Optimization of Extraction Solvent and Fast Blue BB Assay for Comparative Analysis of Antioxidant Phenolics from Cucumis melo L.
Abstract
:1. Introduction
2. Results
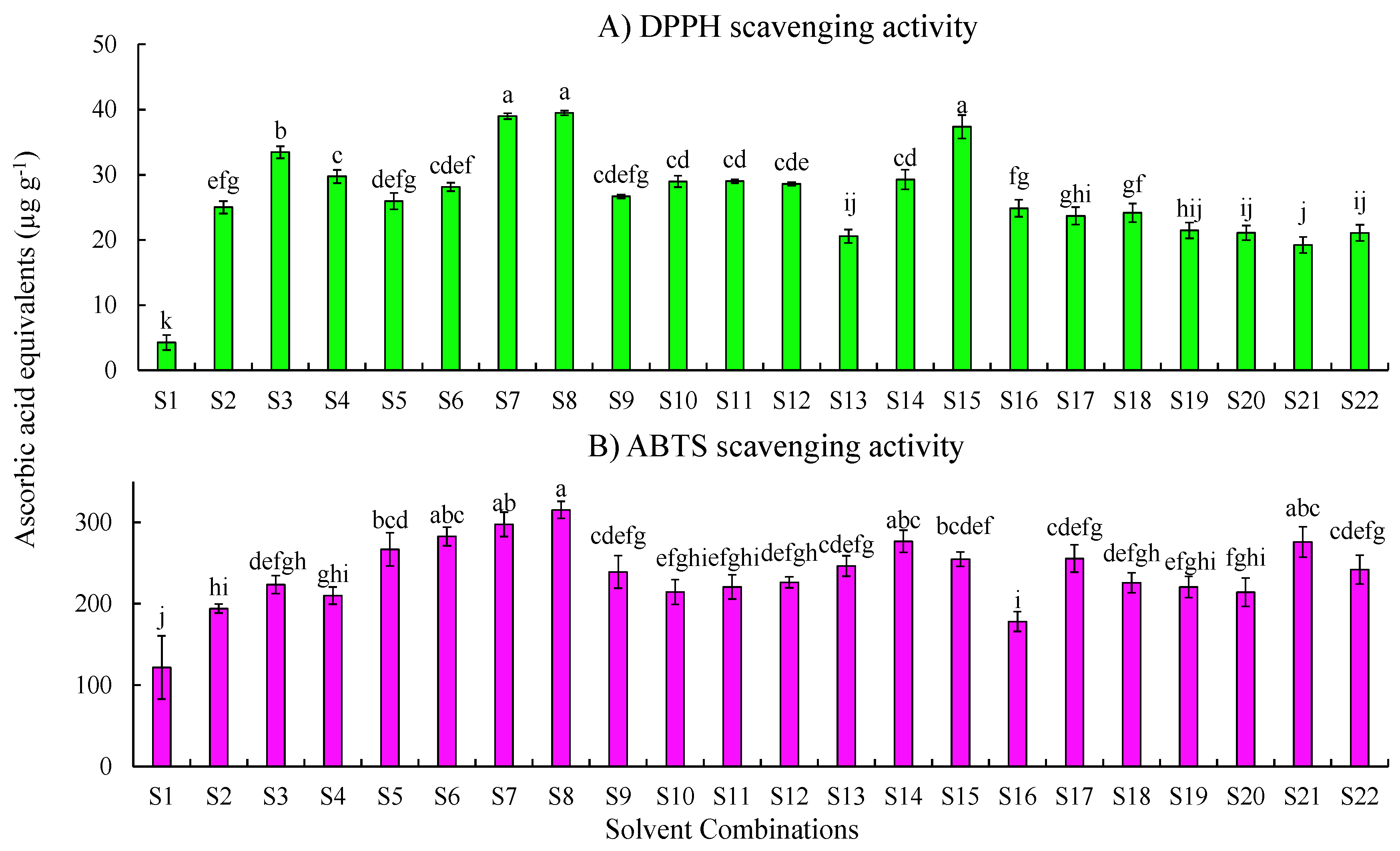
2.1. Effect of the Extraction Solvent on Free Radical Scavenging Activities
2.1.1. DPPH Scavenging Activity
2.1.2. ABTS Scavenging Activity
2.2. Optimization of the FB Assay
2.2.1. Optimization of the Base Type, Concentration, and Incubation Time
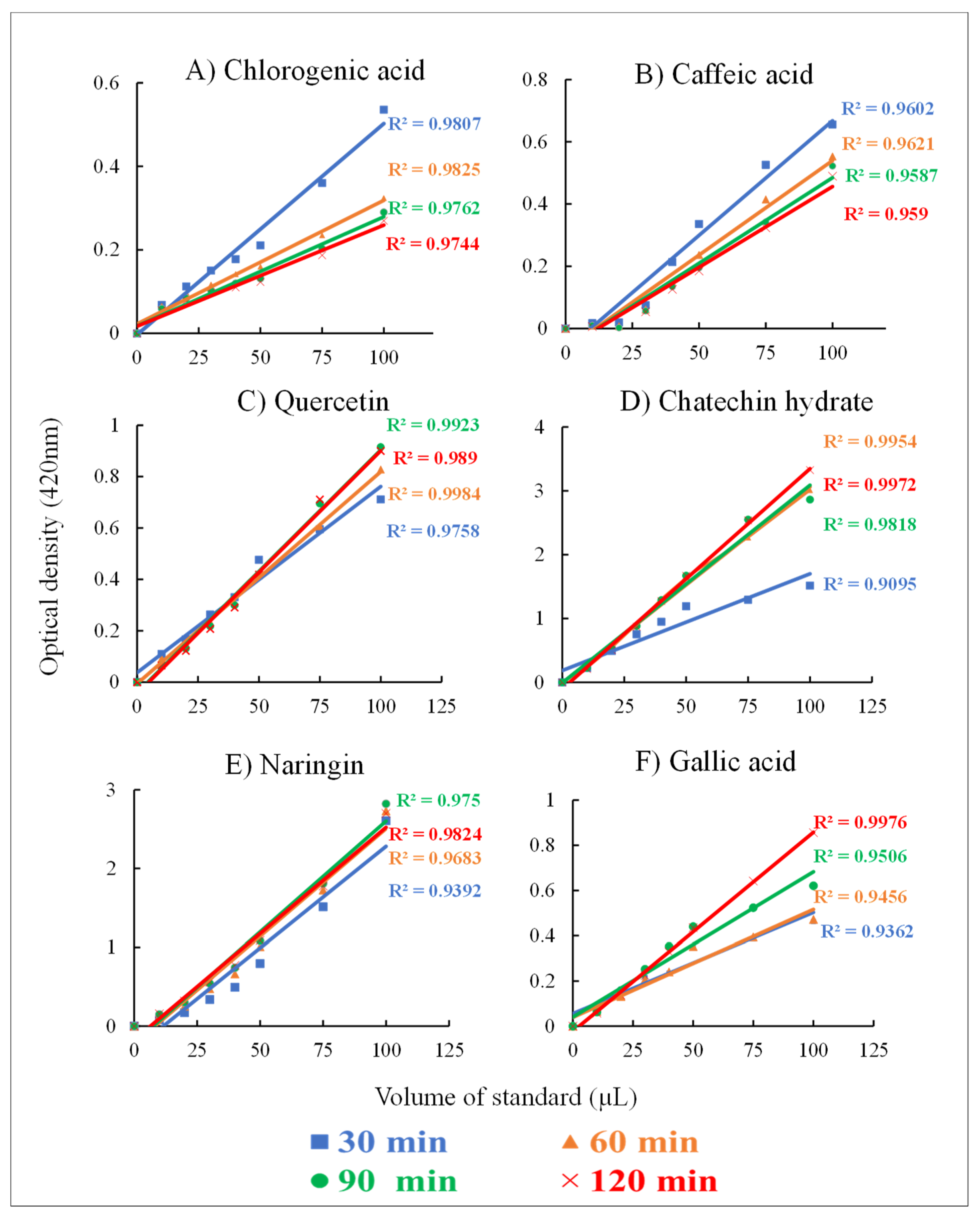
2.2.2. Evaluation of Standards Using the FB Assay
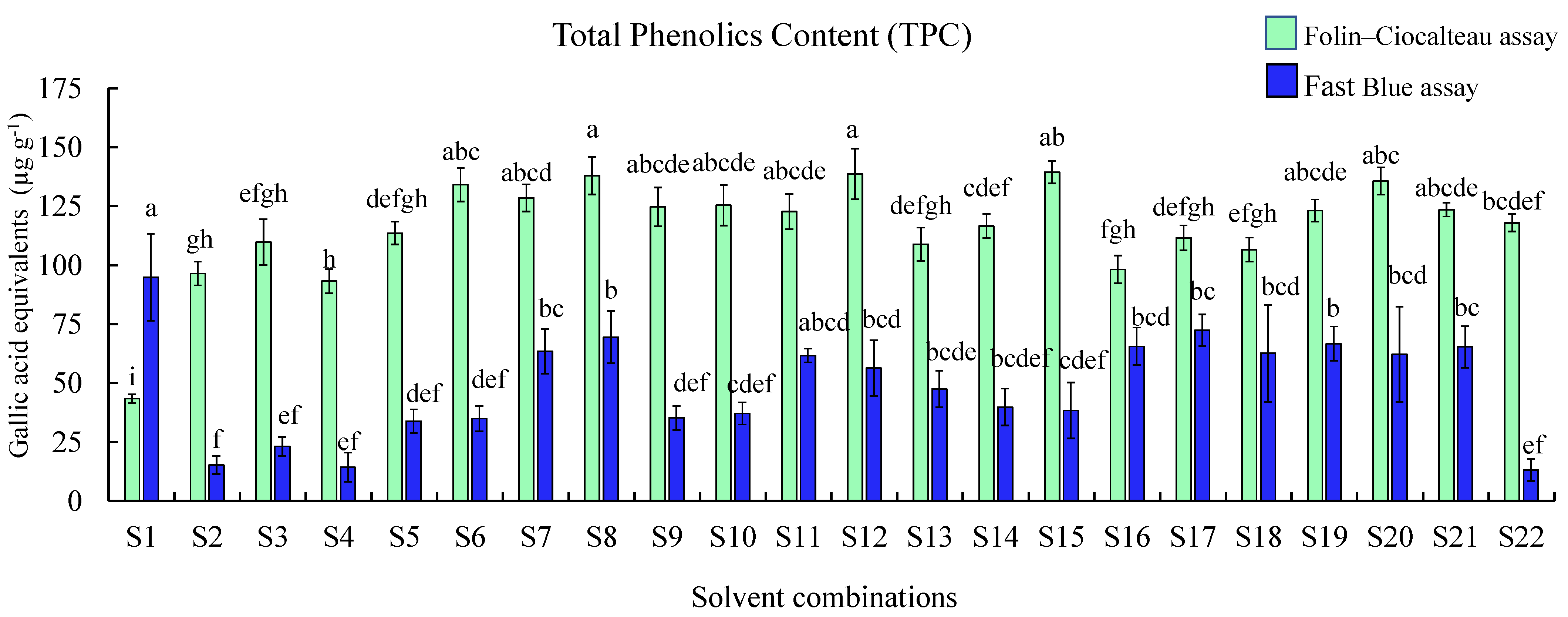
2.3. Comparison of Total Phenolic Contents Using Folin–Ciocalteu (F–C) and FB Assays
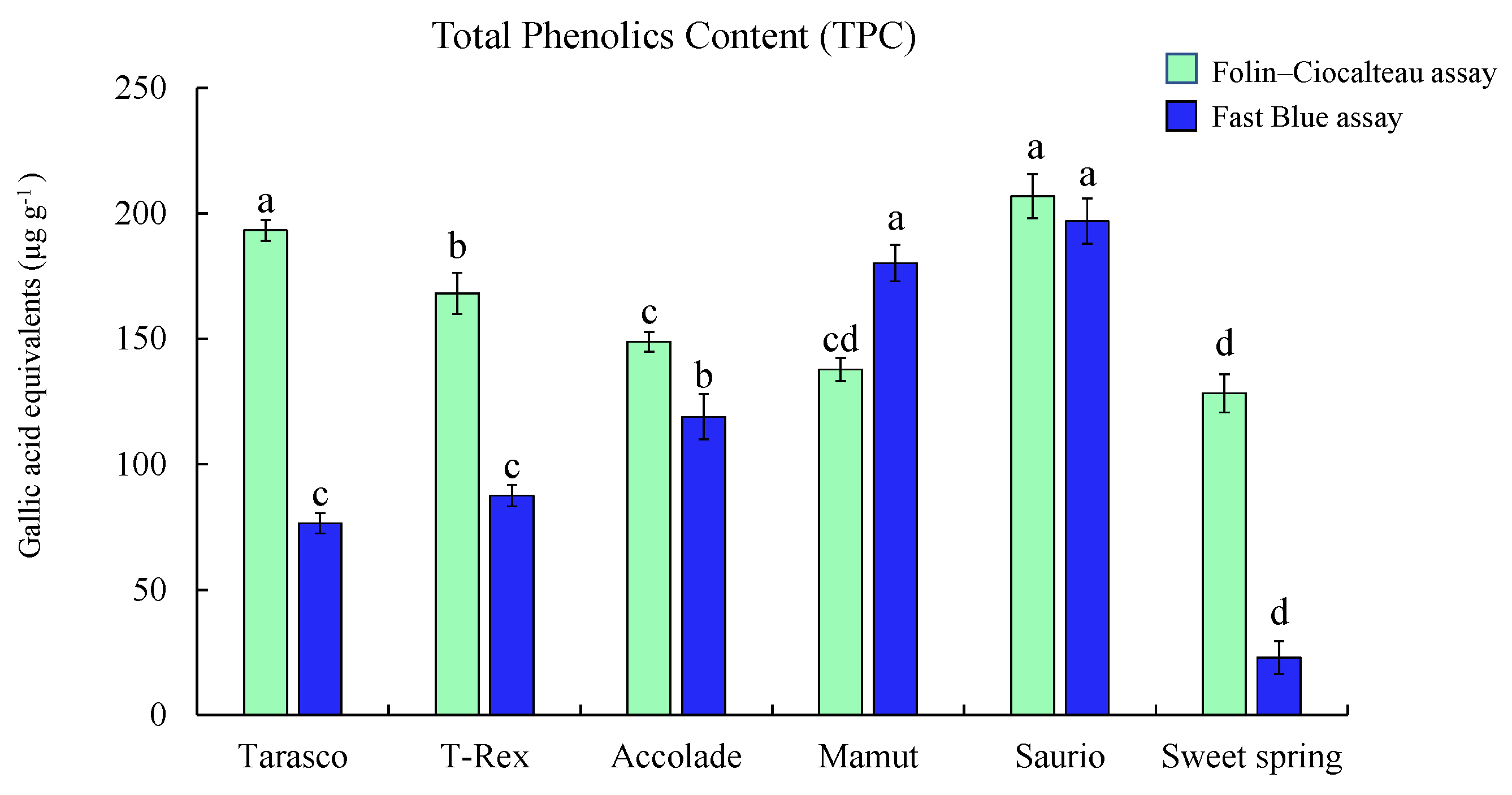
2.4. Application of the Optimized FB Assay on Melon Varieties
2.5. Application of F–C and Optimized FB Assays on Commercial Juice Samples
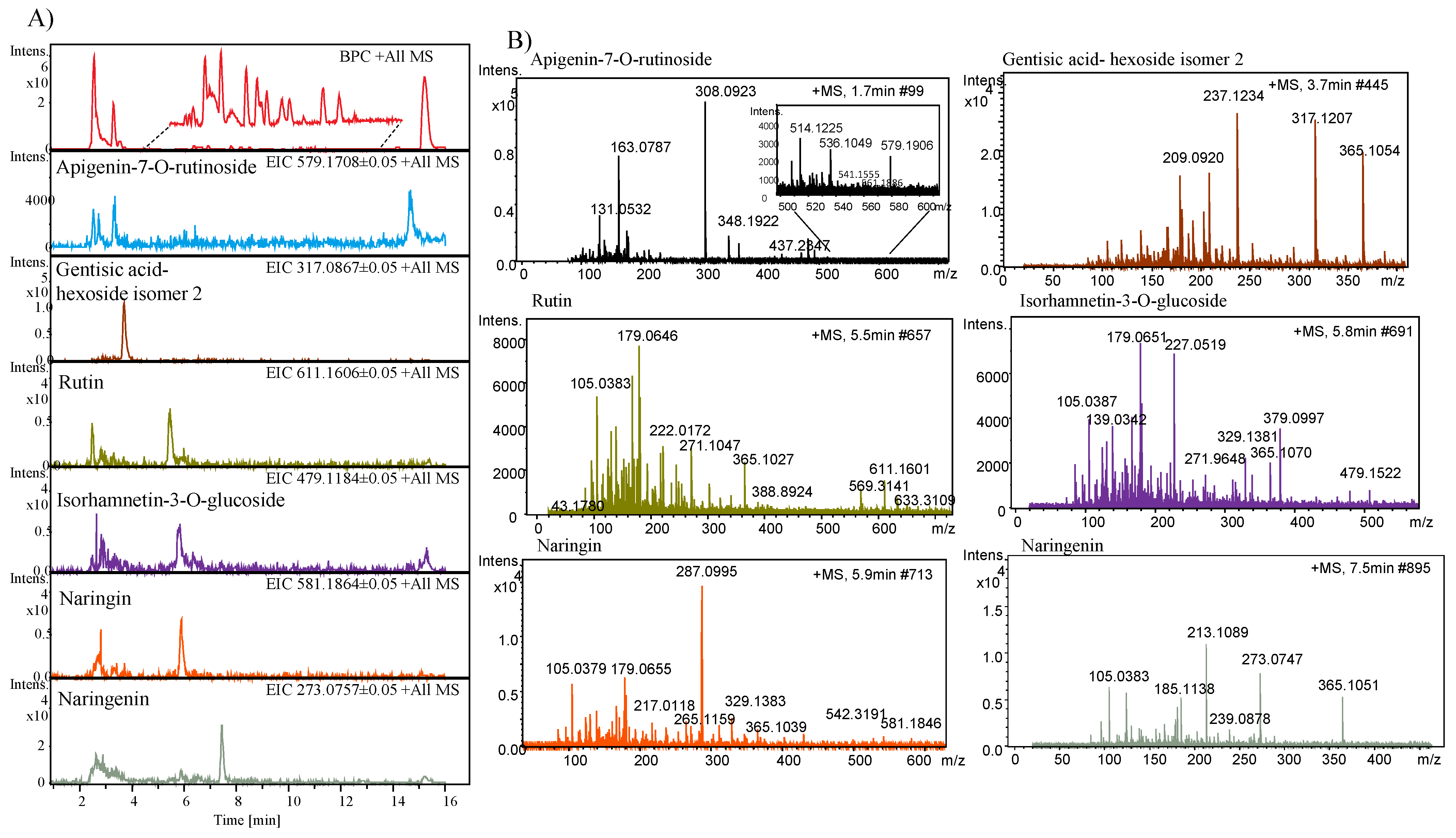
2.6. Identification of Phenolic Compounds by LC/ESI-HR-QTOF-MS
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Extraction Solvents
4.3. Antioxidant Assays
4.3.1. DPPH Assay
4.3.2. ABTS Radical Scavenging Activity
4.4. Folin–Ciocalteu Method to Measure Total Phenolic Contents
4.5. Optimization of the Microplate Assay Using Fast Blue B Salt
4.5.1. Optimization of Base Type and Concentration
4.5.2. Evaluation of Standards Using the Optimized Fast Blue Assay
4.6. Application of The Optimized FB Assay to Measure Total Phenolic Content in Melons
4.7. Application of The Optimized FB Assay to Assess Total Phenolic Content in Commercial Juices
4.8. Identification of Phenolic Compounds by Liquid Chromatography/Electrospray Ionization High-Resolution Quadrupole Time-of-Flight Tandem Mass Spectrometry (LC/ESI-HR-QTOF-MS)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villanueva, M.J.; Tenorio, M.D.; Esteban, M.A.; Mendoza, M. Compositional changes during ripening of two cultivars of muskmelon fruits. Food Chem. 2004, 87, 179–185. [Google Scholar] [CrossRef]
- Amaro, A.L.; Fundo, J.F.; Oliveira, A.; Beaulieu, J.C.; Fernández-Trujillo, J.P.; Almeida, D.P. 1-Methylcyclopropene effects on temporal changes of aroma volatiles and phytochemicals of fresh-cut cantaloupe. J. Sci. Food Agric. 2013, 93, 828–837. [Google Scholar] [CrossRef]
- Albert R. Mann Library. U.S. Cantaloupe Statistics. Available online: https://usda.library.cornell.edu/concern/publications/bv73c0402 (accessed on 27 June 2021).
- Maietti, A.; Tedeschi, P.; Stagno, C.; Bordiga, M.; Travaglia, F.; Locatelli, M.; Arlorio, M.; Brandolini, V. Analytical traceability of melon (Cucumis melo var reticulatus): Proximate composition, bioactive compounds, and antioxidant capacity in relation to cultivar, plant physiology state, and seasonal variability. J. Food Sci 2012, 77, C646–C652. [Google Scholar] [CrossRef]
- Mehra, M.; Pasricha, V.; Gupta, R.K. Estimation of nutritional, phytochemical and antioxidant activity of seeds of musk melon (Cucumis melo) and water melon (Citrullus lanatus) and nutritional analysis of their respective oils. Int. J. Pharmacogn. Phytochem. 2015, 3, 98–102. [Google Scholar]
- Fundo, J.F.; Miller, F.A.; Garcia, E.; Santos, J.R.; Silva, C.L.; Brandão, T.R. Physicochemical characteristics, bioactive compounds and antioxidant activity in juice, pulp, peel and seeds of Cantaloupe melon. J. Food Meas 2018, 12, 292–300. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Raslan, M.; Salama, M.M.; Menze, E.T.; El Hawary, S.S. In vivo anti-inflammatory activity and UPLC-MS/MS profiling of the peels and pulps of Cucumis melo var. cantalupensis and Cucumis melo var. reticulatus. J. Ethnopharmacol. 2019, 237, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Purwaningroom, D.L.; Ishii, T.; Setiowati, T.; Permatasari, G.W.; Rosjidi, C.H.; Munawaroh, S. Potential of the methanol extract from Cucumis melo var. cantalupensis as an antihypertensive agent possibly through the interference of glutathione S-transferase. Drug Invent. Today 2019, 12, 2910–2918. [Google Scholar]
- Ismail, H.I.; Chan, K.W.; Mariod, A.A.; Ismail, M. Phenolic content and antioxidant activity of cantaloupe (Cucumis melo) methanolic extracts. Food Chem. 2010, 119, 643–647. [Google Scholar] [CrossRef]
- Soni, A.; Sosa, S. Phytochemical analysis and free radical scavenging potential of herbal and medicinal plant extracts. J Pharmacogn Phytochem. 2013, 2, 22–29. [Google Scholar]
- Caretto, S.; Linsalata, V.; Colella, G.; Mita, G.; Lattanzio, V. Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. Int. J. Mol. Sci. 2015, 16, 26378–26394. [Google Scholar] [CrossRef] [Green Version]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 157–168. [Google Scholar]
- Giacco, R.; Costabile, G.; Fatati, G.; Frittitta, L.; Maiorino, M.I.; Marelli, G.; Parillo, M.; Pistis, D.; Tubili, C.; Vetrani, C. Effects of polyphenols on cardio-metabolic risk factors and risk of type 2 diabetes. A joint position statement of the Diabetes and Nutrition Study Group of the Italian Society of Diabetology (SID), the Italian Association of Dietetics and Clinical Nutrition (ADI) and the Italian Association of Medical Diabetologists (AMD). Nutr. Metab. Cardiovasc. Dis. 2020, 30, 355–367. [Google Scholar] [PubMed]
- Bhebhe, M.; Füller, T.N.; Chipurura, B.; Muchuweti, M. Effect of solvent type on total phenolic content and free radical scavenging activity of black tea and herbal infusions. Food Anal. Methods 2016, 9, 1060–1067. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Sepahpour, S.; Selamat, J.; Abdul Manap, M.Y.; Khatib, A.; Abdull Razis, A.F. Comparative analysis of chemical composition, antioxidant activity and quantitative characterization of some phenolic compounds in selected herbs and spices in different solvent extraction systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef] [Green Version]
- Meneses, N.G.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Motomura, Y.; Sugawara, J.; Aikawa, T.; Nara, K.; Nishizawa, T. Changes in free and bound phenolic contents and antioxidant activities of melon flesh dried at different stages of fruit growth. Acta Hortic. 2018, 1213, 505–510. [Google Scholar] [CrossRef]
- Rolim, P.; Fidelis, G.; Padilha, C.; Santos, E.; Rocha, H.; Macedo, G. Phenolic profile and antioxidant activity from peels and seeds of melon (Cucumis melo L. var. reticulatus) and their antiproliferative effect in cancer cells. Braz. J. Med. Biol. Res. 2018, 51, e6069. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.P.; Stathopoulos, C.; Parks, S.; Roach, P. An optimised aqueous extract of phenolic compounds from bitter melon with high antioxidant capacity. Antioxidants 2014, 3, 814–829. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Singh, V.; Shukla, S.; Rai, A. Phenolic content and antioxidant capacity of selected cucurbit fruits extracted with different solvents. J. Nutr. Food Sci. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Dorta, E.; Lobo, M.G.; Gonzalez, M. Reutilization of mango byproducts: Study of the effect of extraction solvent and temperature on their antioxidant properties. J. Food Sci. 2012, 77, C80–C88. [Google Scholar] [CrossRef]
- Salandanan, K.; Bunning, M.; Stonaker, F.; Külen, O.; Kendall, P.; Stushnoff, C. Comparative analysis of antioxidant properties and fruit quality attributes of organically and conventionally grown melons (Cucumis melo L.). HortScience 2009, 44, 1825–1832. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Tachakittirungrod, S.; Okonogi, S.; Chowwanapoonpohn, S. Study on antioxidant activity of certain plants in Thailand: Mechanism of antioxidant action of guava leaf extract. Food Chem. 2007, 103, 381–388. [Google Scholar] [CrossRef]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Hinojosa-Nogueira, D.; Muros, J.; Rufián-Henares, J.A.; Pastoriza, S. New method to estimate total polyphenol excretion: Comparison of Fast Blue BB versus Folin–Ciocalteu performance in urine. J. Agric. Food Chem. 2017, 65, 4216–4222. [Google Scholar] [CrossRef]
- Pico, J.; Pismag, R.Y.; Laudouze, M.; Martinez, M.M. Systematic evaluation of the Folin–Ciocalteu and Fast Blue BB reactions during the analysis of total phenolics in legumes, nuts and plant seeds. Food Funct. 2020, 11, 9868–9880. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Comparative characterization of phenolic and other polar compounds in Spanish melon cultivars by using high-performance liquid chromatography coupled to electrospray ionization quadrupole-time of flight mass spectrometry. Food Res. Int. 2013, 54, 1519–1527. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Metrani, R.; Jayaprakasha, G.K.; Crosby, K.M.; Ravishankar, S.; Patil, B.S. Multivariate Analysis of Amino Acids and Health Beneficial Properties of Cantaloupe Varieties Grown in Six Locations in the United States. Plants 2020, 9, 1058. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Boussoussa, H.; Hamia, C.; Djeridane, A.; Boudjeniba, M.; Yousfi, M. Effect of different solvent polarity on extraction of phenolic compounds from Algerian Rhanterium adpressum flowers and their antimicrobial and antioxidant activities. Curr. Chem. Biol. 2014, 8, 43–50. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Lester, G.E.; Lewers, K.S.; Medina, M.B.; Saftner, R.A. Comparative analysis of strawberry total phenolics via Fast Blue BB vs. Folin–Ciocalteu: Assay interference by ascorbic acid. J. Food Compos. Anal. 2012, 27, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Medina, M.B. Determination of the total phenolics in juices and superfruits by a novel chemical method. J. Funct. Foods 2011, 3, 79–87. [Google Scholar] [CrossRef]
- Medina, M.B. Simple and rapid method for the analysis of phenolic compounds in beverages and grains. J. Agric. Food Chem. 2011, 59, 1565–1571. [Google Scholar] [CrossRef]
- Palombini, S.V.; Claus, T.; Maruyama, S.A.; Carbonera, F.; Montanher, P.F.; Visentainer, J.V.; Gomes, S.; Matsushita, M. Optimization of a new methodology for determination of total phenolic content in rice employing fast blue BB and QUENCHER procedure. J. Braz. Chem. Soc. 2016, 27, 1188–1194. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Singh, M.; Jha, A.; Kumar, A.; Hettiarachchy, N.; Rai, A.K.; Sharma, D. Influence of the solvents on the extraction of major phenolic compounds (punicalagin, ellagic acid and gallic acid) and their antioxidant activities in pomegranate aril. J. Food Sci. Technol. 2014, 51, 2070–2077. [Google Scholar] [CrossRef] [Green Version]
- Zieliński, H.; Kozłowska, H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J. Agric. Food Chem. 2000, 48, 2008–2016. [Google Scholar] [CrossRef]
- Metrani, R.; Singh, J.; Acharya, P.; K Jayaprakasha, G.; S Patil, B. Comparative metabolomics profiling of polyphenols, nutrients and antioxidant activities of two red onion (Allium cepa L.) Cultivars. Plants 2020, 9, 1077. [Google Scholar] [CrossRef] [PubMed]
- Barkat, N.; Singh, J.; Jayaprakasha, G.K.; Patil, B.S. Effect of harvest time on the levels of phytochemicals, free radical-scavenging activity, α-amylase inhibition and bile acid-binding capacity of spinach (Spinacia oleracea). J. Sci. Food Agric. 2018, 98, 3468–3477. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jayaprakasha, G.; Patil, B.S. Chemometric characterization of 30 commercial thermal and cold processed juices using UPLC-QTOF-HR-MS fingerprints. LWT 2019, 114, 108322. [Google Scholar] [CrossRef]
- Singh, J.; Jayaprakasha, G.; Patil, B.S. Rapid ultra-high-performance liquid chromatography/quadrupole time-of-flight tandem mass spectrometry and selected reaction monitoring strategy for the identification and quantification of minor spinacetin derivatives in spinach. Rapid Commun. Mass Spectrom. 2017, 31, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Jayaprakasha, G.; Patil, B.S. An optimized solvent extraction and characterization of unidentified flavonoid glucuronide derivatives from spinach by UHPLC-HR-QTOF-MS. Talanta 2018, 188, 763–771. [Google Scholar] [CrossRef]
| Base | Concentration (N) | 0 min | 30 min | 60 min | 90 min | 120 min |
|---|---|---|---|---|---|---|
| NaOH | 0.5 | 0.832 | 0.956 | 0.970 | 0.981 | 0.990 |
| 1 | 0.858 | 0.960 | 0.985 | 0.991 | 0.995 | |
| 2 | 0.789 | 0.906 | 0.902 | 0.945 | 0.969 | |
| KOH | 0.5 | 0.822 | 0.975 | 0.991 | 0.989 | 0.991 |
| 1 | 0.796 | 0.920 | 0.943 | 0.965 | 0.992 | |
| 2 | 0.804 | 0.808 | 0.923 | 0.907 | 0.901 | |
| Na2CO3 | 0.5 | 0.898 | 0.955 | 0.989 | 0.991 | 0.990 |
| 1 | 0.858 | 0.967 | 0.989 | 0.991 | 0.995 | |
| 2 | 0.831 | 0.980 | 0.988 | 0.978 | 0.973 | |
| K2CO3 | 0.5 | 0.827 | 0.854 | 0.937 | 0.970 | 0.982 |
| 1 | 0.662 | 0.835 | 0.931 | 0.959 | 0.976 | |
| 2 | 0.735 | 0.876 | 0.943 | 0.967 | 0.977 |
| Juice ID | FB Assay (µg GAE g−1) | F–C Assay (µg GAE g−1) |
|---|---|---|
| J1 | 301.18 ± 8.33 f,g | 347.01 ± 9.24 h,i |
| J2 | 41.42 ± 1.88 h | 127.31 ± 12.39 k |
| J3 | 93.56 ± 7.49 g,h | 150.87 ± 13.88 k |
| J4 | 321.7 ± 12.81 f | 379.82 ± 18.7 g,h |
| J5 | 135.31 ± 9.3 f,g,h | 289.17 ± 8.02 i,j |
| J6 | 267.77 ± 28.62 f,g,h | 298.07 ± 19.13 i,j |
| J7 | 676.84 ± 30.29 e | 287.5 ± 17.23 i,j |
| J8 | 950.11 ± 10.41 c,d | 267.9 ± 12.4 1,j |
| J9 | 738.28 ± 22.13 d,e | 567.89 ± 15.34 f |
| J10 | 1010.97 ± 30.29 c | 836.12 ± 26.62 c |
| J11 | 343.88 ± 18.24 f | 409.07 ± 19.07 g |
| J12 | 594.3 ± 19.93 e | 435.57 ± 11.54 g |
| J13 | 716.92 ± 11.44 e | 798.65 ± 20.32 d |
| J14 | 730.77 ± 13.64 e | 659.77 ± 21.1 e |
| J15 | 4565.08 ± 114.91 b | 1649.39 ± 40.88 b |
| J16 | 5634.19 ± 26.83 a | 1756.86 ± 23.23 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravindranath, V.; Singh, J.; Jayaprakasha, G.K.; Patil, B.S. Optimization of Extraction Solvent and Fast Blue BB Assay for Comparative Analysis of Antioxidant Phenolics from Cucumis melo L. Plants 2021, 10, 1379. https://doi.org/10.3390/plants10071379
Ravindranath V, Singh J, Jayaprakasha GK, Patil BS. Optimization of Extraction Solvent and Fast Blue BB Assay for Comparative Analysis of Antioxidant Phenolics from Cucumis melo L. Plants. 2021; 10(7):1379. https://doi.org/10.3390/plants10071379
Chicago/Turabian StyleRavindranath, Varsha, Jashbir Singh, Guddarangavvanahally K. Jayaprakasha, and Bhimanagouda S. Patil. 2021. "Optimization of Extraction Solvent and Fast Blue BB Assay for Comparative Analysis of Antioxidant Phenolics from Cucumis melo L." Plants 10, no. 7: 1379. https://doi.org/10.3390/plants10071379






