Siberian Wildrye (Elymus sibiricus L.) Abscisic Acid-Insensitive 5 Gene Is Involved in Abscisic Acid-Dependent Salt Response
Abstract
:1. Introduction
2. Results
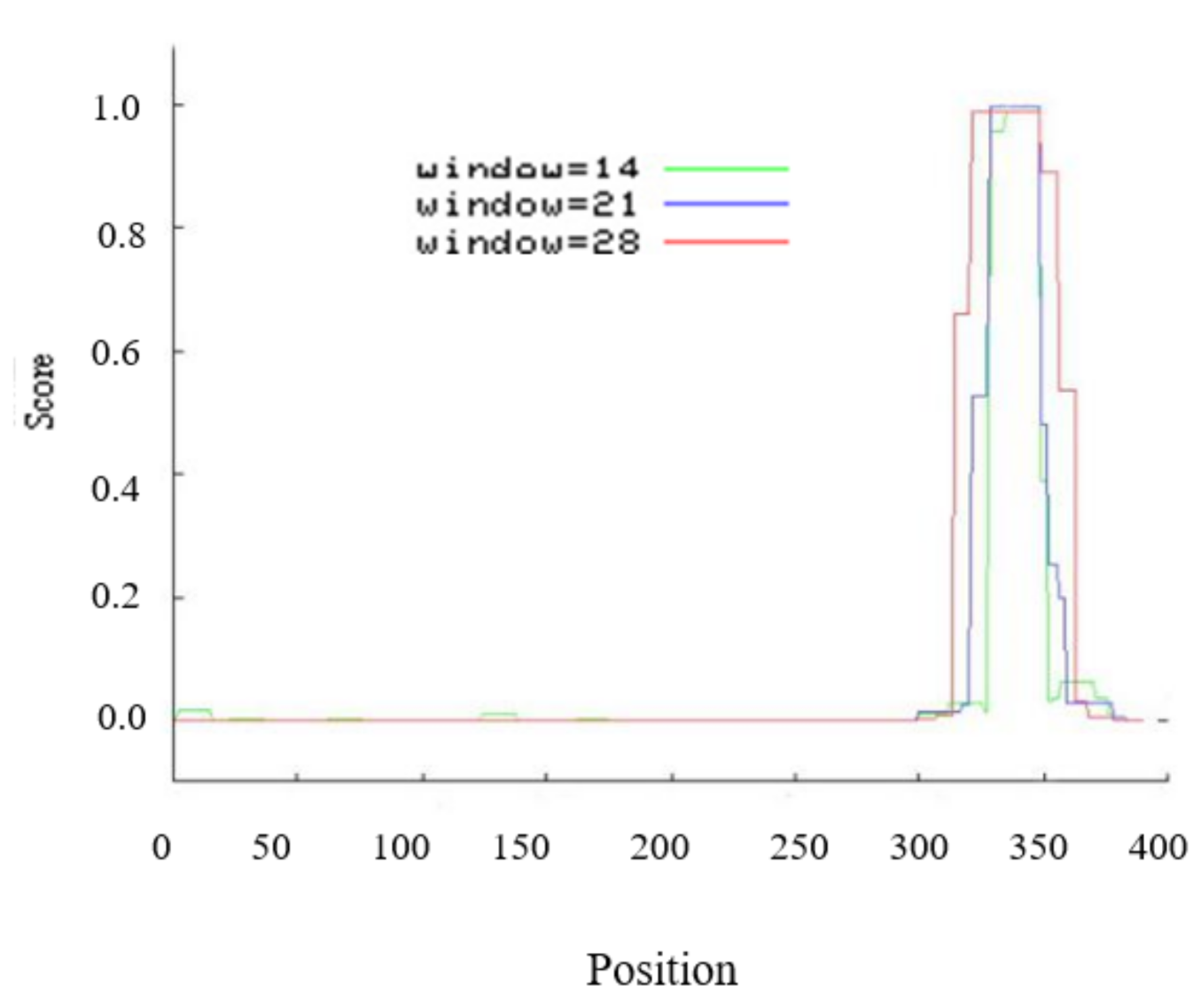
2.1. Isolation and Basic Analysis of EsABI5
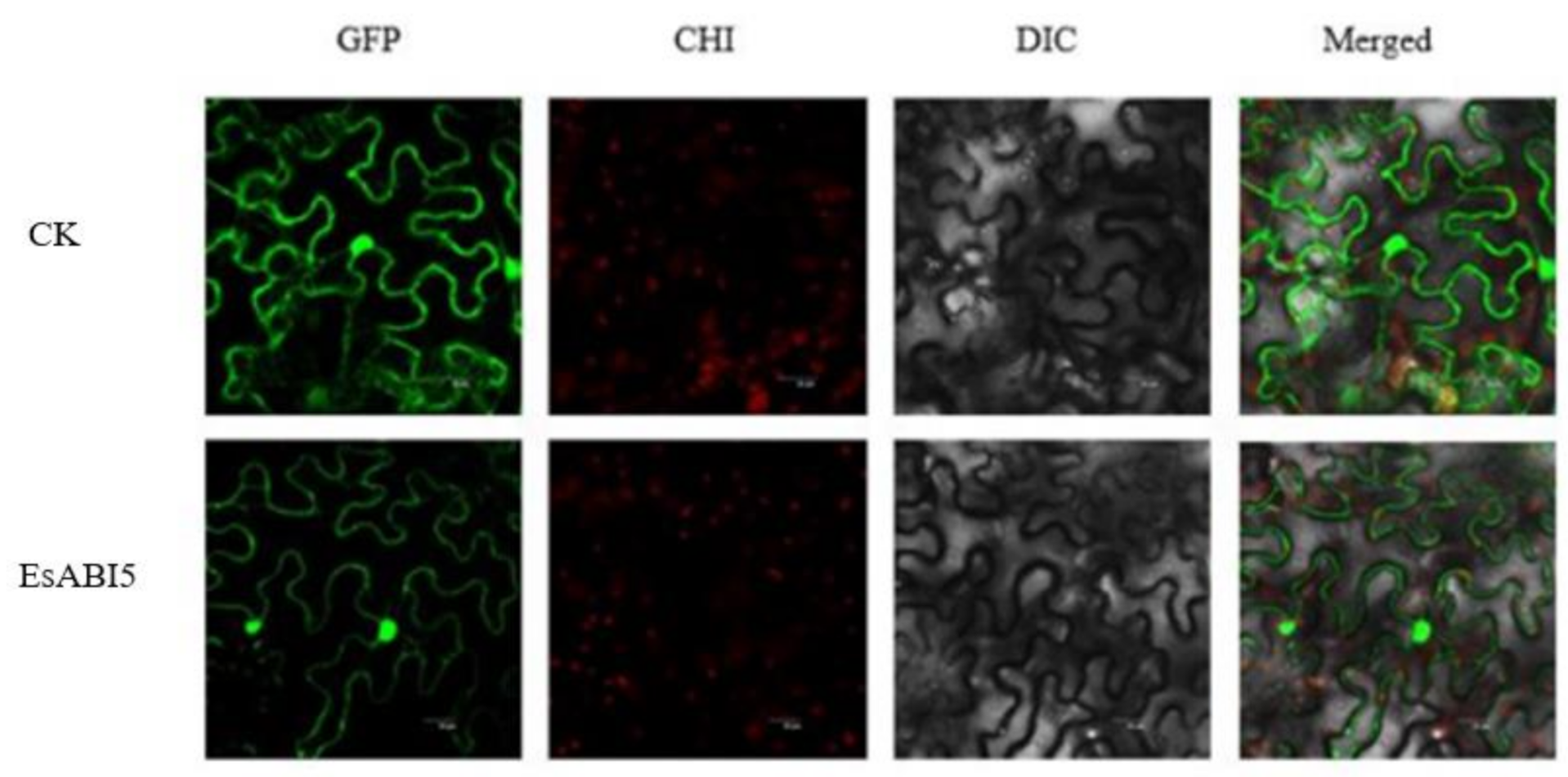
2.2. Subcellular Localization of EsABI5
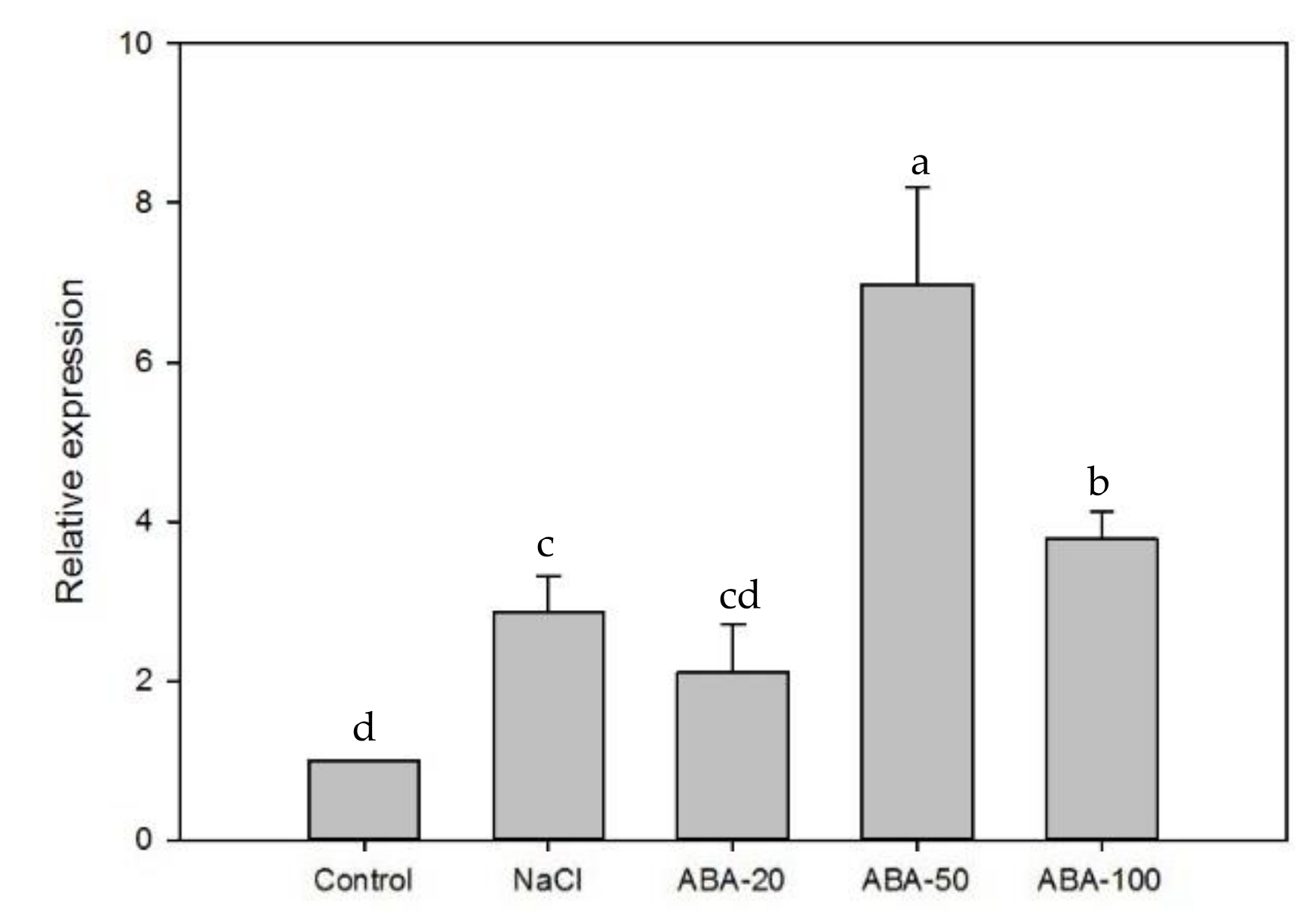
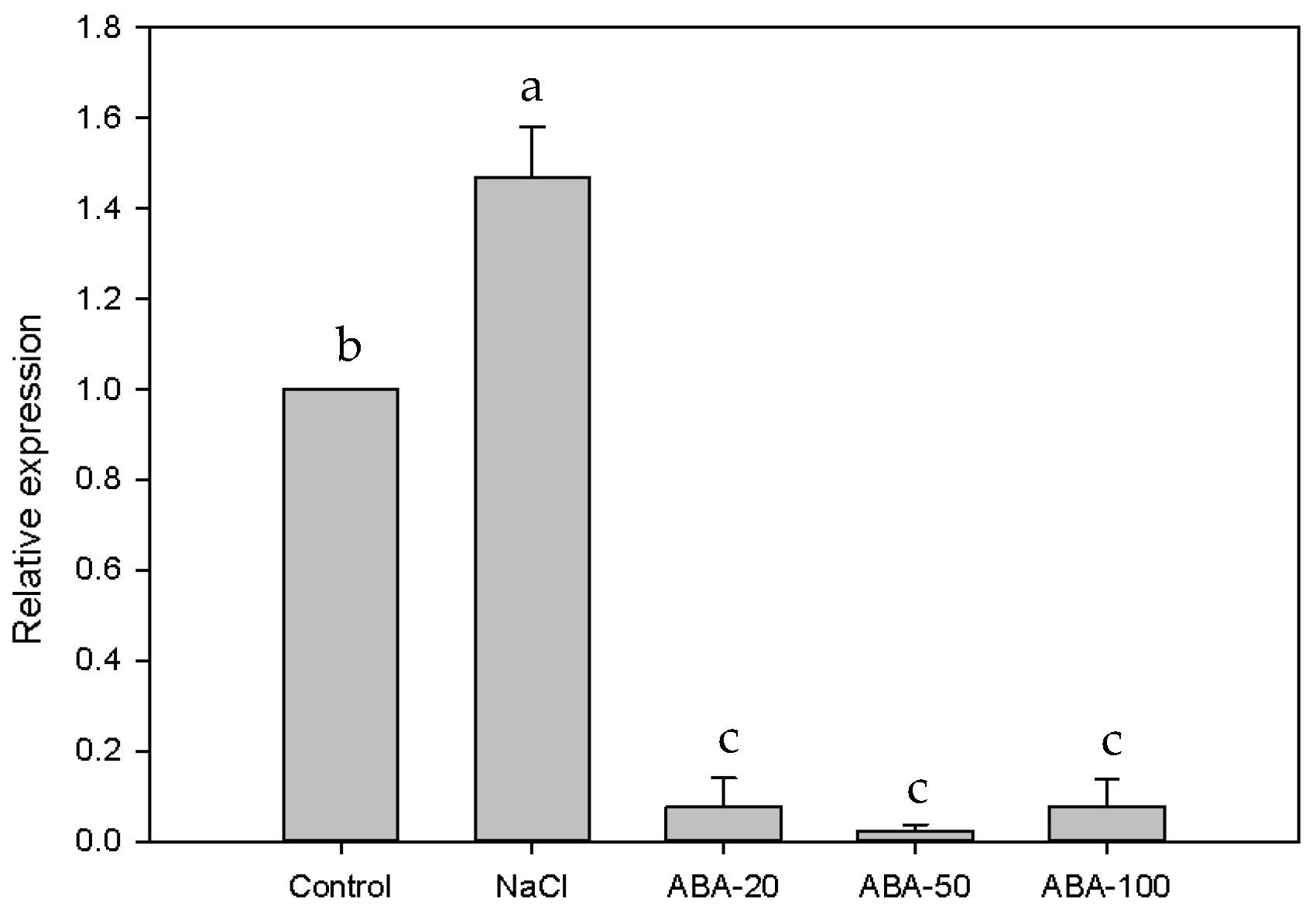
2.3. Expression of EsABI5 under Salt and ABA Treatment
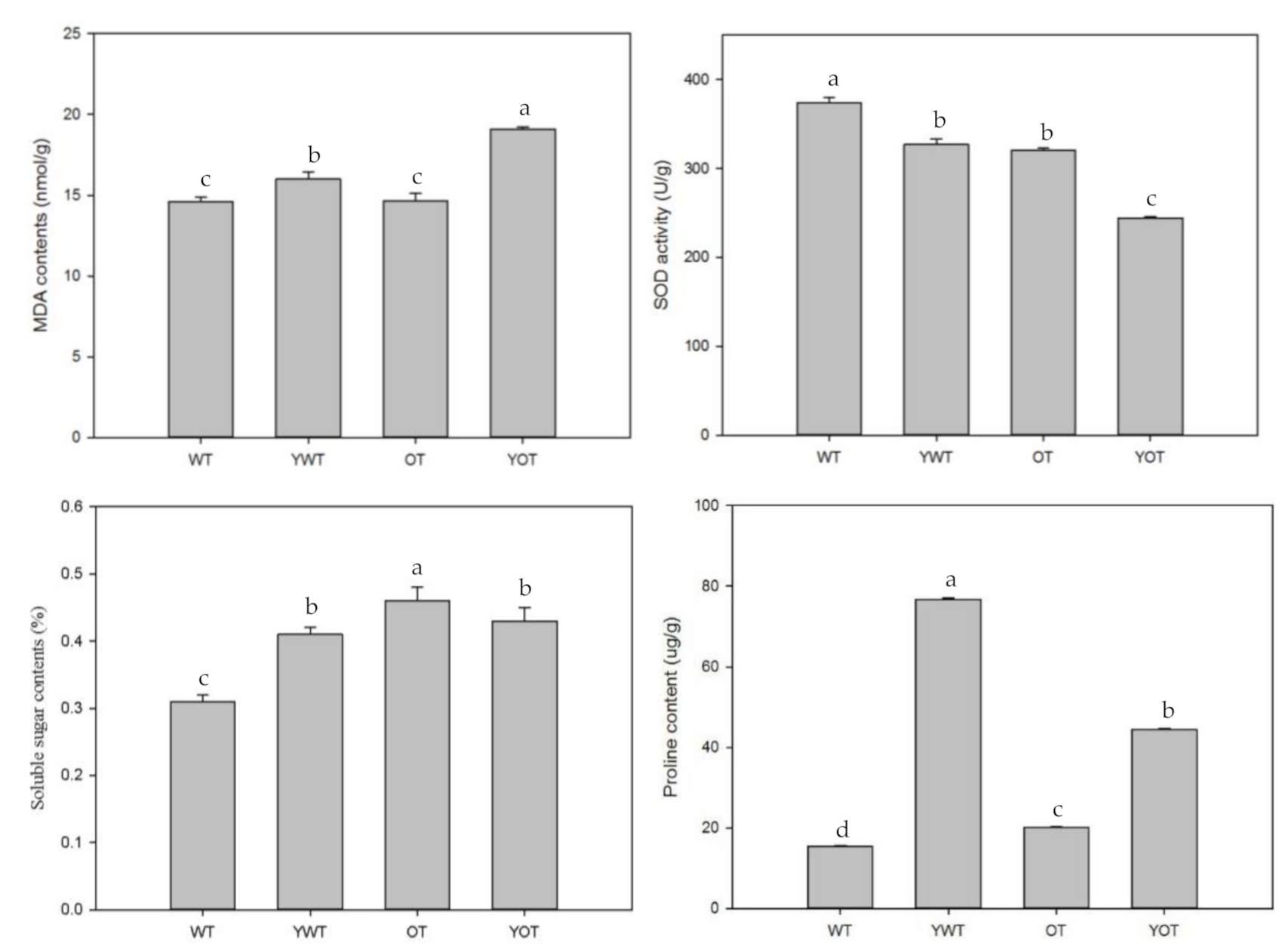
2.4. Decreased Salt Tolerance of EsABI5 Transgenic Tobacco
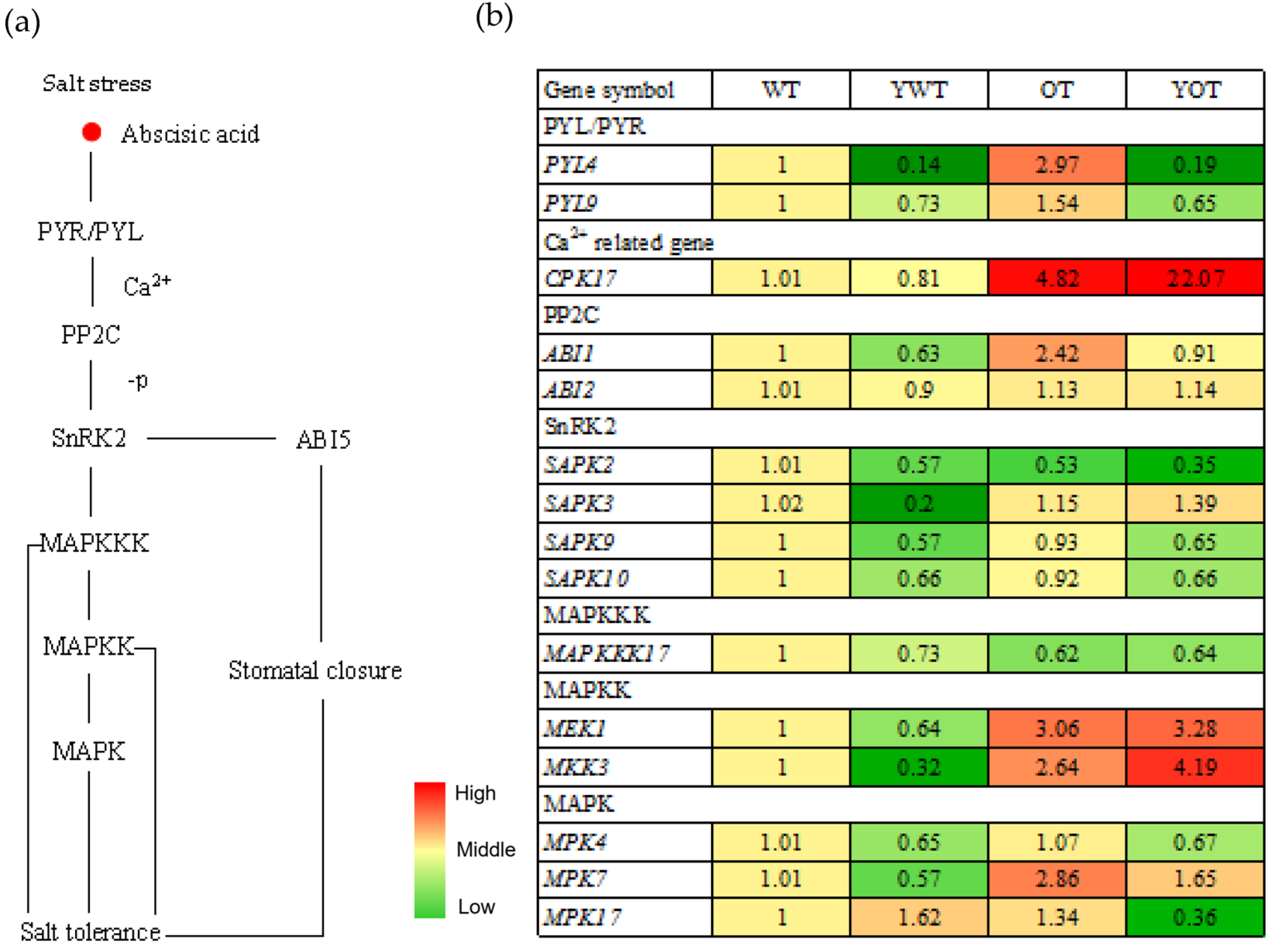
2.5. Involvement of EsABI5 in ABA and MAPK Signaling Pathways in Response to Salt Stress
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Primer Design
4.3. Reverse Transcription Quantitative PCR, PCR Product Purification, and Clone Sequencing
4.4. Bioinformatics Analysis of EsABI5
4.5. Subcellular Localization
4.6. Real-Time Fluorescence Quantitative PCR
4.7. Overexpression Vector Construction, Tobacco Transformation, and Line Propagation
4.8. Physiological Indexes and Data Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uno, Y.C.; Furihata, T.; Abe, H.; Yoshida, R.; Shinozaki, K.; Shinozaki, K.Y. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc. Natl. Acad. Sci. USA 2000, 97, 11632–11637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, M.J.; Guan, Y.C.; Ren, H.B.; Zhang, F.; Chen, F.A. bZIP transcription factor, OsABI5, is involved in rice fertility and stress tolerance. Plant Mol. Biol. 2008, 66, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, Y.N.; Xue, L.J.; Zou, M.J.; Liu, J.Y.; Chen, F.; Xue, H.W. Rice ABI5-like1 regulates abscisic acid and auxin responses by affecting the expression of ABRE-containing genes. Plant Physiol. 2011, 156, 1397–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RuizPartida, R.; Rosario Sttefany, M.; LozanoJuste, J. An update on crop ABA receptors. Plants 2021, 10, 1087. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic Stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Wasilewska, A.; Vlad, F.; Sirichandra, C.; Redko, Y.; Jammes, F.; Valon, C.; Frey, N.F.D.; Leung, J. An update on abscisic acid signaling in plants and more. Mol. Plant 2008, 1, 198–217. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [Green Version]
- Hubbard, K.E.; Nishimura, N.; Hitomi, K.; Getzoff, E.D.; Schroeder, J.I. Early abscisic acid signal transduction mechanisms: Newly discovered components and newly emerging questions. Gene Dev. 2010, 24, 1695–1708. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, R.R.; Lynch, T.J. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 2000, 12, 599–609. [Google Scholar] [CrossRef] [Green Version]
- Lopez, M.L.; Mongrand, S.; Chua, N.H. A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc. Natl. Acad. Sci. USA 2001, 98, 4782–4787. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, N.; Yoshida, T.; Kitahata, N.; Asami, T.; Shinozaki, K.; Hirayama, T. ABA-hypersensitive germination 1 encodes a protein phosphatase 2C, an essential component of abscisic acid signaling in Arabidopsis seed. Plant J. 2007, 50, 935–949. [Google Scholar] [CrossRef]
- Chen, T.T.; Liu, F.F.; Xiao, D.W.; Jiang, X.Y.; Li, P.; Zhao, S.M.; Hou, B.K.; Li, Y.J. The Arabidopsis UDP-glycosyltransferase75B1, conjugates abscisic acid and affects plant response to abiotic stresses. Plant Mol. Biol. 2020, 102, 389–401. [Google Scholar] [CrossRef]
- Chang, H.C.; Tsai, M.C.; Wu, S.S.; Chang, I. Regulation of ABI5 expression by ABF3 during salt stress responses in Arabidopsis thaliana. Bot. Stud. 2019, 60, 16. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.W.; Chen, Y.H.; Qian, L.F.; Mu, R.; Yuan, X.; Fang, H.M.; Huang, X.; Xu, E.S.; Zhang, H.S.; Huang, J. A novel RNA-binding protein involves ABA signaling by post-transcriptionally repressing ABI2. Front. Plant Sci. 2017, 24, 24. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.X.; Stone, S.L. Regulation of ABI5 turnover by reversible post-translational modifications. Plant Signal. Behav. 2014, 9, e27577. [Google Scholar] [CrossRef] [Green Version]
- Seo, K.I.; Lee, J.H.; Nezames, C.D.; Zhong, S.W.; Song, E.Y.; Byun, M.O. ABD1 is an Arabidopsis DCAF substrate receptor for CUL4-DDB1-based E3ligases that acts as a negative regulator of abscisic acid signaling. Plant Cell 2014, 26, 695–711. [Google Scholar] [CrossRef] [Green Version]
- Han, J.J.; Yang, X.Y.; Wang, Q.; Tang, L.; Yu, F.F.; Huang, X.H.; Wang, Y.C.; Liu, J.X.; Xie, Q. The β5 subunit is essential for intact 26S proteasome assembly to specifically promote plant autotrophic growth under salt stress. New Phytol. 2019, 221, 1359–1368. [Google Scholar] [CrossRef] [Green Version]
- Skubacz, A.; Daszkowska, G.A.; Szarejko, I. The role and regulation of ABI5 (ABA-insensitive 5) in plant development, abiotic stress responses and phytohormone crosstalk. Front. Plant Sci. 2016, 7, 1884. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Ma, J.Z.; Perret, P.; Li, Z.S.; Thomas, T.L. Arabidopsis ABI5 subfamily members have distinct DNA-binding and transcriptional activities. Plant Physiol. 2002, 130, 688–697. [Google Scholar] [CrossRef] [Green Version]
- Garcia, M.E.; Lynch, T.; Peeters, J.; Finkelstein, R. A small plant-specific protein family of ABI five binding proteins (AFPs) regulates stress response in germinating Arabidopsis seeds and seedlings. Plant Mol. Biol. 2008, 67, 643–658. [Google Scholar] [CrossRef]
- Finkelstein, R. Abscisic acid: A seed maturation and stress response hormone. In Plant Physiology, 5th ed.; Taiz, L., Zeiger, E., Eds.; Sinauer Associates Press: Sunderland, UK, 2010; pp. 573–698. [Google Scholar]
- Collin, A.; Golec, A.D.; Kurowska, M.; Szarejko, I. Barley ABI5 (Abscisic acid insensitive 5) is involved in abscisic acid-dependent drought response. Front. Plant Sci. 2020, 11, 1138. [Google Scholar] [CrossRef]
- Bai, Y.L.; Zhu, W.B.; Hu, X.C.; Sun, C.C.; Li, Y.L.; Wang, D.D.; Wang, Q.H.; Pei, G.L.; Zhang, Y.F.; Guo, A.G.; et al. Genome-wide analysis of the bZIP gene family identifies two ABI5-like bZIP transcription factors, BrABI5a and BrABI5b, as positive modulators of ABA signaling in Chinese cabbage. PLoS ONE 2016, 11, e0158966. [Google Scholar] [CrossRef]
- Yan, F.; Deng, W.; Wang, X.M.; Yang, C.W.; Li, Z.G. Maize (Zea mays L.) homologue of ABA-insensitive (ABI) 5 gene plays a negative regulatory role in abiotic stresses response. Plant Growth Regul. 2012, 68, 383–393. [Google Scholar] [CrossRef]
- Liu, M.; Gong, J.R.; Zhang, Z.Y.; Wang, Y.H. Progress in drought resistance and cold tolerance of artificial pastures in northern arid areas. J. NW Univ. 2015, 43, 56–62. [Google Scholar]
- Zhang, J.C.; Xie, W.G.; Yu, X.X.; Zhang, Z.Y.; Zhao, Y.Q.; Wang, N.; Wang, Y.R. Selection of suitable reference genes for RT-qPCR gene expression analysis in Siberian wild rye (Elymus sibiricus) under different experimental conditions. Genes 2019, 10, 451. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.; Liu, W.H.; Zhao, J.M.; You, M.H.; Xiong, C.H.; Xiong, Y.; Xiong, Y.L.; Yu, Q.Q.; Bai, S.Q.; Ma, X. Comparative physiological and proteomic analysis reveals different involvement of proteins during artificial aging of Siberian wildrye seeds. Plants 2020, 9, 1370. [Google Scholar] [CrossRef]
- Hossain, M.A.; Cho, J.I.; Han, M.; Ahn, C.H.; Jeon, J.S.; An, G.; Park, P.B. The ABRE-binding bZIP transcription factor OsABF2 is a positive regulator of abiotic stress and ABA signaling in rice. J. Plant Physiol. 2010, 167, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Fujita, Y.; Sayama, H.; Kidokoro, S.; Maruyama, K.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 2010, 61, 672–685. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, H.; Li, X.H.; Xiao, J.H.; Xiong, L.Z. Constitutive activation of transcription factor OsbZIP46 improves drought tolerance in rice. Plant Physiol. 2012, 158, 1755–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.; Moon, S.J.; Min, M.K.; Choi, E.H.; Kim, J.A.; Koh, E.Y.; Yoon, I.; Byun, M.O.; Yoo, S.D.; Kim, B.G. Functional characterization and reconstitution of ABA signaling components using transient gene expression in rice protoplasts. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Tang, N.; Ma, S.; Zong, W.; Yang, N.; Lv, Y.; Yan, C.; Guo, Z.; Li, J.; Li, X.; Xiang, Y.; et al. MODD mediates deactivation and degradation of OsbZIP46 to negatively regulate ABA signaling and drought resistance in Rice. Plant Cell 2016, 28, 2161–2177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, G.Y.; Ye, L.F.; Chen, X.B. Research progress of Arabidopsis B3 transcription factor gene superfamily. Chem. Life 2013, 33, 287–293. [Google Scholar]
- Tang, R.J.; Wang, C.; Li, K.L.; Luan, S. The CBL-CIPK calcium signaling network: Unified paradigm from 20 years of discoveries. Trends Plant Sci. 2020, 25, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Chong, Y.H.; Gupta, R.; Luan, S. Interaction specificity of Arabidopsis calcineur in B-like calcium sensors and their target kinases. Plant Physiol. 2000, 124, 1844–1853. [Google Scholar] [CrossRef] [Green Version]
- Batistic, O.; Kudla, J. Integration and channeling of calcium signaling through the CBL calcium sensor/CIPK proteins kinase network. Planta 2004, 219, 915–924. [Google Scholar] [CrossRef]
- Kim, K.N.; Cheong, Y.H.; Grant, J.J.; Pandey, G.K.; Luan, S. CIPK3, a calcium sensor-associated protein kinase that regulates abscisic acid and cold signal transduction in Arabidopsis. Plant Cell 2003, 5, 411–427. [Google Scholar] [CrossRef] [Green Version]
- Kolukisaoglu, U.; Weinl, S.; Blazevic, D.; Batistic, O.; Kudla, J. Calcium sensors and their 32 protein kinases: Genomics of the Arabidopsis and rice CBL-CIPK signaling networks. Plant Physiol. 2004, 134, 43–58. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Zhang, H.; Tian, S.; Chang, X.; Jing, R. TaSnRK2.4, an SNF1-type serine/threonine protein kinase of wheat (Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis. J. Exp. Bot. 2010, 61, 683–696. [Google Scholar] [CrossRef] [Green Version]
- Diédhiou, C.J.; Popova, O.V.; Dietz, K.J.; Golldack, D. The SNF1-type serine/threonine protein kinase SAPK4 regulates stress responsive gene expression in rice. BMC Plant Biol. 2008, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.T.; Liu, H.; Stone, S.; Callis, J. ABA and the ubiquitin E3 ligase KEEP ON GOING affect proteolysis of the Arabidopsis thaliana transcription factors ABF1 and ABF3. Plant J. 2013, 75, 965–976. [Google Scholar] [CrossRef] [Green Version]
- Lyzenga, W.J.; Liu, H.; Schofield, A.; Muise, H.A.; Muise-Hennessey, A.; Stone, S.L. Arabidopsis CIPK26 interacts with KEG, components of the ABA signaling network and is degraded by the ubiquitin-proteasome system. J. Exp. Bot. 2013, 64, 2779–2791. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Stone, S.L. Abscisic acid increases Arabidopsis ABI5 transcription factor levels by promoting KEG E3 ligase self-ubiquitination and proteasomal degradation. Plant Cell 2010, 22, 2630–2641. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.Y.; Sun, P.W.; Wang, W.; Tang, D.Z. Arabidopsis E3 ligase KEG associates with and ubiquitinates MKK4 and MKK5 to regulate plant immunity. J. Integr. Plant Biol. 2020, 63, 327–339. [Google Scholar] [CrossRef]
- Lyzenga, W.J.; Sullivan, V.; Liu, H.X.; Stone, S.L. The kinase activity of calcineurin B-like interacting protein kinase 26 (CIPK26) influences its own stability and that of the ABA-regulated ubiquitin ligase, keep on going (KEG). Front. Plant Sci. 2017, 8, 502. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Min, J.R. Structure and function of WD40 domain proteins. Protein Cell 2011, 2, 202–214. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Paek, K.H.; Kwon, S.Y.; Cho, H.S.; Kim, S.J.; Park, J.M. A novel WD40 protein, Bn SWD1, is involved in salt stress in Brassica napus. Plant Biotechnol. Rep. 2010, 4, 165–172. [Google Scholar] [CrossRef]
- Mishra, A.K.; Puranik, S.; Bahadur, R.P.; Prasad, M. The DNA-binding activity of an AP2 protein is involved in transcriptional regulation of a stress-responsive gene, Si WD40, in foxtail millet. Genomics 2012, 100, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.Y.; Xiong, L.Z. Putative megaenzyme DWA1 plays essential roles in drought resistance by regulating stress-induced wax deposition in rice. Proc. Natl. Acad. Sci. USA 2013, 110, 17790–17795. [Google Scholar] [CrossRef] [Green Version]
- Wasternack, C. Jasmonates, an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.Z.; Wan, W.; Jiang, G.B.; Xi, Y.; Huang, H.J.; Cai, J.J.; Chang, Y.N.; Duan, C.G.; Mangrauthia, S.K.; Peng, X.X.; et al. Nucleocytoplasmic trafficking of the Arabidopsis WD40 repeat protein XIW1 regulates ABI5 stability and abscisic acid responses. Mol. Plant 2019, 12, 1598–1611. [Google Scholar] [CrossRef]
- Chen, C.T.; Wu, C.G.; Miao, J.M.; Lei, Y.X.; Zhao, D.X.; Sun, D.; Yang, G.D.; Huang, J.G.; Zheng, C.C. Arabidopsis SAG protein containing the MDN1 domain participates in seed germination and seedling development by negatively regulating ABI3 and ABI5. J. Exp. Bot. 2014, 65, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.; Davison, T.S.; Henz, S.R.; Pape, U.J.; Demar, M.; Vingron, M.; Schlkopf, B.; Weigel, D.; Lohmann, J.U. A gene expression map of Arabidopsis thaliana development. Nat. Genet. 2005, 37, 501–506. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Zhou, Y.F.; Ruan, Y.Y.; Gong, X.; Zhang, J.; Huang, R.D. Inhibition of SbABI5 expression in roots by ultra-high endogenous ABA accumulation results in Sorghum sensitivity to salt Stress. Int. J. Agric. Biol. 2016, 18, 146–154. [Google Scholar] [CrossRef]
- Gietler, M.; Fidler, J.; Labudda, M.; Nykiel, M.; Marta, G.; Justyna, F.; Mateusz, L.; Malgorzata, N. Abscisic acid-enemy or saviour in the response of cereals to abiotic and biotic stresses? Int. J. Mol. Sci. 2020, 21, 4607. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.; Lambert, A.; Herouart, D.; Boncompagni, E. Identification of new up-regulated genes under drought stress in soybean nodules. Gene 2008, 426, 15–22. [Google Scholar] [CrossRef]
- Brown, P.O.; Botstein, D. Exploring the new world of the genome with DNA microarrays. Nat. Genet. 1999, 21, 33–37. [Google Scholar] [CrossRef]
- Xing, Y.; Jia, W.S.; Zhang, J.H. AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J. 2008, 54, 440–451. [Google Scholar] [CrossRef]
- Zong, X.J.; Li, D.P.; Gu, L.K.; Li, D.Q.; Liu, L.X.; Hu, X.L. Abscisic acid and hydrogen peroxide induce a novel maize group C MAP kinase gene, ZmMPK7, which is responsible for the removalof reactive oxygen species. Planta 2009, 229, 485–495. [Google Scholar] [CrossRef]
- Su, S.H.; Suarez-Rodriguez, M.C.; Krysan, P. Genetic interaction and phenotypic analysis of the Arabidopsis MAP kinase pathway mutation mekk1 and mpk4 suggests signaling pathway complexity. FEBS Lett. 2007, 581, 3171–3177. [Google Scholar] [CrossRef] [Green Version]
- Mizoguchi, T.; Hayashida, N.; Yamaguchi-Shinozaki, K.; Kamada, H.; Shinozaki, K. ATMPKs, a gene family of plant MAP kinases in Arabidopsis thaliana. FEBS Lett. 1993, 336, 440–444. [Google Scholar] [CrossRef] [Green Version]
- Ichimura, K.; Mizoguchi, T.; Yoshida, R.; Yuasa, T.; Shinozaki, K. Various abiotic stresses vapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J. 2000, 24, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Wang, Y.; Liu, H.X.; Lei, L.; Yang, H.L.; Liu, G.Q.; Ren, D.T. Activation of MAPK kinase 9 induces ethylene and camalexin biosynthesis and enhances sensitivity to salt stress in Arabidopsis. J. Biol. Chem. 2008, 283, 26996–27006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Guo, H.W. Paradigms and paradox in the ethylene signaling pathway and interaction network. Mol. Plant. 2011, 4, 626–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, M.J.; Lee, S.K.; Kim, B.G.; Kwon, T.R.; Cho, W.S.; Park, Y.T.; Lee, J.O.; Kwon, H.B.; Byun, M.O.; Park, S.C. A rice (Oryza sativa L.) MAP kinase gene, OsMAPK44, is involved in response to abiotic stresses. Plant Cell Tissue Org. Culture 2006, 85, 151–160. [Google Scholar] [CrossRef]
- Shi, J.; An, H.L.; Zhang, L.A.; Gao, Z.; Guo, X.Q. GhMPK7, a novel multiple stress-responsive cotton group C MAPK gene, has a role in broad spectrum disease resistance and plant development. Plant Mol. Biol. 2010, 72, 1–17. [Google Scholar] [CrossRef]
- Ding, H.D.; Zhang, X.H.; Xu, S.C.; Sun, L.L.; Jiang, M.Y.; Zhang, A.Y.; Jin, Y.G. Induction of protection against paraquat-induced oxidative damage by abscisic acid in maize leaves is mediated through mitogen-activated protein kinase. J. Integr. Plant Biol. 2009, 51, 961–972. [Google Scholar] [CrossRef]
- Xie, W.G.; Zhang, J.C.; Zhao, X.H.; Zhang, Z.Y.; Wang, Y.R. Transcriptome profiling of Elymus sibiricus, an important forage grass in Qinghai-Tibet plateau, reveals novel insights into candidate genes that potentially connected to seed shattering. BMC Plant Biol. 2017, 17, 78. [Google Scholar] [CrossRef]
- Pombo, M.A.; Ramos, R.N.; Zheng, Y.; Fei, Z.J.; Martin, G.B.; Rosli, H.G. Transcriptome-based identification and validation of reference genes for plant-bacteria interaction studies using Nicotiana benthamiana. Sci. Rep. 2019, 9, 742–744. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
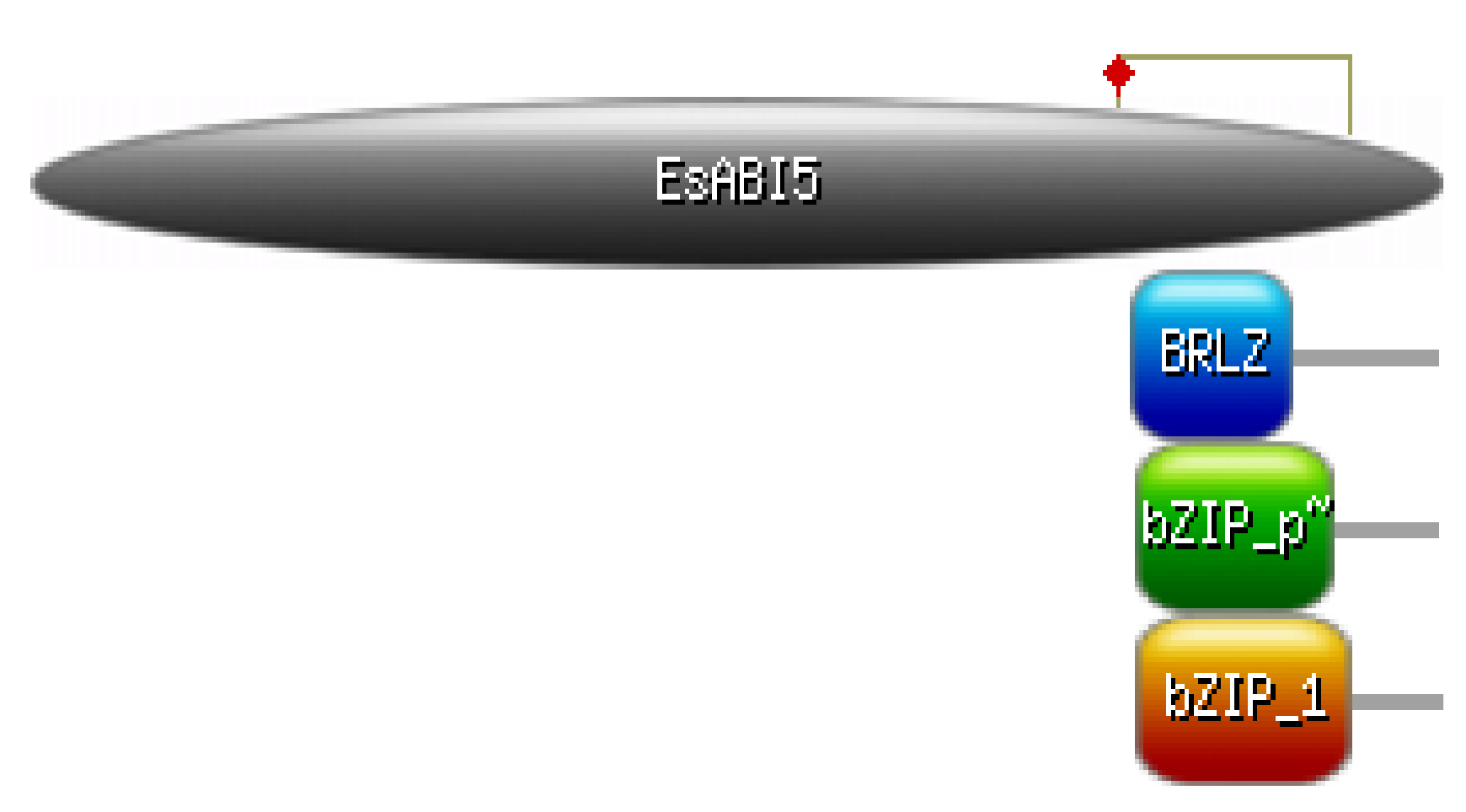


| Name | Position Interval | E Value |
|---|---|---|
| bZIP_plant_BZIP46 | 305–359 | 3.67 × 10−23 |
| BRLZ | 304–348 | 3.09 × 10−14 |
| bZIP_1 | 305–364 | 1.44 × 10−10 |
| Name | Sequence 5′→3′ | Application |
|---|---|---|
| CTG0957 F1 | GCGGCAGTCTTCCATCTTCG | Homologous gene acquisition |
| CTG0957 R1 | TCCTCACCTTCGGCAACG | Homologous gene acquisition |
| CTG0957-1 F3 | ACAGATGAACCCCGCGCAGCAGG | 3′ RACE |
| CTG0957-1 F5 | TGACGCAGGCTGACATGATGAACT | 3′ RACE |
| CTG0957-1 F9 | GATGATGGAACAGTCCAAGG | 3′ RACE |
| CTG0957-2 R4 | GAACATGCCGTTGGCCGGTGCCATC | 5′ RACE |
| CTG0957-2 R6 | CATGGACCATGCCGACCGGCACAG | 5′ RACE |
| CTG0957-2 YZF1 | CACAAGGCAAGCATATCGAG | RACE verification |
| CTG0957-1 YZR1 | CCGCTCCGAAATGATAAGGT | RACE verification |
| CTG0957-1 YZR2 | GTTATGATAGCTGAATGGCA | RACE verification |
| XhoI-EsABI5-F | CCGCTCGAGATGGCGTCGGCGATGAGCAA | EsABI5 CDS cloning |
| XbaI-EsABI5-R | GCTCTAGATCACACGTGGTGGTGGTGGT | EsABI5 CDS cloning |
| Gene Symbol | GenBank Accession Number | Primer Name | Sequence (5′→ 3′) |
|---|---|---|---|
| NbUbe35 | SRP118889 | NbUbe35-F | CTTCAGATTCGCACCGTTCT |
| NbUbe35-R | CCAATGCTTCGCAATGTTCTC | ||
| Gapdh | AF251217.1 | Gapdh-F | ATGAGGACCTTGTTTCCACTGACTT |
| Gapdh-R | GTGCTGTATCCCCACTCGTTGT | ||
| EsABI5 | MN607227.1 | EsABI5-F | ACGCAGGCTGACATGATGA |
| EsABI5-R | CGGCTGACTCACGGTTCTT | ||
| EsABI5 | MN607227.1 | EsABI5-F1 | CGGCAGTCTTCCATCTTCG |
| EsABI5-R1 | GGAACTCCTCGGCATTCCAG | ||
| PYL4 | XM_019392528.1 | PYL4-F | TCCGCGTTGTTTCTGGC |
| PYL4-R | GTTTCTTCCTTAGTATTCCCTTGTG | ||
| PYL9 | XM_019409396.1 | PYL9-F | GTTTGGTCATTAGTGAGGAGGTTT |
| PYL9-R | TTGCTTGTGGTAGCTGGGAG | ||
| ABI1 | NM_001336190.1 | ABI1-F | CGCCTCTTGTGACCTTGCT |
| ABI1-R | CCGCTTTCGGGTTCTGTTA | ||
| ABI2 | XM_019393623.1 | ABI2-F | GGTAGGAGGGCTTGGTAGTGA |
| ABI2-R | TGGACAACGGCATGGGTA | ||
| SAPK2 | XM_033652288.1 | SNF1-F | AGTGGCAAGGCTTATGAGGG |
| SNF1-R | CTCTTTGAATCTGACTATGTTAGGGTG | ||
| SAPK3 | XM_019398597.1 | SAPK3-F | CAAAGGAGCTTGTTGCTGTCA |
| SAPK3-R | GAGCCTCATCTTCACTAAATCTACC | ||
| SAPK9 | EH367211.1 | SAPK9-F | ATTCTACTCGACGGAAGTGCTG |
| SAPK9-R | GATCGCTTGAATCTTGAAATGG | ||
| SAPK10 | XM_019398090.1 | SRK2E-F | AGTGGCAAGGCTTATGAGGG |
| SRK2E-R | CTCTTTGAATCTGACTATGTTAGGGTG | ||
| MAPKKK17 | NM_001325618.1 | NPK1-F | TCCTGGTGGCTCAATCTCG |
| NPK1-R | GTCAACAAGTATGTTTGCTCCCT | ||
| MPK4 | XM_019391189.1 | MPK4-F | GTCGTAACACGGTGGTATCGG |
| MPK4-R | CTGGCATCATCAGGTGATCCTAT | ||
| MPK7 | XM_019380635.1 | MPK7-F | CGAATAGAATTGATGCGCTGAG |
| MPK7-R | GGATAAAGGCTGCGACGACT | ||
| MPK17 | NM_001325663.1 | MPK17-F | TCCCATCTGCCAGTGAGGTT |
| MPK17-R | TTGCTGCGAGGCTTTGAGT | ||
| MKK3 | XM_019380228.1 | MKK3-F | TTTCTCACCTGCCTCTACATCG |
| MKK3-R | ACACTACTTGCACCGCTACCTAT | ||
| MEK1 | NM_001326016.1 | MEK1-F | CTTTCACGACGGCGATTTAC |
| MEK1-R | GAACAACACCACCACTTCCCT | ||
| CPK17 | XM_019401998.1 | CPK17-F | AGGATGGAGAAGCACCAGATACAC |
| CPK17-R | CACCCTGCAATTACCCGAAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De, Y.; Shi, F.; Gao, F.; Mu, H.; Yan, W. Siberian Wildrye (Elymus sibiricus L.) Abscisic Acid-Insensitive 5 Gene Is Involved in Abscisic Acid-Dependent Salt Response. Plants 2021, 10, 1351. https://doi.org/10.3390/plants10071351
De Y, Shi F, Gao F, Mu H, Yan W. Siberian Wildrye (Elymus sibiricus L.) Abscisic Acid-Insensitive 5 Gene Is Involved in Abscisic Acid-Dependent Salt Response. Plants. 2021; 10(7):1351. https://doi.org/10.3390/plants10071351
Chicago/Turabian StyleDe, Ying, Fengling Shi, Fengqin Gao, Huaibin Mu, and Weihong Yan. 2021. "Siberian Wildrye (Elymus sibiricus L.) Abscisic Acid-Insensitive 5 Gene Is Involved in Abscisic Acid-Dependent Salt Response" Plants 10, no. 7: 1351. https://doi.org/10.3390/plants10071351





