in our study when compared to some other studies.
2.3. Antioxidant Activity of Cladode Extracts
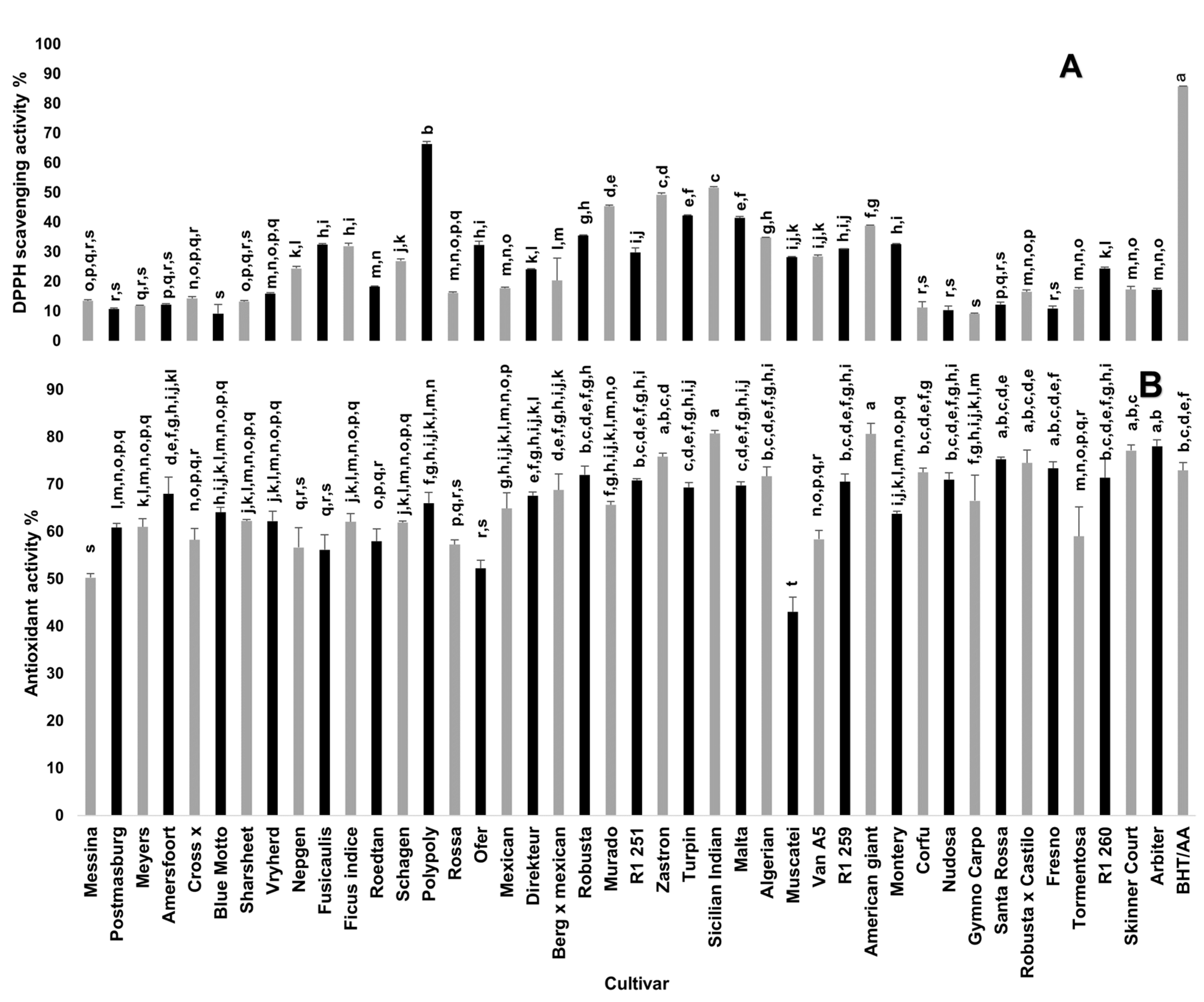
Figure 2 shows the free radical scavenging and antioxidant activities of cladode methanolic extracts from 42 spineless cactus pear cultivars. A statistically significant variation was observed among the cultivars. Polypoly cultivar showed the highest free radical scavenging activity (66.37%), which was approximately seven-fold what was recorded for cultivars with low free radical scavenging activity including Messina, Postmasburg, Meyers, Amersfoort, Fresno, Gymno Carpo, Blue Motto and Corfu. A previous study [27] indicated free radical scavenging activity ranging from 83.77–95.53% in cladodes of cactus pear cultivars, which is higher than that recorded in this study (9.1–66.37%). Haile et al. [28] recorded the radical scavenging activity of cactus pear cladodes that ranged from 59.3–85.8%. The concentrations at which the extracts were evaluated in each study may be a confounding factor in the results reported. Nevertheless, the current study indicated variations in the cultivar antioxidant activity.
Figure 2.
Antioxidant activity of cladode methanolic extracts from 42 spineless cactus pear cultivars. (A) DPPH free radical scavenging activity (%) at 10 mg/mL extract concentration. (B) Antioxidant activity (%) based on bleaching rate in β-carotene-linoleic acid assay. Bars bearing different letters in each graph indicate significant differences (p = 0.05) according to Duncan’s Multiple Range Test (DMRT). Values are means ± standard errors (n = 3). Butylated hydroxytoluene (BHT) was used as a standard for total antioxidant activity while ascorbic acid (AA) was used as a standard for DPPH free radical scavenging activity.
The inclusion of antioxidant agents, particularly from natural sources such as plants, is of great importance in the cosmetic and food industry [29]. Although antioxidants are essential for protecting cells against free radicals, it is important to have a balanced system of oxidants and antioxidants [30]. Oxidants present at acceptable levels play an important role in the production of new skin cells by initiating cell-signaling pathways, resulting in the removal of UV-damaged cells [31].
Due to the complex mechanism of action of antioxidants, the use of at least two different assays has become standard practice [32]. Antioxidant activity based on β-carotene-linoleic acid assay of the cultivars ranged from 43 to 80%. The highest antioxidant activity (80%), which was significantly higher than that of butylated hydroxytoluene (BHT), was recorded in Sicilian Indian Fig and American giant cultivars whilst the Muscatei cultivar had the lowest activity (43%). Twenty-three of the cultivars exhibited antioxidant activity comparable to that of BHT. BHT is a synthetic antioxidant usually used as a food additive to prevent the damage caused by free radicals during the oxidation processes [33]. The use of BHT as a food additive has potential health hazards for consumers including long-term toxic effects on the liver and lungs [34,35]. In addition to their antioxidant potential as food additives, natural antioxidants such as cactus pear cladodes may be used as a food colorant and could positively influence the sensory characteristics of stabilized food [36]. The cladodes of selected spineless cactus pear cultivars (such as Polypoly, Sicilian Indian Fig and American giant), which demonstrated high in vitro antioxidant activities in this study, can be used in the food industry as nutraceutical and functional foods, and potentially in the cosmetic industry to protect against reactive oxygen species that cause skin disorders [37].
2.4. Alpha-Glucosidase Inhibitory Activity
Table 2 presents the alpha-glucosidase inhibitory activity of the cladode extracts. The extracts significantly inhibited alpha-glucosidase with IC50 values ranging from 0.06 to 1.85 mg/mL. The lower the IC50, the stronger the inhibitory activity. Of the 42 cultivars investigated, 27 cultivars exhibited stronger alpha-glucosidase inhibitory activity with IC50 values significantly lower than that of acarbose (1.07 mg/mL), which is a widely used drug for the treatment of type-2 diabetes [38]. Eleven of the cultivars (Berg X Mexican, Blue Motto, Cross X, Ficus Indice, Messina, Nepgen, Ofer, Polypoly, Postmasburg, Roedtan and Sharsheret) demonstrated alpha-glucosidase inhibitory activity (IC50 < 0.1 mg/mL) that was 10-fold stronger) when compared to acarbose. Alpha-glucosidase is a known key enzyme in carbohydrate digestion and its inhibition is considered as a therapeutic target for the modulation of postprandial hyperglycemia, a common abnormality in type-2 diabetes [39]. Alpha-glucosidase inhibitors can be effective in the management of hyperglycemia by delaying the effects of postprandial hyperglycemia [40]. The noteworthy in vitro alpha-glucosidase activity of the cladode extracts indicates their potential use as a functional food in the effective management of diabetes, even if it varies due to cultivar.

Table 2.
Alpha-glucosidase inhibitory activity of cladode extracts from 42 spineless cactus pear cultivars.
2.5. Correlation Analysis of Phytochemical Content, Antioxidant and Alpha-Glucosidae Inhibitory Activities
Significantly, positive and moderate correlations were established between the total phenolic and flavonoid contents, flavonoid content and DPPH radical scavenging activity, as well as between antioxidant and antidiabetic activities (Table 3). This observation is in line with previous studies demonstrating the positive roles of flavonoids and phenolic compounds in free radical scavenging [11,13] as well as the roles of antioxidants, particularly via inhibition of lipid peroxidation in the treatment or management of diabetes [41,42,43,44]. Direct selection of cultivars with a high flavonoid content may result in increased free radical scavenging while selection for cultivars with strong antioxidants (through inhibition of lipid peroxidation) may lead to increased antidiabetic properties. This finding is also significant in cultivar selection during breeding programs.

Table 3.
Pearson correlation coefficient analysis for the phytochemical, antioxidant, and antidiabetic properties of cladodes from the 42 spineless cactus pear cultivars.
2.6. Antibacterial Activity
The antibacterial activities of extracts from selected cultivars against two Gram-positive and two Gram-negative bacteria are presented in Table 4. The lower the minimum inhibitory concentration (MIC) values, the stronger the antibacterial activity in terms of potency. In general, some noteworthy antibacterial activities were observed against B. subtilis and E. coli with MIC values below 1 mg/mL [45]. Weak activity was observed against K. pneumoniae.

Table 4.
Antibacterial activity (minimum inhibitory concentration; mg/mL) of cladodes from 20 selected spineless cactus pear cultivars.
Low antibacterial activity against Gram-negative bacteria may be due to the thick murein layer in their structure preventing the entry of inhibitors [46]. Poor activity in some of the extracts may be due to low concentrations of antibacterial compounds in the extracts [47]. The differential antibacterial activity of Opuntia dillenii (Ker Gawl.) Haw. extracts against two Gram-negative bacteria, E. coli and Salmonella typhi, has also been reported [48].
Petroleum ether extracts generally had better antibacterial activity in terms of potency, when compared to 50% methanol extracts. This is mostly associated with the difference in polarity of the solvents. Umar et al. [48] similarly observed a considerably improved antimicrobial activity against Gram-positive B. subtilis and S. aureus with non-polar extracts of O. dillenii when compared with polar extracts. Similar observations regarding the superior activity/potency of non-polar extracts against both Gram-positive and Gram-negative bacteria have been reported [49,50]. In terms of antibacterial potency, the best MIC value was recorded with petroleum ether extract of Malta cultivar (0.39 mg/mL) against B. subtilis and E. coli, and 0.78 mg/mL against S. aureus. Overall, the cultivars demonstrating potent antibacterial activity (MIC < 1.0 mg/mL) against at least two bacteria are Malta, Roedtan, Sicilian Indian Fig and Turpin whereas Murado and R1251 demonstrated potent antibacterial activity against one bacterium (B. subtilis and E. coli, respectively). In a similar study, methanol, ethanol, and chloroform extracts of Opuntia ficus-indica cladodes exhibited considerable antibacterial activity against Streptococcus pneumoniae, S. typhi, B. subtilis and E. coli [9]. In addition, Kim et al. [19,20] reported antibacterial activity of Opuntia ficus-indica methanol extracts against Enterococcus faecium, E. coli, Salmonella spp., Pseudomonas aeruginosa, and S. aureus.
Total activity (or minimum inhibitory dilution), which is the volume to which the bioactive compounds in one gram can be diluted and still inhibit bacteria growth, provides a measure of antibacterial efficacy while the minimum inhibitory concentration indicates potency [51,52,53]. Table 5 presents the total antibacterial activity of the cladode extracts against the bacteria evaluated in this study. The polar (methanol) extract demonstrated better efficacy than the non-polar (petroleum ether) extract, owing to the higher extraction yield with the polar extract. At least one extract of the cultivars Malta, Polypoly and R1 251 showed the highest total activity against one of the bacteria. Therefore, in terms of both antibacterial potency and efficacy of the selected 20 cultivars, the cultivar Malta demonstrated superior antibacterial activity. Moreover, the strong antibacterial activity of some of the spineless cladodes documented in this study suggests the potential use of spineless cladodes against food spoilage or pathogenic microorganisms, although a careful cultivar selection may be required.

Table 5.
Total antibacterial activity (minimum inhibitory dilution; mL/g) of cladode extracts from 20 selected spineless cactus pear cultivars.
3. Materials and Methods
3.1. Plant Material Collection and Preparation
Cladodes were collected from one-year old 42 spineless cactus pear cultivars grown under the same glasshouse conditions at the Agricultural Research Council, Roodeplaat Research farm, South Africa (25°36′1″ S 28°21′42″ E). Two cultivars (Robusta and Monterey) belonged to Opuntia robusta while the remaining cultivars were from Opuntia ficus-indica. Each cultivar consisted of five plants and selection was done on good quality cladodes with no bruises or discoloration. For each cultivar, cladodes from the first, third and fifth plant were harvested. The harvested cladodes were sliced into small pieces and oven-dried at 50 °C in the dark. The material was then ground into fine powder using a pulverizing mill. To determine the percentage yield of extracts from the cladodes of 42 spineless cactus pear cultivars, 20 g dry weight (DW) of each cultivar was extracted separately with 300 mL of 50% (v/v) methanol and petroleum ether in order to obtain methanolic (MeOH) and petroleum ether (PE) extracts, respectively. Each mixture (plant material and solvent) was sonicated for an hour in an ultrasonic water bath (Branson, 5510E-MT, Lasec, South Africa) and filtered through Whatman No.1 filter paper, followed by in vacuo concentration using a rotary evaporator (Stuart, RE300DB, Lasec, South Africa) at 40 °C and air-drying in a fume hood.
3.2. Total Phenolic and Flavonoid Content Determination
The extraction procedure was carried out as described by Amoo et al. [54] Plant material (0.2 g) was extracted by sonication in an ultrasonic bath containing ice-cold water for 30 min using 10 mL of 50% MeOH, followed by centrifuging at 1073.3× g for 2 min. Total phenolic content was determined using the Folin-Ciocalteu method [55], with modifications. A reaction mixture containing 50 µL of the sample extract, 450 µL distilled water, 250 µL of Folin-Ciocalteu reagent and 1250 µL sodium carbonate (2% w/v) was briefly vortexed and incubated for 40 min at room temperature. Thereafter, absorbance was recorded using a spectrophotometer (Specord 210 plus, Analytik Jena, Jena, Germany) at 725 nm. The assay was done in triplicate and a calibration curve was prepared using gallic acid as a standard. Results were expressed in mg gallic acid equivalent (GAE) per gram dry weight (DW).
Flavonoid content was determined using an aluminum chloride method [56], with modifications. A reaction mixture containing 250 µL sample extract, 1.6 mL of distilled water, 75 µL (5% w/v) sodium nitrite, 75 µL of aluminum chloride (10% w/v), and 0.5 mL (1 M) NaOH was briefly vortexed and absorbance measured at 510 nm. The assay was done in triplicates and a calibration curve was prepared using catechin as a standard. Results were expressed in mg catechin equivalent (CE) per gram DW.
3.3. Antioxidant Assays
An approach into antioxidant investigation of natural compounds can be a strenuous process due to their diverse chemical structures, biological roles and different modes of actions [57]. Hence, different antioxidant procedures may give different results because each assay has its own thermodynamics and kinetics [32]. A selection of reliable antioxidant procedures that measure different properties such as radical scavenging, phase distribution equilibria, proton and electron transfer, and relate to food and biological systems is of importance in antioxidant investigation [32].
3.3.1. DPPH (2,2-diphenyl-1-picrylhydrazyl) Free Radical Scavenging Activity
Samples were extracted using the method described previously by Amoo et al. [54], with slight modifications. An amount of 20 g dried powdered cladode from each cultivar was extracted with 300 mL of 50% MeOH by sonication for 1 h. The extract volume was condensed on a rotary evaporator at 40 °C before air-drying. The antioxidant activity was determined using the DPPH method [58], with modifications [53]. At different known concentrations, 30 µL of 50% MeOH extracts were diluted with 720 µL MeOH followed by an addition of 750 µL DPPH solution. Ascorbic acid was used as a positive control. The mixture was incubated at room temperature (25 ± 2 °C) for 40 min before recording absorbance at 517 nm. The assay was done in triplicate and the percentage free radical scavenging activity (RSA) was calculated using Equation (1):
where Abs517 nm; Sample is the absorbance of the sample mixture; Abs517 nm Neg Control is the absorbance of the negative control (MeOH); and Abs517 nm Blank is the absorbance of the blank (50% MeOH in place of DPPH).
3.3.2. Antioxidant Activity Using β-Carotene Linoleic Acid Assay
Following the extraction procedure described above in Section 3.3, antioxidant activity using β-carotene linoleic acid assay was determined [53,59]. An aliquot (2.4 mL) of β-carotene emulsion consisting of β-carotene (5 mg) dissolved in chloroform (1 mL), linoleic acid (100 µL), Tween 20 (1 mL), and distilled water (248 mL) was dispensed into reaction tubes containing 100 µL of sample extract at a predetermined concentration. Butylated hydroxytoluene was prepared as a positive control at 6.25 mg/mL. Aqueous methanol (50%) in place of the sample was used as a negative control. The assay was conducted in triplicates. Absorbance was measured at 470 nm immediately and then a second absorbance reading at 470 nm was done after incubation in a water bath at 50 °C for 1 h. β-carotene bleaching rate was calculated using Equation (2):
where At=0 is the absorbance of the emulsion at 0 min and At=t is the absorbance of the emulsion at 60 min. The bleaching rate was used to calculate the percentage antioxidant activity (ANT) expressed as a percentage inhibition of the rate of β-carotene bleaching using Equation (3):
where Rcontrol and Rsample are the average β-carotene bleaching rates for the control and plant extract or BHT, respectively.
3.4. Alpha-Glucosidase Inhibitory Activity
Alpha-glucosidase inhibitory activity was determined using a method described by Li et al. [60], with modifications. Yeast alpha-glucosidase (0.5 unit/mL) was dissolved in 0.1 M potassium phosphate buffer (pH 6.8) and the substrate (5 mM p-nitrophenyl-α-d-glucopyranoside) was prepared in the same buffer (pH 6.8). Different concentrations of the samples were prepared using dimethyl sulfoxide (DMSO). Sample wells contained 20 µL sample, 100 µL 0.1 M potassium phosphate buffer (pH 6.8), and 20 µL yeast alpha-glucosidase (0.5 unit/mL) enzyme solution. Sample blank wells contained 20 µL sample, 100 µL 0.1 M potassium phosphate buffer (pH 6.8) and 20 µL DMSO. Negative control wells contained 20 µL DMSO, 100 µL 0.1 M potassium phosphate buffer (pH 6.8), and 20 µL yeast alpha-glucosidase (0.5 unit/mL) enzyme solution. The plates were pre-incubated at 37 °C for 5 min after which 20 µL of the substrate was added to initialize the reaction. After further incubation at 37 °C for 30 min, the reaction was stopped by adding 80 µL of 0.2 M sodium carbonate (prepared in the same potassium phosphate buffer). The tests were performed in triplicates and acarbose was used as a positive control. The amount of p-nitrophenol (pNP) released was quantified using a 96-well microplate reader at 405 nm. The alpha-glucosidase inhibitory rate (%) was calculated using Equation (4).
where Abssample is the absorbance of the sample mixture, Abssample blank is the absorbance of Sample blank and Absnegative control is the absorbance of the negative control.
3.5. Antibacterial Activity
After preliminary experiments, twenty cultivars were selected based on their total phenolic and flavonoid content (seven of the highest, six intermediate and seven of the lowest content) and further profiled for their antibacterial activity. Antibacterial activity was determined using a serial micro-plate dilution assay [61]. Extracts were tested against two Gram-positive—Staphylococcus aureus (ATCC 9144), Bacillus subtilis (ATCC 6051)—and two Gram-negative—Escherichia coli (ATCC 8739) and Klebsiella pneumonia (ATCC 13883)—bacteria. The bacterial cultures were maintained on Mueller Hinton agar medium in petri dishes and an inoculum of each microorganism was grown in Mueller Hinton broth and incubated at 37 °C for 24 h. An equal volume (100 µL) of distilled water and plant extract was transferred into first row wells and two-fold serially diluted through the 96-well plates to prepare extracts with different concentrations. A hundred microliters of the bacterial solution were then added to all the wells and ciproflaxin was used as a positive control. For negative control, 50% MeOH and petroleum ether were used. The plates were incubated for 24 h at 37 °C. After incubation, 40 µL of p-iodonitrotetrazolium chloride (INT) was added and minimum inhibitory concentration (MIC) values were determined as the lowest concentration where there was no color change. Bacterial growth was indicated by pink color, whilst bacterial inhibition was indicated by no color change after addition of INT.
3.6. Data Analysis
The IC50, which is the concentration of the extract required to inhibit 50% of the alpha-glucosidase, was determined for each extract using GraphPad Prism software (version 4.03). The data were log-transformed, normalized, and fitted into a nonlinear regression for IC50 determination. Data were subjected, as appropriate, to one-way analysis of variance using Statsoft (Statistica 8) software. The mean values were compared based on Duncan’s multiple range test and a significant difference was established at p = 0.05. Pearson correlation coefficient analysis was computed using SPSS software (version 16) and significant correlation was established at p ≤ 0.05.
4. Conclusions
The significant influence of cultivar when using cactus pear as a potential functional and nutraceutical food product was highlighted in this study. Strong antidiabetic activity coupled with the observed antioxidant and antibacterial activities, although varied with cultivars, indicate the potential of using cladodes as a functional food and in applications against food spoilage in place of synthetic compounds. Although the cultivars exhibited different levels of activity, some cultivars are superior in terms of their phytochemical content and/or biological properties. Turpin and Berg x Mexican had both the highest phenolic and flavonoid content, whilst the non-polar extract of Turpin also exhibited a higher antibacterial activity against B. subtillis and E. coli. Sicilian Indian Fig was amongst the cultivars with a higher antioxidant activity whilst also showing great inhibition against B. subtillis and E. coli. Polypoly was among the superior cultivars showing strong antioxidant and antidiabetic activities, while its polar extract showed the highest total antibacterial activity against B. subtilis. The non-polar extract of Malta demonstrated superior antibacterial activity in terms of both potency and efficacy against three bacteria (B. subtilis, S. aureus, and E. coli). The cultivars with high phytochemical contents and biological activities have potential to be used as food additives against food spoilage and in the fight against diabetes and pathogenic organisms.
Author Contributions
Conceptualization, M.B.M. and S.O.A.; methodology, M.B.M. and S.O.A.; investigation, M.B.M.; writing—original draft preparation, M.B.M.; writing—review and editing, S.O.A., T.K., C.P.D.P. and S.L.V.; supervision, S.O.A. and T.K.; funding acquisition, S.L.V., C.P.D.P., T.K. and S.O.A. All authors have read and agreed to the published version of the manuscript.
Funding
Funding from the Agricultural Research Council, South Africa and National Research Foundation, South Africa (Grant UID: 111966 and 118927) are gratefully acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data related to this study are already presented in this publication.
Acknowledgments
We acknowledge the ARC, DUT, and UFS collaboration consortium and Herman Fouche for his assistance with the cactus pear cultivars.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shai, L.; Masoko, P.; Mokgotho, M.; Magano, S.; Mogale, A.; Boaduo, N.; Eloff, J. Yeast alpha glucosidase inhibitory and antioxidant activities of six medicinal plants collected in Phalaborwa, South Africa. S. Afr. J. Bot. 2010, 76, 465–470. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; IDF: Brussels, Belgium, 2019. [Google Scholar]
- World Health Organization. Noncommunicable Diseases Country Profiles 2018; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Rao, M.M.V.; Hariprasad, T.P.N. In silico analysis of a potential antidiabetic phytochemical erythrin against therapeutic targets of diabetes. Silico Pharmacol. 2021, 9, 5. [Google Scholar] [CrossRef]
- Talib, A.; Manzoor, K.N.; Ali, W.; Saeed, M.; Gondal, M.A.; Badshah, M.; Khan, A.A. Biogenic copper nanoparticles as a nanoscale solution to address multiple drug resistance in bacteria. Pak. J. Zool. 2021, 53, 201–208. [Google Scholar]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef]
- Logan, N. Bacillus and relatives in foodborne illness. J. Appl. Microbiol. 2011, 112, 417–429. [Google Scholar] [CrossRef]
- Welegerima, G.; Zemene, A.; Tilahun, Y. Phytochemical composition and antibacterial activity of Opuntia Ficus Indica cladodes extracts. J. Med. Plants Stud. 2018, 6, 243–246. [Google Scholar]
- Alarcon-Aguilar, F.J.; Valdes-Arzate, A.; Xolalpa-Molina, S.; Banderas-Dorantes, T.; Jimenez-Estrada, M.; Hernandez-Galicia, E.; Roman-Ramos, R. Hypoglycemic activity of two polysaccharides isolated from Opuntia Ficus Indica and O. streptacantha. Proc. West. Pharmacol. Soc. 2003, 46, 139–142. [Google Scholar]
- Abdel-Hameed, E.-S.S.; Nagaty, M.A.; Salman, M.S.; Bazaid, S.A. Phytochemicals, nutritionals and antioxidant properties of two prickly pear cactus cultivars (Opuntia ficus indica Mill.) growing in Taif, KSA. Food Chem. 2014, 160, 31–38. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, W.; Sheng, C.; Zheng, C.; Yao, J.; Miao, Z. Chemical Composition and Antidiabetic Activity of Opuntia Milpa Alta Extracts. Chem. Biodivers. 2010, 7, 2869–2879. [Google Scholar] [CrossRef]
- Du Toit, A.; de Wit, M.; Osthoff, G.; Hugo, A. Antioxidant properties of fresh and processed cactus pear cladodes from selected Opuntia ficus-indica and O. robusta cultivars. S. Afr. J. Bot. 2018, 118, 44–51. [Google Scholar] [CrossRef]
- Tesoriere, L.; Fazzari, M.; Allegra, M.; Livrea, M.A. Biothiols, Taurine, and Lipid-Soluble Antioxidants in the Edible Pulp of Sicilian Cactus Pear (Opuntia ficus-indica) Fruits and Changes of Bioactive Juice Components upon Industrial Processing. J. Agric. Food Chem. 2005, 53, 7851–7855. [Google Scholar] [CrossRef]
- Osuna-Martínez, U.; Reyes-Esparza, J.; Rodríguez-Fragoso, L. Cactus (Opuntia ficus-indica): A review on its antioxidants properties and potential pharmacological use in chronic diseases. Nat. Prod. Chem. Res. 2014, 2, 6. [Google Scholar]
- Stintzing, F.C.; Schieber, A.; Carle, R. Phytochemical and nutritional significance of cactus pear. Eur. Food Res. Technol. 2001, 212, 396–407. [Google Scholar] [CrossRef]
- El Feghali, P.A.R.; Ibrahim, R.; Nawas, T. Antibacterial activity of Curcuma longa, Opuntia ficus-indica and Linum usitatissimum. MOJ Toxicol. 2018, 4, 214–220. [Google Scholar]
- Sánchez, E.; Dávila-Aviña, J.; Castillo, S.L.; Heredia, N.; Vázquez-Alvarado, R.; Garcia, S. Antibacterial and Antioxidant Activities in Extracts of Fully Grown Cladodes of 8 Cultivars of Cactus Pear. J. Food Sci. 2014, 79, M659–M664. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, N.; Kim, J.; Lim, J.; Bae, W.; Kim, J.; Noh, K.; Hur, J.; Jung, W.; Park, K. Antimicrobial activity of natural product made by Opuntia ficus-indica var. Saboten against Salmonella spp. and Escherichia coli O157: H7. J. Food Hyg. Saf. 2002, 17, 71–78. [Google Scholar]
- Kim, H.-N.; Kwon, D.-H.; Jun, H.-K. Antimicrobial Activities of Opuntia ficus-indica var. saboten Makino Methanol Extract. J. Life Sci. 2005, 15, 279–286. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.; Kudanga, T. Opuntia (Cactaceae) plant compounds, biological activities and prospects—A comprehensive review. Food Res. Int. 2018, 112, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, T.M.; El-Hady, E.-S.A.A.; Omran, H.T.; El-Samahy, S.K.; Kroh, L.W.; Rohn, S. Influence of cultivar and origin on the flavonol profile of fruits and cladodes from cactus Opuntia ficus-indica. Food Res. Int. 2014, 64, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.-H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the Use of Different Solvents for Phytochemical Constituents, Antioxidants, and In Vitro Anti-Inflammatory Activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 8178294. [Google Scholar] [CrossRef]
- Alves, F.A.L.; De Andrade, A.P.; Bruno, R.D.L.A.; Silva, M.G.D.V.; Souza, M.D.F.V.D.; Dos Santos, D.C. Seasonal variability of phenolic compounds and antioxidant activity in prickly pear cladodes of Opuntia and Nopalea genres. Food Sci. Technol. 2017, 37, 536–543. [Google Scholar] [CrossRef]
- Guevara-Figueroa, T.; Jiménez-Islas, H.; Reyes-Escogido, M.L.; Mortensen, A.G.; Laursen, B.B.; Lin, L.W.; De León-Rodríguez, A.; Fomsgaard, I.S.; Barba de la Rosa, A.P. Proximate composition, phenolic acids, and flavonoids characterization of commercial and wild nopal (Opuntia spp.). J. Food Comps. Anal. 2010, 23, 525–532. [Google Scholar] [CrossRef]
- Rodríguez-Garcia, M.E.; De Lira, C.; Hernández-Becerra, E.; Villegas, M.D.L.A.C.; Palacios-Fonseca, A.J.; Rojas-Molina, I.; Reynoso, R.; Quintero, L.C.; Del-Real, A.; Zepeda, T.A.; et al. Physicochemical Characterization of Nopal Pads (Opuntia ficus indica) and Dry Vacuum Nopal Powders as a Function of the Maturation. Plant Foods Hum. Nutr. 2007, 62, 107–112. [Google Scholar] [CrossRef]
- De Wit, M.; du Toit, A.; Osthoff, G.; Hugo, A. Cactus pear antioxidants: A comparison between fruit pulp, fruit peel, fruit seeds and cladodes of eight different cactus pear cultivars (Opuntia ficus-indica and Opuntia robusta). J. Food Meas. Charact. 2019, 13, 2347–2356. [Google Scholar] [CrossRef]
- Haile, K.; Mehari, B.; Atlabachew, M.; Chandravanshi, B.S. Phenolic composition and antioxidant activities of cladodes of the two varieties of cactus pear (Opuntia ficus-indica) grown in Ethiopia. Bull. Chem. Soc. Ethiop. 2017, 30, 347. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Tepe, B.; Yamaç, M. Evaluation of the antioxidant activity of four edible mushrooms from the Central Anatolia, Eskisehir—Turkey: Lactarius deterrimus, Suillus collitinus, Boletus edulis, Xerocomus chrysenteron. Bioresour. Technol. 2008, 99, 6651–6655. [Google Scholar] [CrossRef] [PubMed]
- Mwinga, J.L.; Asong, J.A.; Amoo, S.; Nkadimeng, S.; McGaw, L.J.; Aremu, A.O.; Otang-Mbeng, W. In vitro antimicrobial effects of Hypoxis hemerocallidea against six pathogens with dermatological relevance and its phytochemical characterization and cytotoxicity evaluation. J. Ethnopharmacol. 2019, 242, 112048. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.; Desikan, R.; Neill, S. Role of reactive oxygen species in cell signalling pathways. Biochem. Soc. Trans. 2001, 29, 345–350. [Google Scholar] [CrossRef]
- Apak, R. Current Issues in Antioxidant Measurement. J. Agric. Food Chem. 2019, 67, 9187–9202. [Google Scholar] [CrossRef]
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Hamid, S.B.A.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): A review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef]
- Kahl, R.; Kappus, H. Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Z. Lebensm. Unters. Forsch. 1993, 196, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Rodriguez, J.M.L.; Sánchez, M.; Amado, I.R.; Franco, D. Effects of natural (grape seed and chestnut extract) and synthetic antioxidants (buthylatedhydroxytoluene, BHT) on the physical, chemical, microbiological and sensory characteristics of dry cured sausage “chorizo”. Food Res. Int. 2013, 54, 611–620. [Google Scholar] [CrossRef]
- Pokorny, J. Are natural antioxidants better—And safer—Than synthetic antioxidants? Eur. J. Lipid Sci. Technol. 2007, 109, 629–642. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Tuyen, D.T.; Yew, G.Y.; Cuong, N.T.; Hoang, L.T.; Yen, H.T.; Hong Thao, P.T.; Thao, N.T.; Sy Le Thanh, N.; Hien Trang, N.T.; Trung, N.T.; et al. Selection, purification, and evaluation of acarbose−an α-glucosidase inhibitor from Actinoplanes spp. Chemosphere 2021, 265, 129167. [Google Scholar] [CrossRef]
- Vadivelan, R.; Krishnan, R.G.; Kannan, R. Antidiabetic potential of Asparagus racemosus Willd leaf extracts through inhibition of α-amylase and α-glucosidase. J. Tradit. Complement. Med. 2019, 9, 1–4. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Aderogba, M.A.; Amoo, S.; Stirk, W.A.; Van Staden, J. Potential antiradical and alpha-glucosidase inhibitors from Ecklonia maxima (Osbeck) Papenfuss. Food Chem. 2013, 141, 1412–1415. [Google Scholar] [CrossRef]
- Abd El-Razek, F.H.; Hassan, A.A. Nutritional value and hypoglycemic effect of prickly cactus pear (Opuntia ficus-indica) fruit juice in alloxan-induced diabetic rats. Aust. J. Basic Appl. Sci. 2011, 5, 356–377. [Google Scholar]
- Abdallah, I.Z. Evaluation of Hypoglycemic Activity of Opuntia dillenii Haw Fruit Juice in Streptozotocin-Induced Diabetic Rats. Egypt. J. Hosp. Med. 2008, 33, 544–558. [Google Scholar] [CrossRef]
- Davì, G.; Falco, A.; Patrono, C. Lipid Peroxidation in Diabetes Mellitus. Antioxid. Redox Signal. 2005, 7, 256–268. [Google Scholar] [CrossRef]
- Fatani, S.H.; Babakr, A.T.; Noureldin, E.M.; AlMarzouki, A.A. Lipid peroxidation is associated with poor control of type-2 diabetes mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, S64–S67. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Matu, E.N.; van Staden, J. Antibacterial and anti-inflammatory activities of some plants used for medicinal purposes in Kenya. J. Ethnopharmacol. 2003, 87, 35–41. [Google Scholar] [CrossRef]
- Rabe, T.; van Staden, J. Antibacterial activity of South African plants used for medicinal purposes. J. Ethnopharmacol. 1997, 56, 81–87. [Google Scholar] [CrossRef]
- Umar, M.I.; Javeed, A.; Ashraf, M.; Riaz, A.; Mukhtar, M.M.; Afzal, S.; Altaf, R. Polarity-based solvents extraction of Opuntia dillenii and Zingiber officinale for in vitro antimicrobial activities. Int. J. Food Prop. 2013, 16, 114–124. [Google Scholar] [CrossRef][Green Version]
- Lim, S.-H.E.; Almakhmari, M.A.; Alameri, S.I.; Chin, S.-Y.; Abushelaibi, A.; Mai, C.-W.; Lai, K.-S. Antibacterial Activity of Clinacanthus nutans Polar and Non-Polar Leaves and Stem Extracts. Biomed. Pharmacol. J. 2020, 13, 1169–1174. [Google Scholar] [CrossRef]
- Rayan, M.; Abu-Farich, B.; Basha, W.; Rayan, A.; Abu-Lafi, S. Correlation between Antibacterial Activity and Free-Radical Scavenging: In-Vitro Evaluation of Polar/Non-Polar Extracts from 25 Plants. Processes 2020, 8, 117. [Google Scholar] [CrossRef]
- Eloff, J.N. A proposal on expressing the antibacterial activity of plant extracts a small first step in applying scientific knowledge to rural primary health care in South Africa. S. Afr. J. Sci. 2000, 96, 116–118. [Google Scholar]
- Eloff, J. Quantification the bioactivity of plant extracts during screening and bioassay guided fractionation. Phytomedicine 2004, 11, 370–371. [Google Scholar] [CrossRef] [PubMed]
- Amoo, S.; Ndhlala, A.; Finnie, J.; Van Staden, J. Antifungal, acetylcholinesterase inhibition, antioxidant and phytochemical properties of three Barleria species. S. Afr. J. Bot. 2011, 77, 435–445. [Google Scholar] [CrossRef]
- Amoo, S.; O Aremu, A.; Moyo, M.; Van Staden, J. Antioxidant and acetylcholinesterase-inhibitory properties of long-term stored medicinal plants. BMC Complement. Altern. Med. 2012, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S. Quantification of Tannins in Tree and Shrub Foliage: A laboratory Manual; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Durazzo, A. Study Approach of Antioxidant Properties in Foods: Update and Considerations. Foods 2017, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Amarowicz, R.; Karamać, M.; Shahidi, F. Antioxidant activity of phenolic fractions of lentil (Lens culinaris). J. Food Lipids 2003, 10, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Zhu, L.-J.; Chen, J.-P.; Shi, C.-Y.; Niu, L.-T.; Zhang, X.; Yao, X.-S. C-Methylated flavanones from the rhizomes of Matteuccia intermedia and their α-glucosidase inhibitory activity. Fitoterapia 2019, 136, 104147. [Google Scholar] [CrossRef]
- Eloff, J.N. A Sensitive and Quick Microplate Method to Determine the Minimal Inhibitory Concentration of Plant Extracts for Bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).