Improving Soil Quality and Potato Productivity with Manure and High-Residue Cover Crops in Eastern Canada
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Characteristics and Treatments
2.2. Cover Crop Sampling and Analyses
2.3. Soil Sampling and Analyses
2.4. Soil Nitrate Dynamics over Winter Following Cover Crop Incorporation Measured Using Anion Exchange Membranes
2.5. Potato Sampling and Harvest
2.6. Verticillium DNA Extraction and qPCR Quantitation and Root-Lesion Nematode Quantification
2.7. Statistical Analyses
3. Results
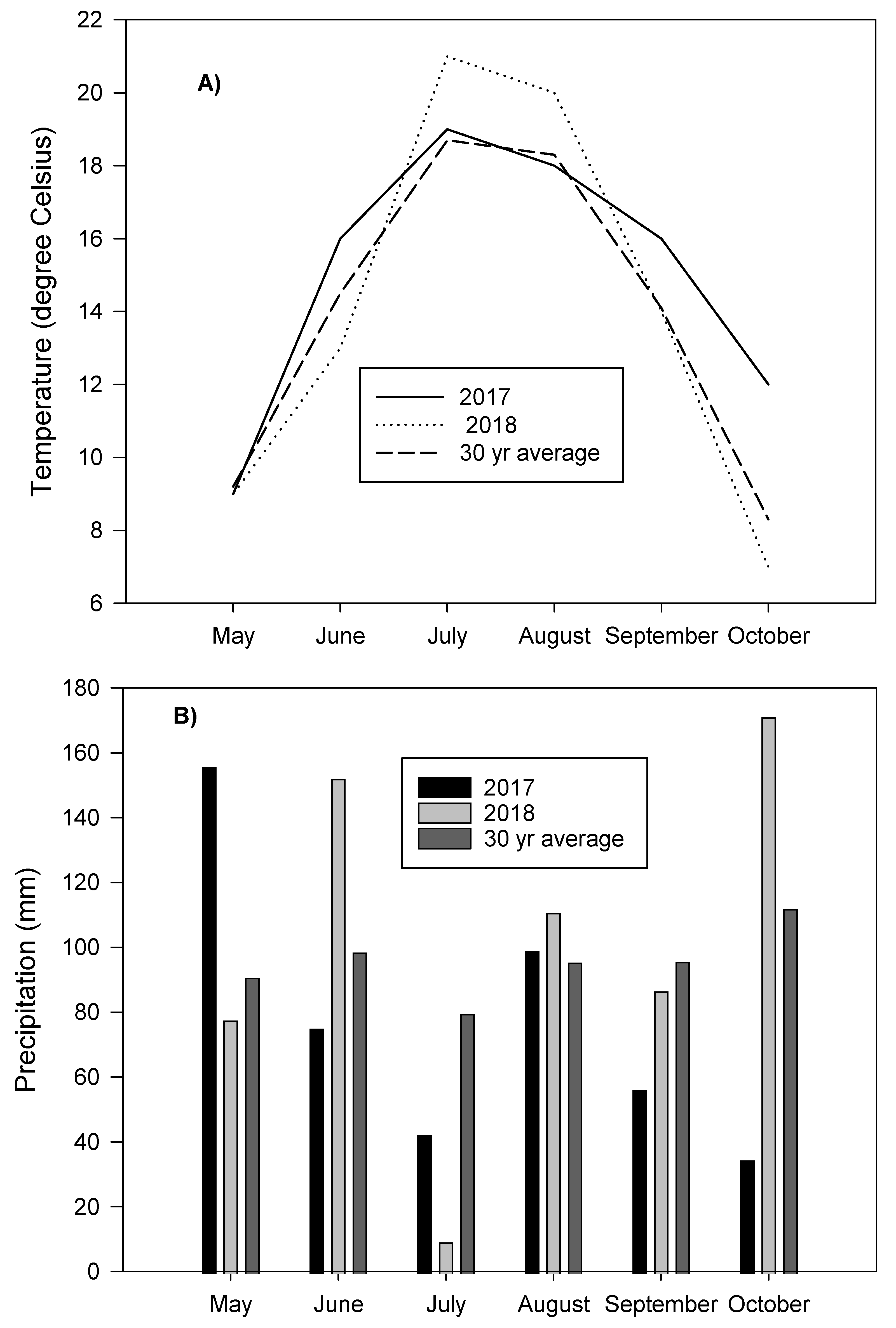
3.1. Air Temperature and Precipitation in 2017 and 2018
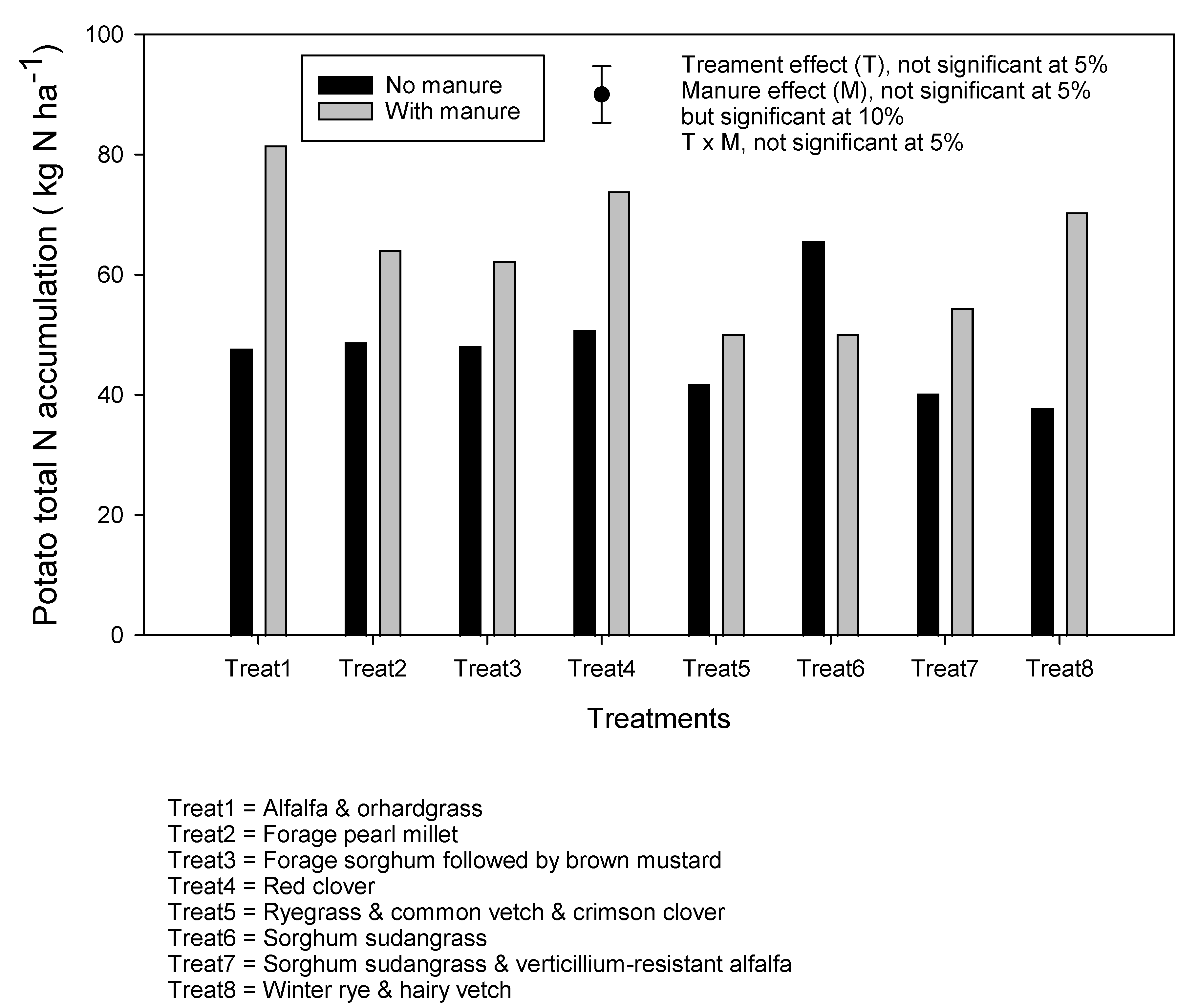
3.2. Effects of Cover Crop Treatment and Manure Application on Cover Crop Dry Matter Biomass, Carbon and Nitrogen Accumulations, and C:N Ratio
3.3. Effect of Manure and Cover Crop Treatments on Soil Nitrate in Fall Before Biomass Incorporation, and Over Winter as Measured With Anion Exchange Membranes during Potato Phase
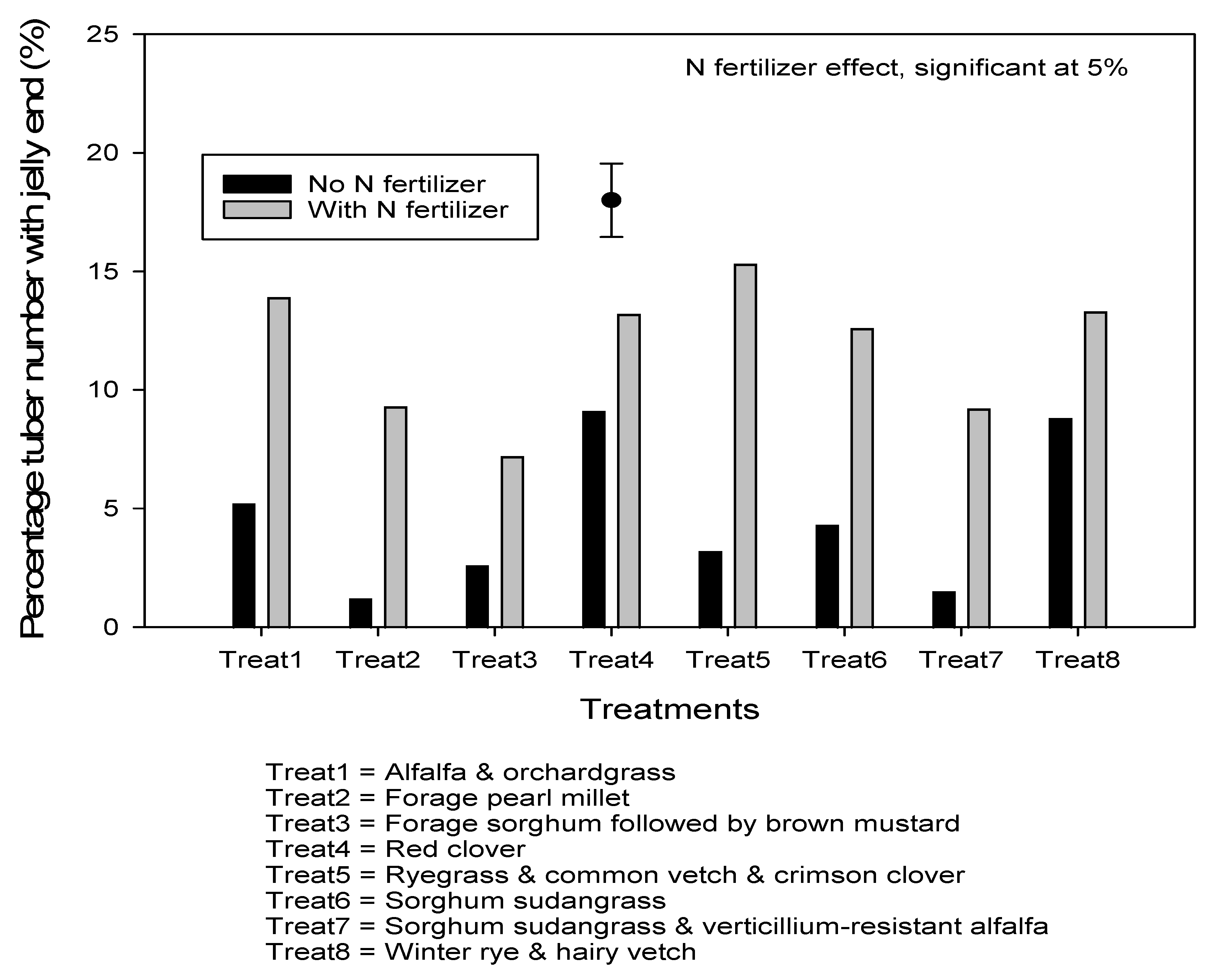
3.4. Effects of Nitrogen Fertilizer Application, Manure and Cover Crop Treatments on Total and Marketable Yield, Specific Gravity, and on Jelly end Incidence
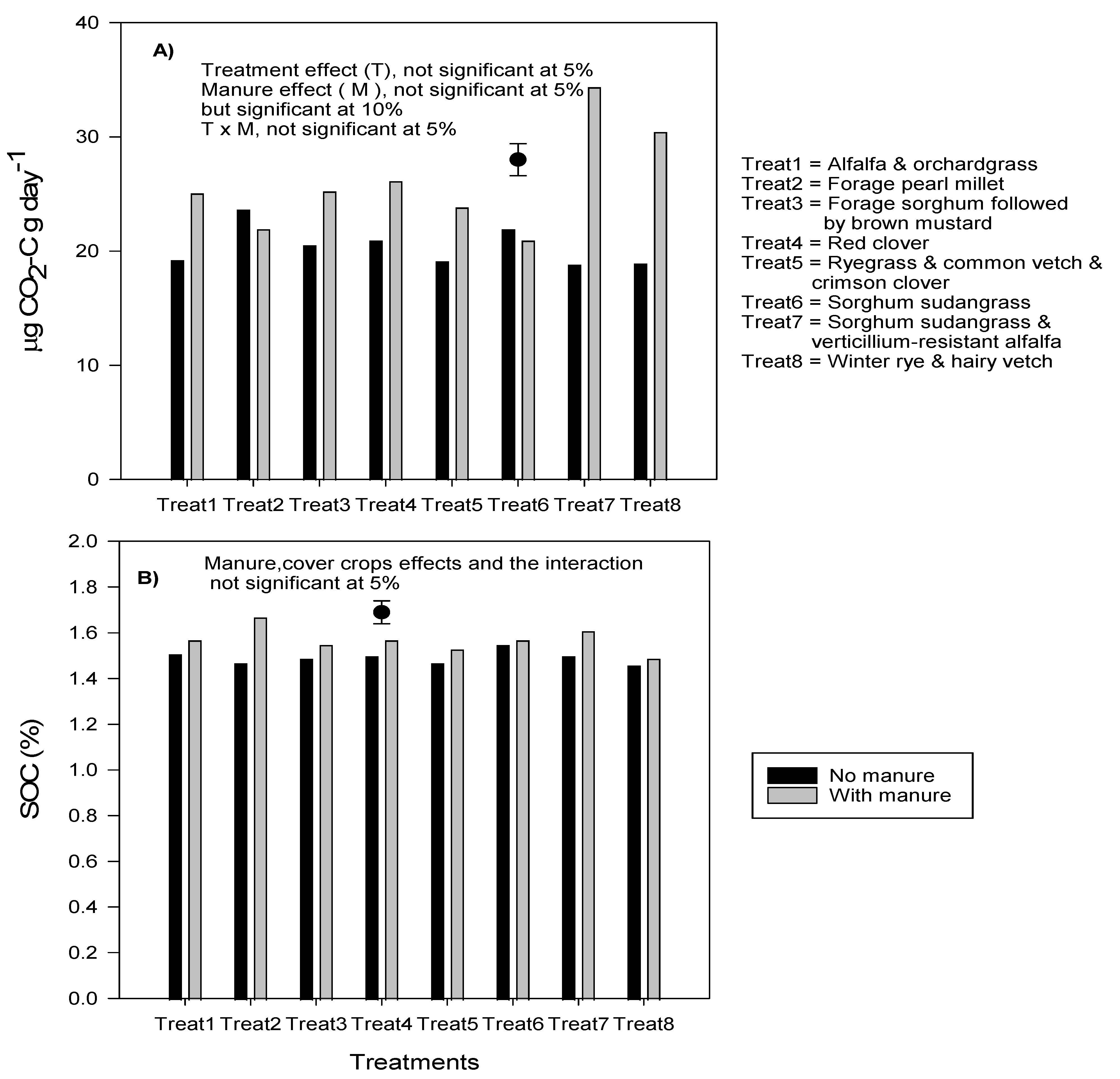
3.5. Effect of Manure and Cover Crop Treatments on Active Carbon, Soil Respiration, Total Earthworm Abundance, Surface Hardness, and Soil N Supply Capacity
3.6. Effects of Manure and Cover Crop Treatments on Population Density of Verticillium and Root-Lesion Nematodes
4. Discussion
4.1. Effect of Manure and Cover Crops on Total N Accumulation in Cover Crops and on Soil Nitrate Dynamics
4.2. Effects of Manure and Cover Crops on Potato Yield, Specific Gravity, and on Jelly End Incidence
4.3. Effects of Manure and Cover Crops on Soil Properties and on Soil N Supply Capacity
4.4. Effects of Manure and Cover Crops on Population Density of Verticillium dahliae and Root-Lesion Nematodes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goyer, A. Maximizing the nutritional potential of potato: The case of folate. Pot. Res. 2017, 60, 319–325. [Google Scholar] [CrossRef]
- Government of PEI. Potato Statistics. Potato Area, Production, Farm Price and Value. 2020. Available online: https://www.princeedwardisland.ca/sites/default/files/publications/af_stat_tab21.pdf (accessed on 16 April 2021).
- Government of PEI. Potato Sector Economic Impact Infographic. 2020. Available online: https://www.princeedwardisland.ca/fr/publication/potato-sector-economic-impact-infographic (accessed on 16 April 2021).
- Stuart, A.G.; Porter, G.A.; Erich, M.S. Organic amendment and rotation crop effects on the recovery of soil organic matter and aggregation in potato cropping systems. Soil Sci. Soc. Am. J. 2002, 66, 1311–1319. [Google Scholar]
- Nyiraneza, J.; Thompson, B.; Geng, X.; He, J.; Jiang, Y.; Fillmore, S.; Stiles, K. Changes in soil organic matter over 18 yr in Prince Edward island, Canada. Can. J. Soil Sci. 2017, 97, 745–756. [Google Scholar]
- Izadi, B.; Ashraf, M.S.; Studer, D.; Cann, I.M.; King, B. A simple model for the prediction of nitrate concentrations in the potato root zone. Agric. Water Manag. 1996, 30, 41–45. [Google Scholar] [CrossRef]
- Sanderson, J.B.; MacLeod, J.A. Soil nitrate profile and response of potatoes fertilizer in relation to time of incorporation of lupin (Lupinus albus). Can. J. Soil Sci. 1994, 74, 241–246. [Google Scholar] [CrossRef]
- Milburn, P.; Richards, J.E.; Gartley, C.; Pollock, T.; O’Neill, H.; Bailey, H. Nitrate leaching from systematically tiled potato fields in New Brunswick, Canada. J. Environ. Qual. 1990, 19, 448–454. [Google Scholar] [CrossRef]
- Honisch, M.; Hellmeier, C.; Weiss, K. Response of surface and subsurface water quality to land use changes. Geoderma 2002, 105, 277–298. [Google Scholar] [CrossRef]
- Savard, M.; Paradis, D.; Somers, G.; Liao, S.; van Bochove, E. Winter nitrification contributes to excess NO3 in groundwater of an agricultural region: A dual-isotope study. Water Res. Res. 2007, 43, 1–10. [Google Scholar] [CrossRef]
- Jiang, Y.; Zebarth, B.J.; Jonathan, L. Long-term simulations of nitrate leaching from potato production systems in Prince Edward Island, Canada. Nutr. Cycl. Agroecosyst. 2011, 91, 307–325. [Google Scholar] [CrossRef]
- Nyiraneza, J.; Snapp, S. Integrated management of inorganic and organic nitrogen and efficiency in potato systems. Soil Sci. Soc. Am. J. 2005, 71, 1508–1515. [Google Scholar] [CrossRef]
- Waddell, J.J.; Gupta, S.C.; Moncief, J.F.; Rosen, C.J.; Steel, D.D. Irrigation and nitrogen management impacts on nitrate leaching under potato. Agron. J. 2000, 91, 991–997. [Google Scholar] [CrossRef]
- Rees, H.W.; Chow, T.L.; Zebarth, B.J.; Xing, Z.; Toner, P.; Lavoie, J.; Daigle, J.-L. Impact of supplemental poultry manure application on potato yield and soil properties on a loam soil in north-western New Brunswick. Can. J. Soil Sci. 2014, 94, 49–65. [Google Scholar] [CrossRef] [Green Version]
- Canali, S.; Ciaccia, C.; Antichi, D.; Bàrberi, P.; Montemurro, F.; Tittarelli, F. Interactions between green manure and amendment type and rate: Effects on organic potato and soil mineral N dynamic. J. Food Agric. Environ. 2010, 8, 537–543. [Google Scholar]
- Nyfeler, D.; Huguenin-Elie, O.; Suter, M.; Frossard, E.; Lüscher, A. Grass–legume mixtures can yield more nitrogen than legume pure stands due to mutual stimulation of nitrogen uptake from symbiotic and non-symbiotic sources. Agric. Ecosyt. Environ. 2011, 140, 155–163. [Google Scholar] [CrossRef]
- Kumar, K.; Goh, K.M. Management practices of antecedent leguminous and non-leguminous crop residues in relation to winter wheat yields, nitrogen uptake, soil nitrogen mineralization and simple nitrogen balance. Europ. J. Agron. 2002, 16, 295–308. [Google Scholar] [CrossRef]
- Jiang, Y.; Nyiraneza, J.; Khakbazan, M.; Geng, X.; Murray, B.J. Nitrate leaching and potato yield under varying plow timing and nitrogen rate. Agrosystm. Geosci. Environ. 2019, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Nyiraneza, J.; Peters, R.D.; Rodd, V.A.; Grimmett, M.G.; Jiang, Y. Improving productivity of managed potato cropping systems in Eastern Canada: Crop rotations and nitrogen source effects. Agron. J. 2015, 107, 1447–1457. [Google Scholar] [CrossRef]
- Ledgard, S.F.; Steele, K.W. Biological nitrogen fixation in mixed legume/grass pastures. Plant Soil 1992, 141, 137–153. [Google Scholar] [CrossRef]
- Ranells, N.N.; Wagger, M.G. Grass-legume bicultures as winter annual cover crops. Agron. J. 1997, 89, 659–665. [Google Scholar] [CrossRef]
- Holderbaum, J.F.; Decker, A.M.; Meisinger, J.J.; Mulford, F.R.; Vough, L.R. Fall seeded legume cover crop for no-tillage corn in the humid East. Agron. J. 1990, 92, 117–124. [Google Scholar] [CrossRef]
- Sullivan, P.G.; Parrish, D.J.; Luna, J.M. Cover crop contributions to N supply and water conservation in corn production. Am. J. Altern. Agric. 1991, 6, 1060–1113. [Google Scholar] [CrossRef]
- Kirwan, L.; Luescher, A.; Sebastià, M.T.; Finn, J.A.; Collins, R.P.; Porqueddu, C.; Helgadóhir, À.; Baadshaug, O.H.; Brophy, C.; Coran Dalmannsdottir, S.; et al. Evenness drives consistent diversity effects in intensive grassland systems across 28 European sites. J. Ecol. 2007, 95, 530–539. [Google Scholar] [CrossRef]
- Nyfeler, D.O.; Huguenin-Elie, O.; Suter, M.; Frossard, E.; Connolly, J.; Lüscher, A. Strong mixture effects among four species in fertilized agricultural grasslands led to persistent and consistent transgressive overyielding. J. Appl. Ecol. 2009, 46, 683–691. [Google Scholar] [CrossRef]
- Rowe, R.C.; Davis, J.R.; Powelson, M.L.; Rouse, D.I. Potato early dying: Causal agents and management strategies. Plant Dis. 1987, 71, 482–489. [Google Scholar] [CrossRef]
- Kimpinski, J.; Sturz, A.V. Potato Tuber Yields and Root-Lesion Nematodes; Factsheet 161/90; Minisrty of Agriculture Fisheries Aquaculture and Forestry: Charlottetown, PE, Canada, 2000.
- Kimpinski, J. Root-lesion nematodes in potatoes. Amer. Potat. J. 1979, 56, 79–86. [Google Scholar] [CrossRef]
- Carter, M.R.; Kunelious, H.T.; Sanderson, J.B.; Kimpinski, J.; Platt, H.W.; Bolinder, M.A. Productivity parameters and soil health dynamics under long-term 2-year potato rotations in Atlantic Canada. Soil Till Res. 2003, 72, 153–168. [Google Scholar] [CrossRef]
- Ball-Coelho, B.; Bruin, A.J.; Roy, R.C.; Riga, E. Forage pearl millet and marigold as rotation crops for biological control of root-lesion nematode in potato. Agron. J. 2003, 95, 282–292. [Google Scholar] [CrossRef]
- Bélair, G.; Dauphinais, N.; Fournier, Y.; Dangi, O.P.; Ciotola, M. Effect of 3-year rotation sequences and pearl millet on population densities of Pratylenchus penetrans and subsequent potato yield. Can. J. Plant Pathol. 2006, 28, 230–235. [Google Scholar] [CrossRef]
- Bélair, G.; Dauphinais, N.; Fournier, Y.; Dangi, O.P.; Clément, M.F. Effect of forage and grain pearl millet on Pratylenchus penetrans and potato yield in Quebec. J. Nematol. 2005, 37, 78–82. [Google Scholar] [PubMed]
- Siritharan, R.; Potter, J.W.; Kumar, K.A.; Dangi, O.P. Crop rotation with forage pearl millet for control of root-lesion nematodes in on-farm trials with potato. J. New Seeds. 2006, 8, 50–61. [Google Scholar] [CrossRef]
- Davis, J.R.; Huisman, O.C.; Westerman, D.T.; Hafez, S.L.; Everson, D.O.; Sorensen, L.H.; Schneider, A.T. Effects of green manures on verticillium wilt of potato. Phytopathology 1996, 86, 444–453. [Google Scholar] [CrossRef]
- Davis, J.R.; Huisman, O.C.; Westerman, D.T.; Hafez, S.L.; Everson, D.O.; Schneider, A.T.; Sorensen, L.H. Some unique benefits with sudangrass for improved U.S. #1 yields and size of Russet Burbank potato. Am. J. Potato Res. 2004, 81, 403–413. [Google Scholar]
- Ochiai, N.; Crowe, F.J.; Dick, R.P.; Powelson, M.L. Effects of green manure type and amendment rate on Verticillium wilt severity and yield of russet Burbank potato. Plant Dis. 2007, 91, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, W.H.; Lalande, H.; Duquette, M. Soil reaction and exchangeable acidity. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Ed.; Taylor & Francis: Abingdon, UK, 2008; pp. 173–178. [Google Scholar]
- Mehlich, A. Mehlich-3 soil test extractant: A modification of Mehlich-2 extractant. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Campbell, C.R.; Plank, C.O. Preparation of plant tissue for laboratory analysis. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press: Raton, FL, USA, 1998; pp. 37–49. [Google Scholar]
- Weil, R.R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Alt. Agric. 2003, 18, 3–17. [Google Scholar]
- Haney, R.L.; Haney, E.B. Simple and rapid laboratory method for rewetting dry soil for incubations. Commun. Soil Sci. Plant Anal. 2010, 41, 1493–1501. [Google Scholar] [CrossRef]
- Ziadi, N.; Simard, R.R.; Allard, G.; Lafond, J. Field evaluation of anion exchange membranes as an N soil testing method for grasslands. Can. J. Soil Sci. 1999, 79, 281–294. [Google Scholar] [CrossRef]
- Conte, J.; Potoczniak, M.J.; Tobe, S.S. Using synthetic oligonucleotides as standards in probe-based qPCR. Biotechniques 2018, 64, 177–179. [Google Scholar] [CrossRef] [Green Version]
- Borza, T.; Beaton, B.; Govindarajan, A.; Gao, X.; Liu, Y.; Ganga, Z.; Wang-Pruski, G. Incidence and abundance of Verticillium dahliae in soil from various agricultural fields in Prince Edward Island, Canada. Europ. J. Plant Path. 2018, 151, 825–830. [Google Scholar] [CrossRef]
- Townsend, J.L. A modification and evaluation of the apparatus for the Oostenbrink direct cottonwool filter extraction method. Nematologica 1963, 9, 106–110. [Google Scholar] [CrossRef] [Green Version]
- SAS Institute Inc. SAS/STAT® 13.2. User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2014. [Google Scholar]
- Francis, G.S. Management practices for minimizing nitrate leaching after ploughing temporary leguminous pastures in Canterbury, New Zealand. J. Contam. Hydrol. 1995, 20, 313–327. [Google Scholar] [CrossRef]
- Fraser, P.M.; Curtin, D.; Harrison-Kirk, T.; Meenken, E.D.; Beare, M.H.; Tabley, F.; Gillespie, R.N.; Francis, G.S. Winter nitrate leaching under different tillage and winter cover crop management practices. Soil Sci. Soc. Am. J. 2013, 77, 1391–1401. [Google Scholar] [CrossRef]
- Liang, K.; Jiang, Y.; Nyiraneza, J.; Fuller, K.; Murnaghan, D.; Meng, F. Nitrogen dynamics and leaching potential under conventional and alternative potato rotations in Atlantic Canada. Field Crop. Res. 2019, 242, 10703. [Google Scholar] [CrossRef]
- Nyiraneza, J.; Bizimungu, B.; Messiga, A.J.; Fuller, K.D.; Fillmore, S.A.E.; Jiang, Y. Potato yield and phosphorus use efficiency of two new potato cultivars in New Brunswick, Canada. Can. J. Plant Sci. 2017, 97, 784–795. [Google Scholar] [CrossRef] [Green Version]
- Nyiraneza, J.; Fuller, K.D.; Messiga, A.J.; Bizimungu, B.; Fillmore, S.; Jiang, Y. Potato response to phosphorus fertilization at two sites in Nova Scotia, Canada. Am. J. Pot. Res. 2017, 94, 357–366. [Google Scholar] [CrossRef]
- Cambouris, A.N.; St. Luce, M.; Zebarth, B.J.; Ziadi, N.; Grant, C.A.; Perron, I. Potato response to nitrogen sources and rates in an irrigated sandy soil. Agron. J. 2016, 108, 391–401. [Google Scholar] [CrossRef]
- Zebarth, B.J.; Tarn, T.R.; de Jong, H.; Murphy, A. Nitrogen use efficiency characteristics of Andigena and diploid potato selections. Am. J. Pot. Res. 2008, 85, 210–218. [Google Scholar] [CrossRef]
- Snowdon, E.; Zebarth, B.J.; Burton, D.L.; Goyer, C.; Rochette, P. Growing season N2O emissions from two-year potato rotations in a humid environment in New Brunswick, Canada. Can. J. Soil Sci. 2013, 93, 279–294. [Google Scholar] [CrossRef] [Green Version]
- Porter, G.A.; Sisson, J.A. Response of Russet Burbank and Shepody potatoes to nitrogen fertilizer in two cropping systems. Am. Potato J. 1991, 68, 425–443. [Google Scholar] [CrossRef]
- Bélanger, G.; Walsh, J.R.; Richards, J.E.; Milbum, P.H.; Ziadi, N. Nitrogen fertilization and irrigation affects tuber characteristics of two potato cultivars. Am. J. Pot. Res. 2002, 79, 269–279. [Google Scholar] [CrossRef]
- Zebarth, B.J.; Leclerc, Y.; Moreau, G.; Botha, E. Rate and timing of nitrogen fertilization of Russet Burbank potato: Yield and processing quality. Can. J. Plant Sci. 2004, 84, 855–863. [Google Scholar] [CrossRef]
- Thompson, A.L.; Stephen, L.L.; Sowokinos, J.R.; Thornton, M.K.; Shock, C.C. Review of the sugar end disorder in potato (Solanum tuberosum, L.). Am. J. Pot. Res. 2008, 85, 375–386. [Google Scholar] [CrossRef]
- Kleinkopf, G.E. Translucent End of Potatoes; Curr. Info Series No 488; University of Idaho: Moscow, ID, USA, 1979. [Google Scholar]
- Kleinkopf, G.E.; Westermann, D.T.; Wille, M.J. Soil temperature effects on sugar end development. Proc. Univ. Ida. Winter Comm. Sch. 1988, 20, 180–181. [Google Scholar]
- Shekhar, V.C.; Iritani, W.M. Influence of moisture stress during growth on 14CO2 fixation and translocation in Solanum tuberosum L. Am. Pot. J. 1979, 56, 307–311. [Google Scholar] [CrossRef]
- Krauss, A.; Marschner, H. Growth rate and carbohydrate metabolism of potato tubers exposed to high temperatures. Pot. Res. 1984, 27, 297–303. [Google Scholar] [CrossRef]
- Shock, C.C.; Zalewski, J.C.; Stieber, T.D.; Burnett, D.S. Impact of early season water deficits on Russet Burbank plant development, tuber yield, and quality. Am. Pot. J. 1992, 69, 793–803. [Google Scholar] [CrossRef]
- Shock, C.C.; Holmes, Z.A.; Stieber, T.D.; Eldredge, E.P.; Zhang, P. The effect of timed water stress on quality, total solids, and reducing sugar content of potatoes. Am. Pot. J. 1993, 70, 227–241. [Google Scholar] [CrossRef]
- Stark, J.C.; McCann, I.R. Optimal allocation of limited water uptake and translocation. Am. Pot. J. 1992, 69, 413–421. [Google Scholar] [CrossRef]
- Eldredge, E.P.; Holmes, Z.A.; Mosley, A.R.; Shock, C.C.; Stieber, T.D. Effects of transitory water stress on potato tuber stem-end reducing sugar and fry color. Am. Pot. J. 1996, 73, 517–530. [Google Scholar] [CrossRef]
- Shock, C.C.; Feibert, E.B.G.; Saunders, L.D. Potato yield and quality response to deficit irrigation. HortScience 1998, 33, 655–659. [Google Scholar] [CrossRef] [Green Version]
- Zebarth, B.J.; Leclerc, Y.; Moreau, G.; Sanderson, J.B.; Arsenault, W.J.; Botha, E.J.; Wang-Pruski, G. Estimation of soil nitrogen supply using a plant bioassay approach. Can. J. Soil Sci. 2005, 85, 377–386. [Google Scholar] [CrossRef]
- Molina, D.I.; Tenuta, M.; El Hadrani, A.; Buckley, K.; Cavers, C.; Daayf, I. Potato early dying and yield response response to compost, green manures, see meal and chemical treatments. Am. J. Potat. Res. 2014, 91, 414–428. [Google Scholar] [CrossRef]
- Franzluebers, A.J.; Haney, R.L.; Honeycutt, C.W.; Schomberg, H.H.; Hons, F.M. Flush of carbon dioxide following rewetting of dried soil relates to active organic pools. Soil Sci. Soc. Am. J. 2000, 64, 613–623. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Arshad, M.A. Soil organic matter pools during early adoption of conservation tillage in northwestern Canada. Soil Sci. Soc. Am. J. 1996, 60, 1422–1427. [Google Scholar] [CrossRef]
- Marumoto, T.; Anderson, J.P.E.; Domsch, K.H. Mineralization of nutrients from soil microbial biomass. Soil Biol. Biochem. 1982, 14, 469–475. [Google Scholar] [CrossRef]
- Berlanger, I.; Powelson, M.L. Verticillium wilt. Plant Health Instr. 2000. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Johnson, D.A. Verticillium dahliae infects, alters plant biomass, and produces inoculum on rotation crops. Phytopathology 2016, 106, 602–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omer, M.; Johnson, D.A.; Rowe, R.C. Recovery of Verticillium dahliae from North American certified seed potatoes and characterization of strains by vegetative compatibility and aggressiveness. Am. J. Pot. Res. 2000, 77, 325–331. [Google Scholar] [CrossRef]
- Ophel-Keller, K.; McKay, A.; Hartley, D.; Curran, J. Development of a routine DNA-based testing service for soilborne diseases in Australia. Australas. Plant Pathol. 2008, 37, 243–253. [Google Scholar] [CrossRef]
- Smiley, R.W.; Machado, S.; Rhinhart, K.E.L.; Wuest, S.B. Rapid quantification of soilborne pathogen communities in wheat-based long-term field experiments. Plant Dis. 2016, 100, 1692–1708. [Google Scholar] [CrossRef]
- Wallon, T.; Sauvageau, A.; Van der Heyden, H. Detection and Quantification of Rhizoctonia solani and Rhizoctonia solani AG1-IB Causing the Bottom Rot of Lettuce in Tissues and Soils by Multiplex qPCR. Plants 2021, 10, 57. [Google Scholar] [CrossRef]
- MacGuidwin, A.E.; Knuteson, D.L.; Connell, T.; Bland, W.L.; Bartelt, K.D. Manipulating inoculum densities of Verticillium dahliae and Pratylenchus penetrans with green manure amendments and solarization influence potato yield. Phytopathology 2012, 102, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Goud, J.C.; Termorshuizen, A.J. Quality of methods to quantify microsclerotia of Verticillium dahliae in soil. Eur. J. Plant Pathol. 2003, 109, 2003. [Google Scholar] [CrossRef]
- Kimpinski, J.; Thompson, L.S. Plant parasitic nematodes and their management in the Maritime provinces of Canada. Phytoprotection 1990, 71, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Everts, K.L.; Sardanelli, S.; Kratochvil, R.J.; Armentrout, D.K.; Gallagher, L.E. Root-knot and root-lesion nematode suppression by cover crops, poultry litter, and poultry litter compost. Plant Dis. 2006, 90, 487–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachel, E.R.; Zasada, I.A.; Hesse, C.; DeVetter, L.W. Brassicaceous seed meal, root removal, and chemical fumigation vary in their effects on soil quality parameters and Pratylenchus penetrans in a replanted floricane raspberry production system. Appl. Soil Ecol. 2019, 133, 44–51. [Google Scholar]


| Sources of Variation | Total Dry Matter | Total Carbon Accumulation | Total Nitrogen Accumulation | C:N Ratio |
|---|---|---|---|---|
| Mg·ha−1 | kg·ha−1 | |||
| Manure application | ||||
| With manure | 4.3 a | 1845.8 a | 93.6 a | 19.1 a |
| Without manure | 4.3 a | 1866.8 a | 98.4 a | 19.2 a |
| Standard error of the mean | 0.22 | 93.7 | 4.8 | 0.42 |
| Cover crop treatments | ||||
| Alfalfa and orchardgrass | 3.7 c | 1577.7 c | 74.2 c | 20.6 ab |
| Forage pearl millet | 6.2 a | 2664.9 a | 112.9 b | 22.3 a |
| Forage sorghum followed by brown mustard | 3.9 c | 1673.0 c | 103.4 b | 17 c |
| Red clover | 4.9 b | 2076.1 b | 145.6 a | 14.4 d |
| Ryegrass and common vetch and crimson clover | 4.7 bc | 2023.9 b | 97.7 b | 19.6 b |
| Sorghum sudangrass | 4.7 bc | 2059.2 b | 90.9 bc | 21.5 ab |
| Sorghum sudangrass and VR alfalfa | 3.6 c | 1588.7 c | 67.7 c | 21.6 ab |
| Winter rye and hairy vetch | 2.7 d | 1186.7 d | 75.8 c | 16.1 cd |
| Standard error of the mean | 0.34 | 147.6 | 8.4 | 0.75 |
| Analysis of variance | ||||
| Manure effect (M) | NS | NS | NS | NS |
| Treatment effect (T) | *** | *** | *** | *** |
| M × T | NS | NS | NS | NS |
| Sources of Variations | May | June | July | August | September | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sampling Depth (cm) | |||||||||||
| 0–15 | 15–30 | 0–15 | 15–30 | 0–15 | 15–30 | 0–15 | 15–30 | 0–15 | 15–30 | ||
| mg NO3-N·kg−1 | |||||||||||
| Manure application | |||||||||||
| With manure | 5.1 a | 6.6 | 10.8 a | 14.5 a | 12.2 a | 16.6 a | 5.3 a | 8.3 a | 5.8 a | 6.6 a | |
| Without manure | 3.5 b | 5.05 | 8.9 a | 11.6 a | 8.6 a | 12.5 a | 4.5 a | 6.3 a | 6.0 a | 6.7 a | |
| Standard error of the mean | 0.43 | 0.53 | 0.67 | 0.83 | 0.82 | 0.98 | 0.58 | 0.97 | 0.56 | 0.72 | |
| Cover crop treatments | |||||||||||
| No manure | With manure | ||||||||||
| Alfalfa and orchardgrass | 5.1 a | 4.9 bc | 6.1 bc | 10.7 a | 13.7 a | 11.5 a | 15.4 a | 6.2 a | 8.6 a | 6.6 a | 5.9 a |
| Forage pearl millet | 3.9 c | 4.9 c | 5.1 d | 9.4 a | 12.9 a | 8.9 a | 13.2 a | 4.1 a | 7.5 a | 6.0 a | 6.6 a |
| Forage sorghum followed by brown mustard | 4.1 bc | 6.1 b | 7.4 ab | 9.9 a | 14.1 a | 10.8 a | 14.1 a | 3.6 a | 5.3 a | 4.2 a | 5.1 a |
| Red clover | 5.1 ab | 7.3 a | 6.6 ab | 10.6 a | 13.0 a | 9.4 a | 13.0 a | 5.4 a | 7.3 a | 7.2 a | 9.4 a |
| Ryegrass and common vetch andcrimson clover | 5.5 a | 2.8 c | 8.4 a | 8.8 a | 10.3 a | 10.9 a | 15.2 a | 5.0 a | 8.9 a | 5.3 a | 6.3 a |
| Sorghum sudangrass | 3.2 c | 4.4 bc | 5.7 cd | 8.6 a | 12.6 a | 10.0 a | 15.1 a | 5.1 a | 8.0 a | 6.5 a | 7.1 a |
| Sorghum sudangrass and VR alfalfa | 4.4 bc | 3.9 bc | 5.4 cd | 10.2 a | 13.6 a | 9.4 a | 14.0 a | 4.5 a | 5.9 a | 5.9 a | 6.3 a |
| Winter rye and hairy vetch | 4.7 bc | 5.4 bc | 6.1 bc | 10.6 | 13.7 a | 12.3 a | 16.7 a | 4.9a | 6.8 a | 5.6 a | 6.1 a |
| Standard error of the mean | 0.55 | 1.063 | 1.063 | 1.087 | 1.34 | 1.6 | 1.91 | 0.82 | 1.37 | 0.98 | 0.99 |
| Analysis of variance | |||||||||||
| Manure effect (M) | * | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Treatment effect (T) | ** | * | NS | NS | NS | NS | NS | NS | NS | NS | |
| M × T | NS | * | NS | NS | NS | NS | NS | NS | NS | NS | |
| Sources of Variations | Total Yield | Marketable Yield | Specific Gravity |
|---|---|---|---|
| Mg·ha−1 | |||
| Manure application | |||
| With manure | 30.3 a | 24.5 a | 1.0786 a |
| Without manure | 23.6 b | 18.1 b | 1.0747 a |
| Standard error of the mean | 1.34 | 1.71 | 0.001 |
| Nitrogen application | |||
| With N fertilizer | 28.9 a | 21.05 a | 1.0732 b |
| Without N fertilizer | 24.9 b | 21.5 a | 1.080 a |
| Standard error of the mean | 1.21 | 1.40 | 0.001 |
| Cover crop treatments | |||
| Alfalfa and orchardgrass | 27.2 ab | 21.05 a | 1.0775 a |
| Forage pearl millet | 29.8 a | 24.2 a | 1.0788 a |
| Forage sorghum followed by brown mustard | 29.9 a | 24.1 a | 1.0715 a |
| Red clover | 24.5 b | 18.8 c | 1.0797 a |
| Ryegrass and common vetch and crimson clover | 23.9 b | 18.7 c | 1.0764 a |
| Sorghum sudangrass | 28.0 a | 22.1 ab | 1.0784 a |
| Sorghum sudangrass and VR alfalfa | 24.8 b | 20.5 bc | 1.0779 a |
| Winter rye and hairy vetch | 27.1 ab | 20.6 bc | 1.0770 a |
| Standard error of the mean | 1.59 | 1.71 | 0.002 |
| Analysis of variance | |||
| Manure effect (M) | * | * | NS |
| Treatment effect (T) | ** | ** | NS |
| M × T | NS | NS | NS |
| N fertilizer (N) | *** | NS | *** |
| T × N | NS | NS | NS |
| M × N | NS | * | NS |
| M × T × N | NS | NS | NS |
| Sources of Variations | Permanganate Oxidazable Carbon | Total Earthworm (m−2) | Surface Hardness (PSI) |
|---|---|---|---|
| (mg C kg−1) | |||
| Manure application | |||
| With manure | 368.4 a | 18.6 a | 216.2 a |
| Without manure | 372.4 a | 12.3 a | 203.1 a |
| Standard error of the mean | 14.3 | 2.2 | 24.3 |
| Cover crop treatments | |||
| Alfalfa and orchardgrass | 375.5 a | 11 a | 189.2 a |
| Forage pearl millet | 378.1 a | 14 a | 190.3 a |
| Forage sorghum followed by brown mustard | 359.8 a | 14 a | 190.0 a |
| Red clover | 383.2 a | 14 a | 230.6 a |
| Ryegrass and common vetch and crimson clover | 360.7 a | 14 a | 242.2 a |
| Sorghum sudangrass | 362.1 a | 22 a | 220.6 a |
| Sorghum sudangrass and VR alfalfa | 374.5 a | 16 a | 196.7 a |
| Winter rye and hairy vetch | 369.6 a | 18 a | 217.5 a |
| Standard error of the mean | 15.13 | 4.4 | 28.4 |
| Analysis of variance | |||
| Manure effect (M) | NS | NS | NS |
| Treatment effect (T) | NS | NS | NS |
| M × T | NS | NS | NS |
| Sampling Time | 2017 Fall | 2018 Spring | 2019 Spring | |
|---|---|---|---|---|
| Sources of Variations | DNA (pg·g−1 Dry Soil) | DNA (pg·g−1 Dry Soil) | DNA (pg·g−1 Dry Soil) | Root-Lesion Nematodes (kg−1 Dry Soil) |
| Manure application | ||||
| With manure | 37.5 a | 26 a | 112.3 a | 3246.2 a |
| Without manure | 49.1 b | 22.4 a | 93.7 a | 2442.5 a |
| Standard error of the mean | 3.9 | 3.1 | 23.4 | 633.5 |
| Cover crop treatments | ||||
| Alfalfa and orchardgrass | 36.9 a | 24.2 a | 83.9 a | 2432.5 a |
| Forage pearl millet | 53.4 a | 28.9 a | 68.5 a | 1410 a |
| Forage sorghum followed by brown mustard | 38.3 a | 18.7 a | 134.6 a | 3390 a |
| Red clover | 45.7 a | 22.6 a | 86.9 a | 3177.5 a |
| Ryegrass and common vetch and crimson clover | 34.4 a | 18.4 a | 57.9 a | 3535 a |
| Sorghum sudangrass | 45.2 a | 29.7 a | 99.3 a | 2335 a |
| Sorghum sudangrass and VR alfalfa | 46.4 a | 22.8 a | 181.9 a | 3117.5 a |
| Winter rye and hairy vetch | 46 a | 28.1 a | 110.9 a | 3357.5 a |
| Standard error of the mean | 7.8 | 6.3 | 46.8 | 858.5 |
| Analysis of variance | ||||
| Manure (M) | * | NS | NS | NS |
| Treatment effect (T) | NS | NS | NS | NS |
| M × T | NS | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyiraneza, J.; Chen, D.; Fraser, T.; Comeau, L.-P. Improving Soil Quality and Potato Productivity with Manure and High-Residue Cover Crops in Eastern Canada. Plants 2021, 10, 1436. https://doi.org/10.3390/plants10071436
Nyiraneza J, Chen D, Fraser T, Comeau L-P. Improving Soil Quality and Potato Productivity with Manure and High-Residue Cover Crops in Eastern Canada. Plants. 2021; 10(7):1436. https://doi.org/10.3390/plants10071436
Chicago/Turabian StyleNyiraneza, Judith, Dahu Chen, Tandra Fraser, and Louis-Pierre Comeau. 2021. "Improving Soil Quality and Potato Productivity with Manure and High-Residue Cover Crops in Eastern Canada" Plants 10, no. 7: 1436. https://doi.org/10.3390/plants10071436
APA StyleNyiraneza, J., Chen, D., Fraser, T., & Comeau, L.-P. (2021). Improving Soil Quality and Potato Productivity with Manure and High-Residue Cover Crops in Eastern Canada. Plants, 10(7), 1436. https://doi.org/10.3390/plants10071436





