Soil Bacteria to Regulate Phoebe bournei Seedling Growth and Sustainable Soil Utilization under NPK Fertilization
Abstract
:1. Introduction
2. Results
2.1. Effect of NPK Fertilization on Phoebe bournei Seedlings’ Soil Chemical Characteristics
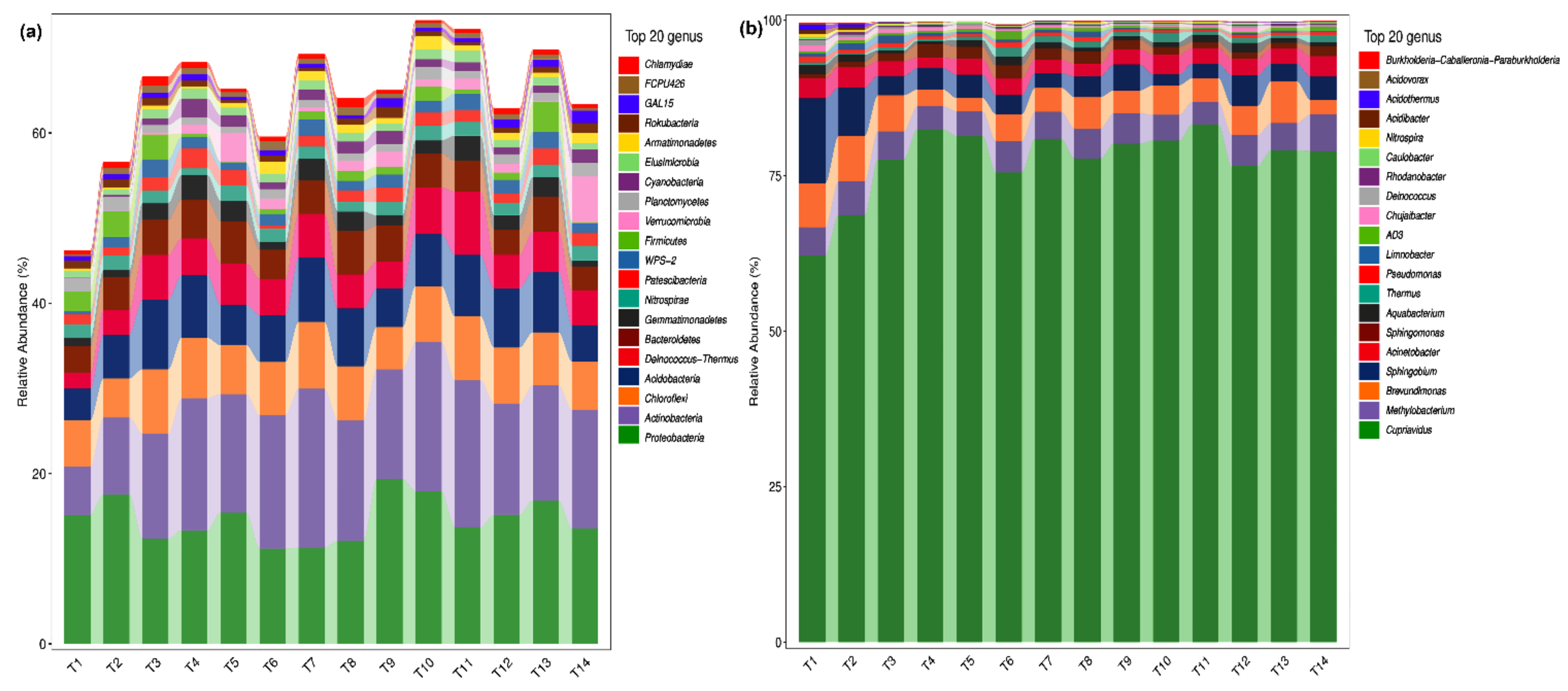
2.2. Analysis of Microbial Species Composition of Phoebe bournei Seedlings’ Soil at the Phylum and Genus Levels
2.3. Effect of NPK Fertilization on Phoebe bournei Seedlings’ Soil Bacterial Diversity
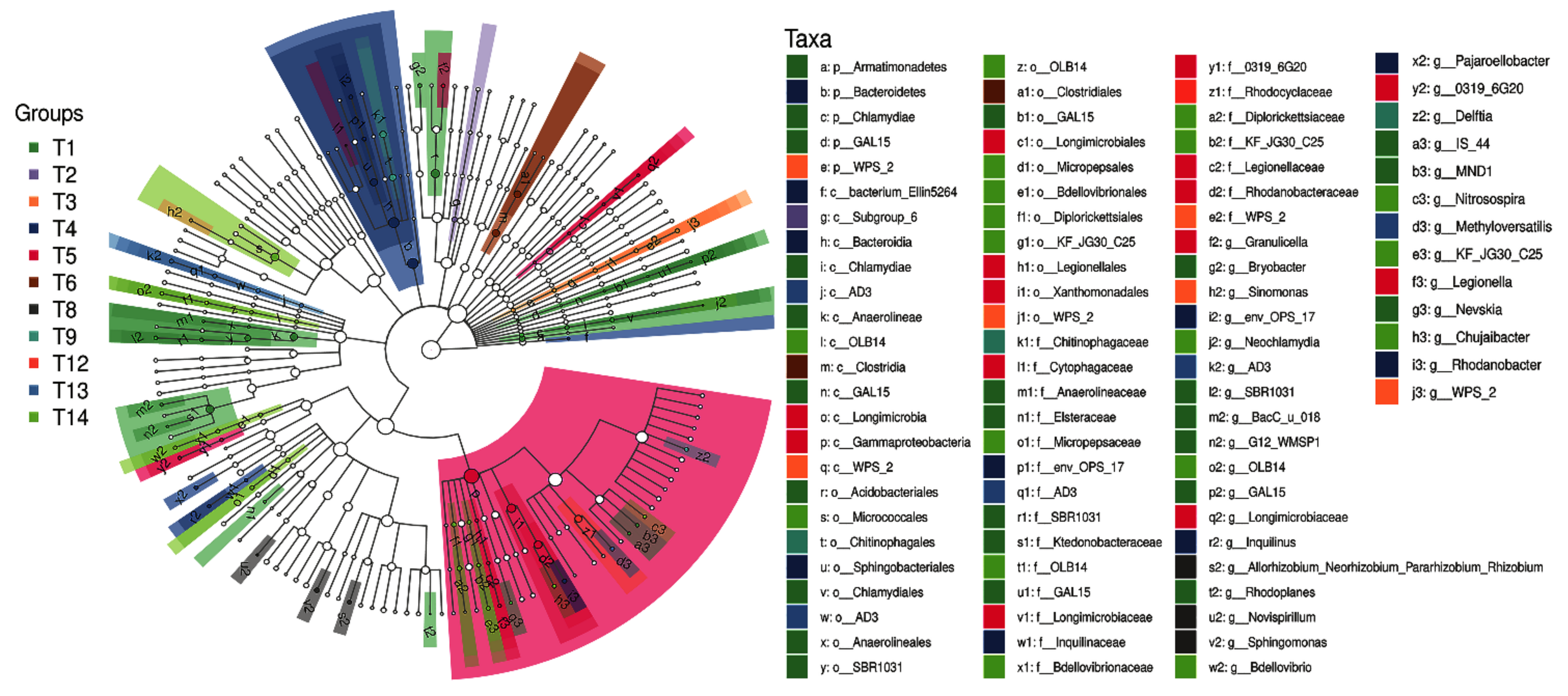
2.4. Differential Groups of Phoebe bournei Seedlings’ Soil Bacteria under NPK Fertilization
2.4.1. Differential Soil Bacteria Groups under Three-Factor NPK Fertilization
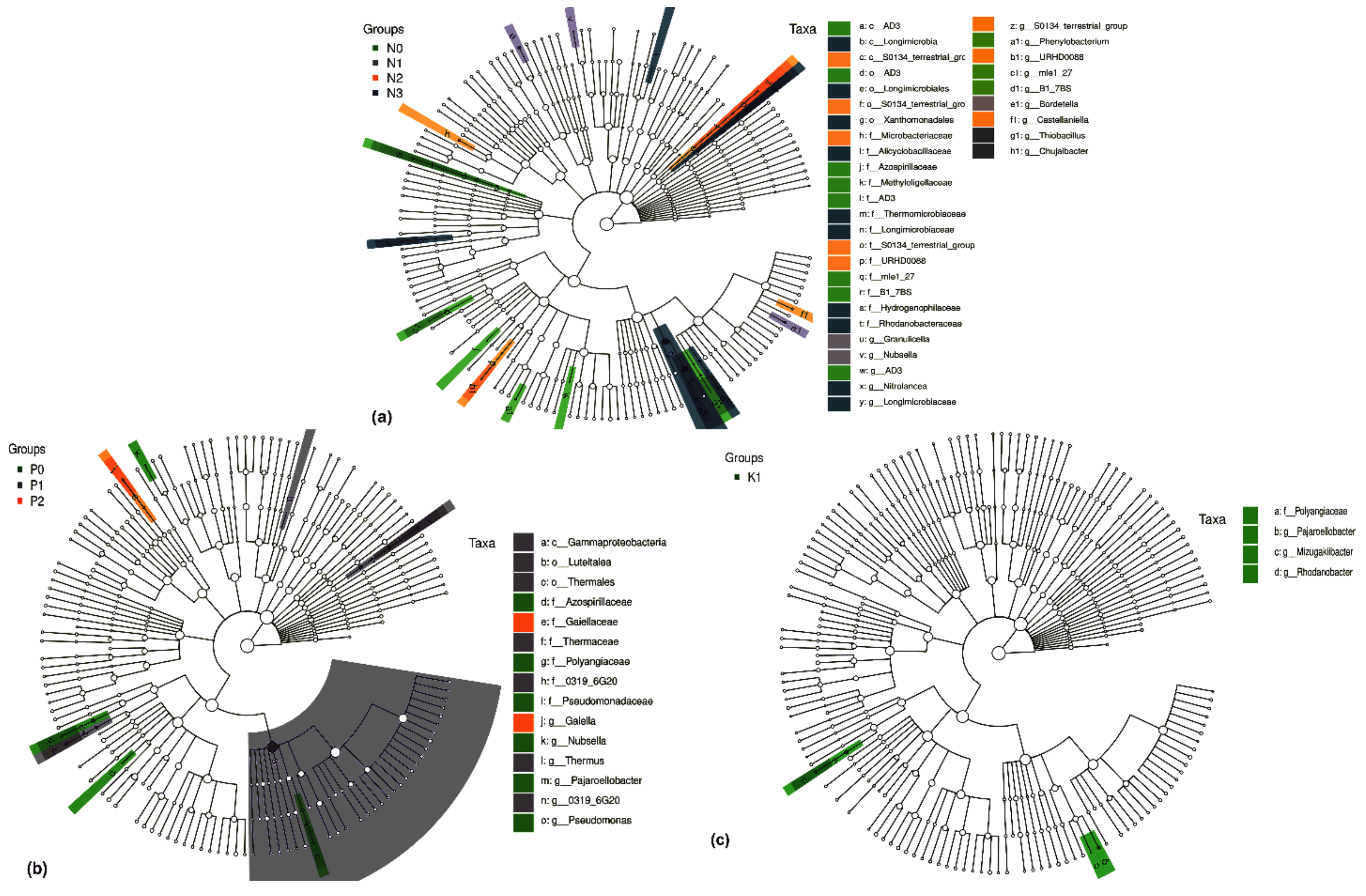
2.4.2. Differential Soil Bacteria Groups under Two-Factor NPK Fertilization
2.4.3. Differential Soil Bacteria Groups under Single-Factor NPK Fertilization
2.5. Network Association Analysis of Phoebe bournei Seedlings’ Soil Microbial Community under NPK Fertilization
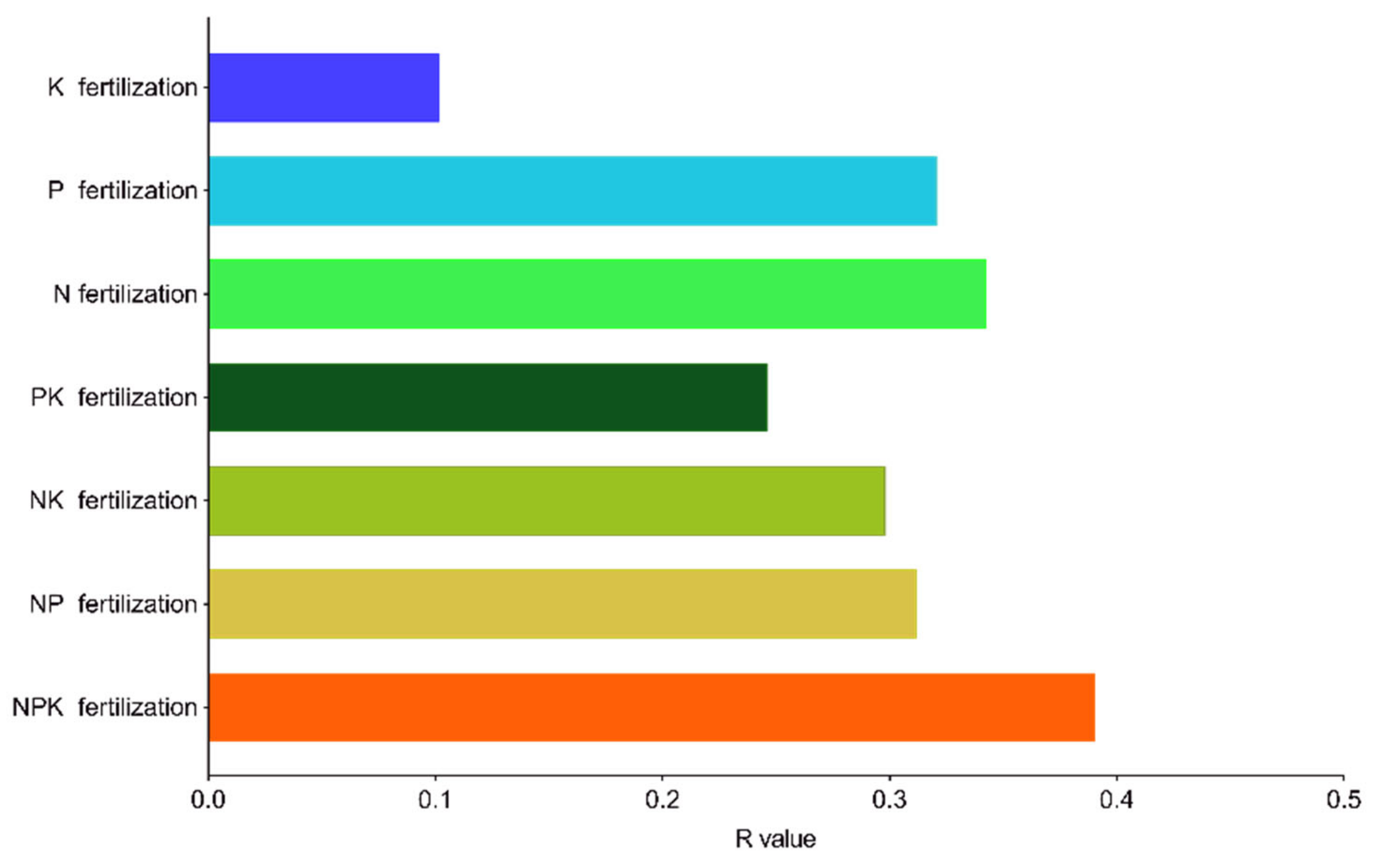
2.6. Driving Factors of Phoebe bournei Seedling Growth under NPK Fertilization
3. Discussion
3.1. NPK Fertilization Regulation of Phoebe bournei Seedlings’ Soil Chemical Characters
3.2. NPK Fertilization Regulation of Phoebe bournei Seedlings’ Soil Bacterial Diversity
3.3. Soil Bacteria Response to Phoebe bournei Seedlings’ NPK Fertilization
3.4. Soil Bacteria Regulate P. bournei Seedling Growth and Soil Development
4. Materials and Methods
4.1. Site Description
4.2. Materials
4.3. Experimental Design
4.4. Experimental Management
4.5. Sample Collection and Processing
4.6. Soil and Seedling Measurement Parameters
4.7. Soil Bacterial Sequencing
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the Soil Microbiome to Increase Soil Health and Plant Fertility. Biol. Fertil. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Armas, C.; Hortal, S.; Casanoves, F.; Pugnaire, F.I. Disentangling above-and below-Ground Facilitation Drivers in Arid Environments: The Role of Soil Microorganisms, Soil Prop-Erties and Microhabitat. New Phytol. 2017, 216, 1236–1246. [Google Scholar] [CrossRef] [Green Version]
- Lamhamedi, M.S.; Renaud, M.; Auger, I.; Fortin, J.A. Granular Calcite Stimulates Natural Mycorrhization and Growth of White Spruce Seedlings in Peat-Based Substrates in Forest Nursery. Microorganisms 2020, 7, 1088. [Google Scholar] [CrossRef]
- Li, J.; Ma, H.; Zhang, E. Influence of Irrigation with Reclaimed Water on Rhizosphere Soil Properties and Leaf Physiology of Different Landscape Plants. Res. Soil. Water Conserv. 2017, 24, 70–76. [Google Scholar]
- Zhou, X.; Chen, L.; Xu, J.; Brookes, P.C. Soil Biochemical Properties and Bacteria Community in a Repeatedly Fumigated-Incubated Soil. Biol. Fertil. Soils 2020, 56, 619–631. [Google Scholar] [CrossRef]
- Van der Wal, A.; De Boer, W. Dinner in the Dark: Illuminating Drivers of Soil Organic Matter Decomposition. Soil Biol. Biochem. 2017, 105, 45–48. [Google Scholar] [CrossRef]
- Singh, J.S.; Gupta, V.K. Soil Microbial Biomass: A Key Soil Driver in Management of Ecosystem Functioning. Sci. Total Environ. 2018, 634, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Muhammad, R.; Zhang, L.; Xia, H.; Cong, M.; Jiang, C. Investigating the Effect of Biochar and Fertilizer on the Composition and Function of Bacteria in Red Soil. Appl. Soil Ecol. 2019, 139, 107–116. [Google Scholar] [CrossRef]
- Wu, J.; Wei, Z.; Zhu, Z.; Zhao, Y.; Jia, L.; Lv, P. Humus Formation Driven by Ammonia-Oxidizing Bacteria During Mixed Materials Composting. Bioresour. Technol. 2020, 311, 123500. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Bai, Y.; Xiang, Q.; Yu, X.; Zhao, K.; Zhang, X.; Li, C.; Liu, S.; Chen, Q. Degradation Shaped Bacterial and Archaeal Communities with Predictable Taxa and Their Association Patterns in Zoige Wetland at Tibet Plateau. Sci. Rep. 2018, 8, 3884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrenfeld, J.G.; Ravit, B.; Elgersma, K. Feedback in the Plant-Soil System. Annu. Rev. Environ. Resour. 2005, 30, 75–115. [Google Scholar] [CrossRef] [Green Version]
- Brandt, K.K.; Frandsen, R.J.N.; Holm, P.E.; Nybroe, O. Development of Pollution-Induced Community Tolerance Is Linked to Structural and Functional Resilience of a Soil Bacterial Community Following a Five-Year Field Exposure to Copper. Soil. Biol. Biochem. 2010, 42, 748–757. [Google Scholar] [CrossRef]
- Miransari, M. Soil Microbes and Plant Fertilization. Appl. Microbiol. Biot. 2011, 92, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zheng, M.; Song, W.; Wen, S.; Wang, B.; Zhu, C.; Shen, R. Impact of 25 Years of Inorganic Fertilization on Diazotrophic Abundance and Community Structure in an Acidic Soil in Southern China. Soil. Biol. Biochem. 2017, 113, 240–249. [Google Scholar] [CrossRef]
- Yang, K.; Luo, S.; Hu, L.; Chen, B.; Xie, Z.; Ma, B.; Ma, W.; Du, G.; Ma, X.; Roux, X.L. Responses of Soil Ammonia-Oxidizing Bacteria and Archaea Diversity to N, P and NP Fertilization: Relationships with Soil Environmental Variables and Plant Community Diversity. Soil. Biol. Biochem. 2020, 145, 107795. [Google Scholar] [CrossRef]
- Daquiado, A.R.; Kuppusamy, S.; Kim, S.Y.; Kim, J.H.; Yoon, Y.E.; Kim, P.J.; Oh, S.H.; Kwak, Y.; Lee, Y.B. Pyrosequencing Analysis of Bacterial Community Diversity in Long-Term Fertilized Paddy Field Soil. Appl. Soil. Ecol. 2016, 108, 84–91. [Google Scholar] [CrossRef]
- Zhang, C.; Song, Z.; Zhuang, D.; Wang, J.; Xie, S.; Liu, G. Urea Fertilization Decreases Soil Bacterial Diversity, but Improves Microbial Biomass, Respiration, and N-Cycling Potential in a Semiarid Grassland. Biol. Fertil. Soils 2019, 55, 229–242. [Google Scholar] [CrossRef]
- Van der Bom, F.; Nunes, I.; Raymond, N.S.; Hansen, V.; Bonnichsen, L.; Magid, J.; Nybroe, O.; Jensen, L.S. Long-Term Fertilization Form, Level and duration Affect the Diversity, Structure and Functioning of Soil Microbial Communities in the Field. Soil. Biol. Biochem. 2018, 122, 91–103. [Google Scholar] [CrossRef]
- Hernández-Fernández, M.; Cordero-Bueso, G.; Ruiz-Muñoz, M.; Cantoral, J.M. Culturable Yeasts as Biofertilizers and Biopesticides for a Sustainable Agriculture: A Comprehensive Review. Plants 2021, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Stephan, A.; Meyer, A.H.; Schmid, B. Plant Diversity Affects Culturable Soil Bacteria in Experimental Grassland Communities. J. Ecol. 2000, 88, 988–998. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Wang, X.; Wei, X.; Wu, G.; Chen, S. Ammonium Application Mitigates the Effects of Elevated Carbon Dioxide on the Carbon/Nitrogen Balance of Phoebe bournei Seedlings. Tree Physiol. 2021, 2. [Google Scholar] [CrossRef]
- Xue, L.; Chen, H.; Xu, Y.; Peng, Y.; Xu, S.; Chen, W. Soil Physical Properties, Microbial Biomass and Enzyme Activity in Mixed Stands. Chin. J. Soil. Sci. 2004, 35, 154–158. [Google Scholar]
- Wu, F.; Wen, S.; He, G.; Zhou, Q. Response of Soil Bacterial Community Structure and Diversity to Different Forest Ages. J. Cent. South. Univ. For. Tech. 2020, 40, 134–143. [Google Scholar]
- Wang, X.; Wang, Y.; Wei, X.; Wu, G. Growth, Physiology and Nutrient Accumulation of Phoebe bournei Seedlings Under Different Amount of Exponential Nitrogen. J. Zhejiang. Agric. For. Univ. 2020, 37, 514–521. [Google Scholar]
- Albertsen, M.; Hugenholtz, P.; Skarshewski, A.; Nielsen, K.L.; Tyson, G.W.; Nielsen, P.H. Genome Sequences of Rare, Uncultured Bacteria Obtained by Differential Coverage Binning of Multiple Metagenomes. Nat. Biotechnol. 2013, 31, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, T.X.; Zhang, X.Z.; Lin, J. Establishment of Fertilization Recommendation Indexes of Rapeseed Soil Based on the “3414” Field Experiments in the Middle of Sichuan Hilly Regions. Sci. Agri. Sin. 2013, 46, 2058–2066. [Google Scholar]
- Zhang, Y.; Shen, H.; He, X.; Thomas, B.W.; Lupwayi, N.Z.; Hao, X.; Thomas, M.C.; Shi, X. Fertilization Shapes Bacterial Community Structure by Alteration of Soil pH. Front. Microbiol. 2017, 8, 325. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, A.; Chakrabarti, K.; Chakraborty, A.; Ghosh, S. Effect of Long-Term Fertilizers and Manure Application on Microbial Biomass and Microbial Activity of a Tropical Agricultural Soil. Biol. Fertil. Soils 2011, 47, 227–233. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Wang, R.; Cai, J.; Zhang, Y.; Huang, S.; Jiang, Y. Impacts of Fertilization Practices on pH and the pH Buffering Capacity of Calcareous Soil. Soil Sci. Plant. Nutr. 2016, 62, 432–439. [Google Scholar] [CrossRef]
- Tan, D.; Jin, J.; Huang, S.; Li, S.; He, P. Effect of Long-Term Application of K Fertilizer and Wheat Straw to Soil on Crop Yield and Soil K under Different Planting Systems. Agric. Sci. China 2007, 6, 200–207. [Google Scholar]
- Gaxiola, R.A.; Sanchez, C.A.; Paez-Valencia, J.; Ayre, B.G.; Elser, J.J. Genetic Manipulation of a “Vacuolar” H+-PPase: From Salt Tolerance to Yield Enhancement under Phosphorus-Deficient Soils. Plant. Physiol. 2012, 159, 3–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wu, H.; Chen, L.; Liu, L.; Wan, X. Maintenance of Mesophyll Potassium and Regulation of Plasma Membrane H+-ATPase Are Associated with Physiological Responses of Tea Plants to Drought and Subsequent Rehydration. Crop. J. 2018, 6, 611–620. [Google Scholar] [CrossRef]
- Ou, J. N, P, K Formulas for Fertilization with Young Phoebe bournei Plantations in Fujian Province. J. Zhejiang Agric. For. Univ. 2015, 32, 92–97. [Google Scholar]
- Major, N.; Schierstaedt, J.; Jechalke, S.; Nesme, J.; Ban, S.G.; Černe, M.; Sørensen, S.J.; Ban, D.; Schikora, A. Composted Sewage Sludge Influences the Microbiome and Persistence of Human Pathogens in Soil. Microorganisms 2020, 7, 1020. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Kumar, G.N.; Parekh, L.J.; Poole, P.S. Role of Soil Microorganisms in Improving P Nutrition of Plants. Plant. Soil 2002, 245, 83–93. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; May, W.E.; Kanashiro, D.A.; Petri, P.M. Soil Bacterial Community Responses to Black Medic Cover Crop and Fertilizer N under No-Till. Appl. Soil. Ecol. 2018, 124, 95–103. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Wang, F.; Xie, Y. Maize (Zea mays) Growth and Nutrient Uptake Following Integrated Improvement of Vermicompost and Humic Acid Fertilizer on Coastal Saline Soil. Appl. Soil. Ecol. 2019, 142, 147–154. [Google Scholar] [CrossRef]
- Naether, A.; Foesel, B.U.; Naegele, V.; Wüst, P.K.; Weinert, J.; Bonkowski, M.; Alt, F.; Oelmann, Y.; Polle, N.; Lohaus, G.; et al. Environmental Factors Affect Acidobacterial Communities below the Subgroup Level in Grassland and Forest Soils. Appl. Environ. Microbiol. 2012, 78, 7398–7406. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.; Su, W.; Chen, H.; Barberan, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X.; et al. Long-Term Nitrogen Fertilization Decreases Bacterial Diversity and Favors the Growth of Actinobacteria and Proteobacteria in Agro-Ecosystems Across the Globe. Glob. Chang. Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef]
- Puig, S.; Serra, M.; Vilar-Sanz, A.; Cabré, M.; Bañeras, L.; Colprim, J.; Balaguer, M.D. Autotrophic Nitrite Removal in the Cathode of Microbial Fuel Cells. Bioresour. Technol. 2011, 102, 4462–4467. [Google Scholar] [CrossRef]
- Yamashita, N.; Tanabata, S.; Ohtake, N.; Sueyosh, K.; Sato, T.; Higuchi, K.; Saito, A.; Ohyama, T. Effects of Different Chemical Forms of Nitrogen on the Quick and Reversible Inhibition of Soybean Nodule Growth and Nitrogen Fixation Activity. Front. Plant. Sci. 2019, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Saidi, S.; Cherif-Silini, H.; Bouket, A.C.; Silini, A.; Eshelli, M.; Luptakova, L.; Alenezi, F.N.; Belbahri, L. Improvement of Medicago sativa Crops Productivity by the Co-Inoculation of Sinorhizobium meliloti-Actinobacteria under Salt Stress. Curr. Microbiol. 2021, 78, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Ai, C.; Liang, G.; Sun, J.; Wang, X.; He, P.; Zhou, W.; He, X. Reduced Dependence of Rhizosphere Microbiome on Plant-Derived Carbon in 32-Year Long-Term Inorganic and Organic Fertilized Soils. Soil. Biol. Biochem. 2015, 80, 70–78. [Google Scholar] [CrossRef]
- Chen, J.; van Groenigen, K.J.; Hungate, B.A.; Terrer, C.; Groenigen, J.V.; Maestre, F.T.; Ying, S.C.; Luo, Y.; Jørgensen, U.; Sinsabaugh, R.L.; et al. Long-Term Nitrogen Loading Alleviates Phosphorus Limitation in Terrestrial Ecosystems. Glob. Chang. Biol. 2020, 26, 5077–5086. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, S.; Zhao, X.; Long, H. Evaluation of Potassium Application on Tomato Performance and Rhizosphere Bacterial Communities under Negative Pressure Irrigation of Greenhouse-Grown. J. Plant. Nutr. 2020, 43, 317–326. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Firestone, M.K. Response of Microbial Community Composition and Function to Soil Climate Change. Microb. Ecol. 2006, 52, 716–724. [Google Scholar] [CrossRef]
- Yang, Z.J.; Wu, X.H.; Grossnickle, S.C.; Chen, L.H.; Yu, X.X.; EI-Kassaby, Y.A.; Feng, J.L. Formula Fertilization Promotes Phoebe bournei Robust Seedling Cultivation. Forests 2020, 11, 781. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Chen, F.; Yao, B. Improving Rice Modeling Success Rate with Ternary Non-Structural Fertilizer Response Model. Sci. Rep. 2018, 8, 9071. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wu, X.; Zhang, L.; Wu, L.; Xu, Y.; Zhou, Z. Effects of Slow-Release Fertilizer Loading on Growth and Construction of Nutrients Reserves of Phoebe chekiangensis and Phoebe bournei Container Seedlings. Sci. Agri. Sin. 2013, 49, 57–63. [Google Scholar]
- Dong, L.J.; Zhu, X.T.; Lin, X.Z.; Xu, Z.D. Effects of Fertilization on the Growth of Three Lauraceae Seedlings in Containers. North. Horti. 2011, 13, 73–77. [Google Scholar]
- Liu, Q.; Chen, M.; Wu, J.; Dong, C.; Huang, X.; Huang, S.; Tang, A.; Huang, M. Nutritional Properties and Fertilizer Demand Rules of Young Phoebe bournei Forest. Agric. Sci. Tech. 2015, 16, 2758–2760, 2781. [Google Scholar]
- Sui, Y.; Gao, J.; Shang, Q. Characterization of Nitrogen Metabolism and Photosynthesis in a Stay-Green Rice Cultivar. Plant Soil Environ. 2019, 65, 283–289. [Google Scholar] [CrossRef]
- Woźniak, G.; Chmura, D.; Małkowski, E.; Zieleźnik-Rusinowska, P.; Sitko, K.; Ziemer, B.; Błońska, A. Is the Age of Novel Ecosystem the Factor Driving Arbuscular Mycorrhizal Colonization in Poa compressa and Calamagrostis epigejos? Plants 2021, 10, 949. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, J.; Fujimura, S.; Kondo, M.; Murai-Hatano, M.; Goto, A.; Shinano, T. Dynamic Changes in the Cs Distribution throughout Rice Plants during the Ripening Period, and Effects of the Soil-K Level. Plant Soil 2018, 429, 503–518. [Google Scholar] [CrossRef]
- Thukral, A.K. A Review on Measurement of Alpha Diversity in Biology. Agric. Res. J. 2017, 54, 1–10. [Google Scholar] [CrossRef]
- Li, B.; Cao, Y.; Guan, X.; Li, Y.; Hao, Z.; Hu, W.; Chen, L. Microbial Assessments of Soil with a 40-Year History of Reclaimed Wastewater Irrigation. Sci. Total Environ. 2019, 651, 696–705. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, Y.; Zhou, F.; Zhang, B.; Wu, J.; Yang, L.; Xu, S.; Stedtfeld, R.; Chen, Q.; Liu, J.; et al. Featured Gut Microbiomes Associated with the Progression of Chronic Hepatitis B Disease. Front. Microbiol. 2020, 11, 383. [Google Scholar] [CrossRef]
- Duan, C.; Razavi, B.S.; Shen, G.; Cui, Y.; Ju, W.; Li, S.; Fang, L. Deciphering the Rhizobium Inoculation Effect on Spatial Distribution of Phosphatase Activity in the Rhizosphere of Alfalfa Under Copper Stress. Soil. Biol. Biochem. 2019, 137, 107574. [Google Scholar] [CrossRef]

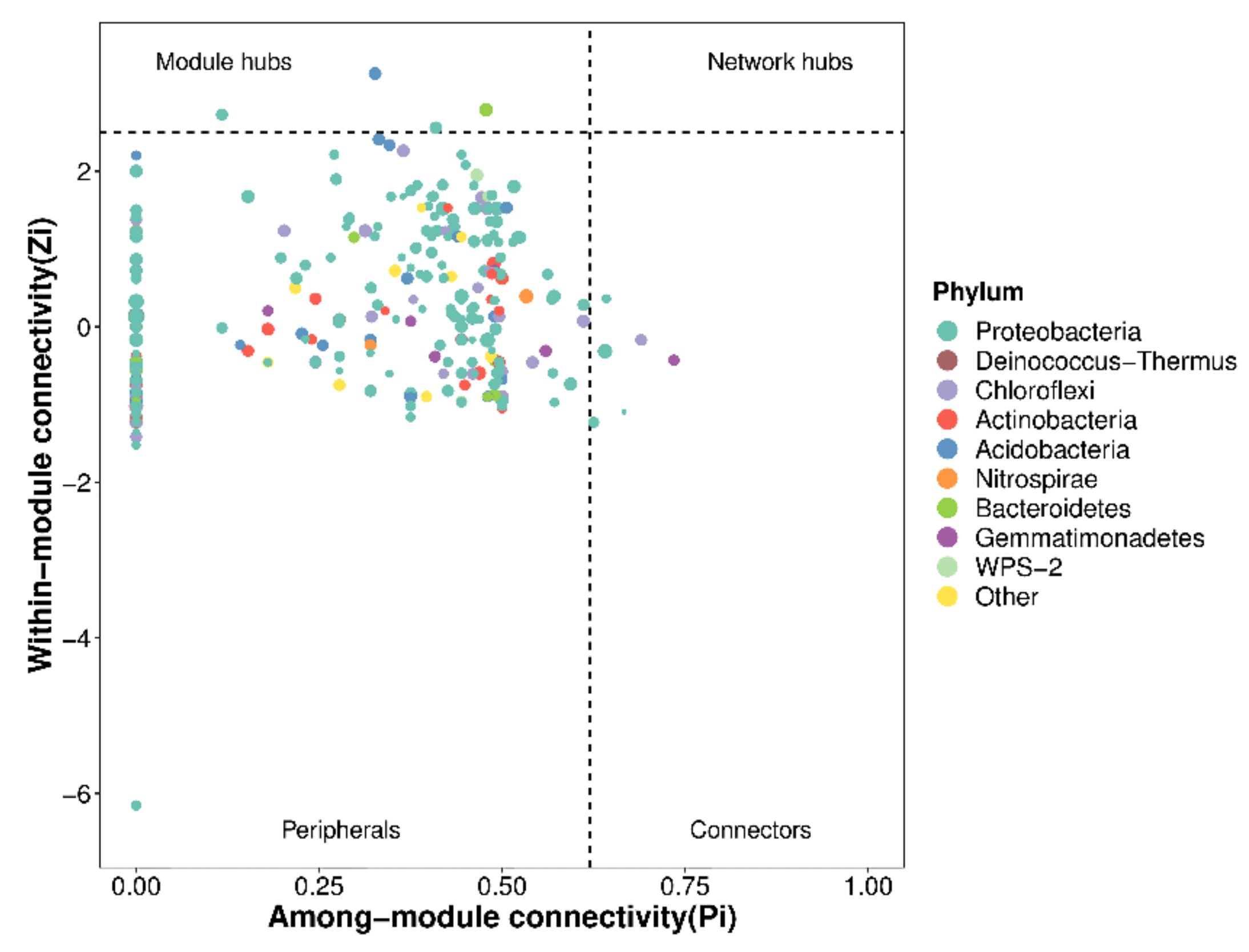
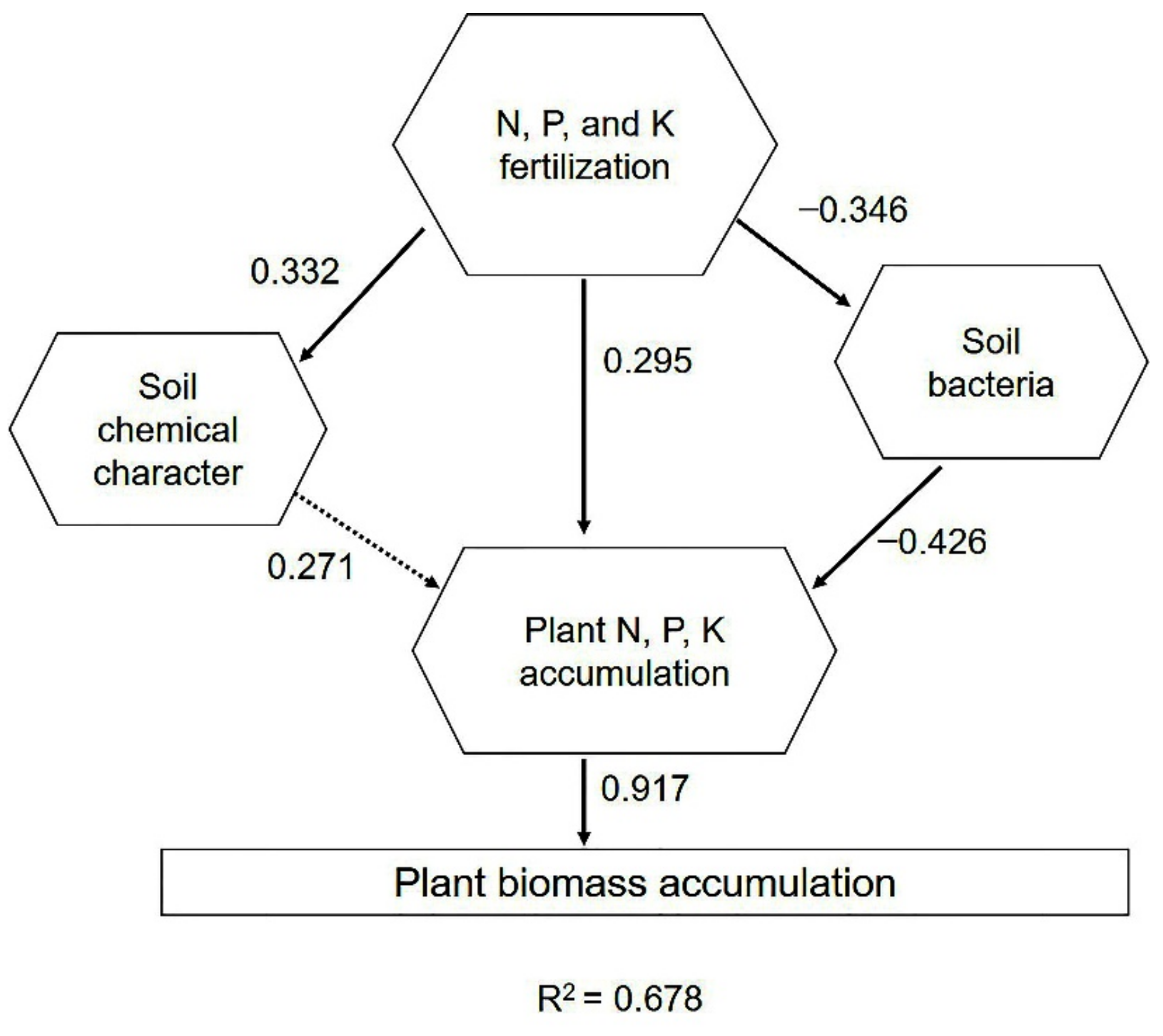
| Number | Treatment | N Content (g·Kg−1) | P Content (g·Kg−1) | K Content (g·Kg−1) | pH |
|---|---|---|---|---|---|
| T1 | N0P0K0 | 2.86 ± 0.35 i | 0.35 ± 0.13 ef | 32.91 ± 1.67 g | 5.29 ± 0.01 b |
| T2 | N0P2K2 | 2.45 ± 0.09 j | 0.18 ± 0.01 gh | 51.91 ± 2.63 cd | 5.59 ± 0.07 a |
| T3 | N1P2K2 | 11.73 ± 0.33 b | 0.74 ± 0.01 c | 49.92 ± 3.88 de | 4.74 ± 0.01 e |
| T4 | N2P0K2 | 4.68 ± 0.04 h | 0.16 ± 0.04 h | 42.79 ± 0.24 ef | 4.43 ± 0.03 h |
| T5 | N2P1K2 | 5.17 ± 0.26 g | 0.25 ± 0.06 fgh | 58.88 ± 4.13 bc | 4.53 ± 0.04 g |
| T6 | N2P2K2 | 6.34 ± 0.19 ef | 0.50 ± 0.08 d | 39.18 ± 1.45 fg | 4.65 ± 0.04 f |
| T7 | N2P3K2 | 4.83 ± 0.14 h | 0.24 ± 0.02 fgh | 69.39 ± 5.50 a | 4.83 ± 0.07 d |
| T8 | N2P2K0 | 8.20 ± 0.08 c | 0.91 ± 0.13 b | 58.19 ± 7.34 bc | 4.97 ± 0.02 c |
| T9 | N2P2K1 | 6.50 ± 0.09 e | 0.45 ± 0.04 de | 48.48 ± 0.58 de | 4.84 ± 0.03 d |
| T10 | N2P2K3 | 5.29 ± 0.08 g | 0.28 ± 0.05 fgh | 43.67 ± 9.15 ef | 4.15 ± 0.04 j |
| T11 | N3P2K2 | 13.47 ± 0.26 a | 2.33 ± 0.18 a | 47.41 ± 3.08 de | 4.64 ± 0.01 f |
| T12 | N1P1K2 | 6.15 ± 0.12 f | 0.18 ± 0.02 gh | 61.86 ± 4.11 b | 4.45 ± 0.04 h |
| T13 | N1P2K1 | 2.79 ± 0.03 i | 0.35 ± 0.00 ef | 33.70 ± 2.50 g | 4.47 ± 0.01 gh |
| T14 | N2P1K1 | 7.42 ± 0.10 d | 0.31 ± 0.04 fg | 34.71 ± 3.04 g | 4.27 ± 0.02 i |
| RN | – | 11.02 | 2.15 | 12.72 | 0.95 |
| RP | – | 1.66 | 0.34 | 30.21 | 0.40 |
| RK | – | 2.90 | 0.62 | 19.01 | 0.82 |
| RNP | – | 11.02 | 2.17 | 30.21 | 1.16 |
| RNK | – | 11.02 | 2.15 | 25.50 | 1.44 |
| RPK | – | 3.52 | 0.75 | 34.68 | 0.82 |
| RNPK | – | 11.02 | 2.17 | 36.69 | 1.44 |
| Number | Treatment | Diversity Index | ||
|---|---|---|---|---|
| Chao1 | Simpson | Shannon | ||
| T1 | N0P0K0 | 1929.96 ± 84.55 a | 0.97 ± 0.01 a | 8.37 ± 0.44 a |
| T2 | N0P2K2 | 1577.62 ± 152.83 ab | 0.96 ± 0.02 ab | 7.60 ± 0.57 ab |
| T3 | N1P2K2 | 1389.66 ± 166.04 ab | 0.96 ± 0.02 ab | 7.10 ± 0.67 ab |
| T4 | N2P0K2 | 1102.84 ± 142.98 b | 0.95 ± 0.01 ab | 6.69 ± 0.43 b |
| T5 | N2P1K2 | 1048.23 ± 87.34 b | 0.95 ± 0.01 ab | 6.52 ± 0.40 b |
| T6 | N2P2K2 | 1380.80 ± 302.08 ab | 0.95 ± 0.04 ab | 7.16 ± 1.65 ab |
| T7 | N2P3K2 | 1281.24 ± 211.79 b | 0.94 ± 0.02 ab | 6.63 ± 0.71 b |
| T8 | N2P2K0 | 1250.01 ± 214.96 b | 0.96 ± 0.03 ab | 7.09 ± 1.21 ab |
| T9 | N2P2K1 | 1112.93 ± 7.42 b | 0.94 ± 0.01 ab | 6.69 ± 0.22 b |
| T10 | N2P2K3 | 1042.75 ± 196.40 b | 0.93 ± 0.02 b | 6.24 ± 0.88 b |
| T11 | N3P2K2 | 1150.40 ± 144.02 b | 0.95 ± 0.01 ab | 6.55 ± 0.60 b |
| T12 | N1P1K2 | 1370.78 ± 336.40 ab | 0.96 ± 0.04 ab | 7.28 ± 1.69 ab |
| T13 | N1P2K1 | 1171.17 ± 75.22 b | 0.95 ± 0.01 ab | 6.72 ± 0.47 b |
| T14 | N2P1K1 | 1056.21 ± 77.82 b | 0.96 ± 0.01 ab | 7.02 ± 0.50 ab |
| RN | – | 427.22 | 0.02 | 1.05 |
| RP | – | 332.58 | 0.01 | 0.64 |
| RK | – | 338.06 | 0.03 | 0.92 |
| RNP | – | 529.39 | 0.02 | 1.08 |
| RNK | – | 534.87 | 0.03 | 1.37 |
| RPK | – | 338.06 | 0.03 | 0.92 |
| RNPK | – | 887.22 | 0.05 | 2.13 |
| Topo | NPK | NP | NK | PK | N | P | K |
|---|---|---|---|---|---|---|---|
| Number of Nodes | 617 | 617 | 617 | 612 | 615 | 545 | 594 |
| Number of Edges | 3593 | 3593 | 3593 | 3444 | 3591 | 2447 | 3223 |
| Closeness Centrality | 0.61 | 0.61 | 0.61 | 0.60 | 0.61 | 0.56 | 0.60 |
| Average Path Length | 4.09 | 4.09 | 4.09 | 4.12 | 4.07 | 4.24 | 4.21 |
| Transitivity | 0.61 | 0.61 | 0.61 | 0.62 | 0.60 | 0.70 | 0.64 |
| Modularity | 0.75 | 0.75 | 0.75 | 0.76 | 0.75 | 0.73 | 0.76 |
| Species | Actinobacteria | Chloroflexi | Acidobacteria | Proteobacteria |
|---|---|---|---|---|
| Cupriavidus | 0.024 | 0.105 | 0.063 | −0.038 |
| Methylobacterium | −0.703 ** | −0.659 ** | −0.689 ** | 0.855 ** |
| Brevundimonas | −0.659 ** | −0.495 ** | −0.496 ** | 0.651 ** |
| Sphingobium | −0.626 ** | −0.350 * | −0.500 ** | 0.643 ** |
| Acinetobacter | −0.641 ** | −0.442 ** | −0.617 ** | 0.732 ** |
| Sphingomonas | −0.157 | −0.132 | −0.370 | 0.298 |
| Species | pH | N Content | P Content | K Content |
|---|---|---|---|---|
| Methylobacterium | −0.383 * | 0.212 | 0.141 | 0.140 |
| Brevundimonas | −0.219 | 0.322 * | 0.178 | 0.080 |
| Sphingobium | −0.154 | 0.240 | 0.165 | 0.293 * |
| Acinetobacter | −0.356 * | 0.433 ** | 0.403 ** | 0.080 |
| No. | Treatment 1 | N 1 (g·Plant−1) | P2O5 1 (g·Plant−1) | K2O 1 (g·Plant−1) |
|---|---|---|---|---|
| T1 | N0P0K0 | 0 (0) | 0 (0) | 0 (0) |
| T2 | N0P2K2 | 0 (0) | 2 (0.1332) | 2 (0.356) |
| T3 | N1P2K2 | 1 (0.266) | 2 (0.1332) | 2 (0.356) |
| T4 | N2P0K2 | 2 (0.532) | 0 (0) | 2 (0.356) |
| T5 | N2P1K2 | 2 (0.532) | 1 (0.0666) | 2 (0.356) |
| T6 | N2P2K2 | 2 (0.532) | 2 (0.1332) | 2 (0.356) |
| T7 | N2P3K2 | 2 (0.532) | 3 (0.1998) | 2 (0.356) |
| T8 | N2P2K0 | 2 (0.532) | 2 (0.1332) | 0 (0) |
| T9 | N2P2K1 | 2 (0.532) | 2 (0.1332) | 1 (0.178) |
| T10 | N2P2K3 | 2 (0.532) | 2 (0.1332) | 3 (0.534) |
| T11 | N3P2K2 | 3 (0.798) | 2 (0.1332) | 2 (0.356) |
| T12 | N1P1K2 | 1 (0.266) | 1 (0.0666) | 2 (0.356) |
| T13 | N1P2K1 | 1 (0.266) | 2 (0.1332) | 1 (0.178) |
| T14 | N2P1K1 | 2 (0.532) | 1 (0.0666) | 1 (0.178) |
| Number | Treatment | Plant Biomass (g·Plant−1) [48] | N Accumulation (mg·Plant−1) | P Accumulation (mg·Plant−1) | K Accumulation (mg·Plant−1) |
|---|---|---|---|---|---|
| T1 | N0P0K0 | 2.89 ± 0.23 e | 31.43 ± 2.05 g | 9.14 ± 0.56 g | 78.36 ± 10.72 i |
| T2 | N0P2K2 | 2.73 ± 0.27 e | 39.87 ± 4.31 f | 11.80 ± 1.39 ef | 132.07 ± 11.24 fg |
| T3 | N1P2K2 | 4.01 ± 0.32 c | 55.50 ± 4.24 d | 14.98 ± 0.32 d | 158.46 ± 3.14 de |
| T4 | N2P0K2 | 4.97 ± 0.14 b | 62.71 ± 1.22 bc | 12.72 ± 0.22 e | 115.07 ± 1.71 h |
| T5 | N2P1K2 | 4.10 ± 0.07 c | 64.96 ± 0.84 b | 16.27 ± 3.25 cd | 220.64 ± 10.78 b |
| T6 | N2P2K2 | 7.27 ± 0.41 a | 82.45 ± 4.85 a | 24.68 ± 0.97 a | 323.91 ± 16.26 a |
| T7 | N2P3K2 | 4.71 ± 0.34 b | 58.85 ± 4.84 cd | 15.69 ± 0.75 cd | 120.47 ± 5.48 gh |
| T8 | N2P2K0 | 3.95 ± 0.14 c | 54.39 ± 0.70 d | 11.08 ± 0.38 ef | 91.79 ± 11.29 i |
| T9 | N2P2K1 | 3.97 ± 0.20 c | 64.83 ± 2.05 bc | 16.09 ± 0.18 cd | 145.80 ± 0.75 ef |
| T10 | N2P2K3 | 3.30 ± 0.12 d | 44.73 ± 5.19 ef | 10.40 ± 0.38 fg | 167.34 ± 7.97 cd |
| T11 | N3P2K2 | 2.73 ± 0.16 e | 47.73 ± 3.81 e | 12.79 ± 0.79 e | 111.10 ± 6.32 h |
| T12 | N1P1K2 | 4.65 ± 0.32 b | 62.46 ± 1.05 bc | 17.07 ± 0.48 bc | 180.19 ± 2.11 c |
| T13 | N1P2K1 | 4.69 ± 0.12 b | 65.76 ± 3.05 b | 18.78 ± 0.99 b | 175.76 ± 7.18 c |
| T14 | N2P1K1 | 4.20 ± 0.12 c | 64.04 ± 1.24 bc | 12.52 ± 1.13 e | 133.77 ± 7.29 fg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.-J.; Wu, X.-H.; Huang, L.-M.; Xie, W.-W.; Chen, Y.; El-Kassaby, Y.A.; Feng, J.-L. Soil Bacteria to Regulate Phoebe bournei Seedling Growth and Sustainable Soil Utilization under NPK Fertilization. Plants 2021, 10, 1868. https://doi.org/10.3390/plants10091868
Yang Z-J, Wu X-H, Huang L-M, Xie W-W, Chen Y, El-Kassaby YA, Feng J-L. Soil Bacteria to Regulate Phoebe bournei Seedling Growth and Sustainable Soil Utilization under NPK Fertilization. Plants. 2021; 10(9):1868. https://doi.org/10.3390/plants10091868
Chicago/Turabian StyleYang, Zhi-Jian, Xiao-Hui Wu, Lan-Ming Huang, Wei-Wei Xie, Yu Chen, Yousry A. El-Kassaby, and Jin-Ling Feng. 2021. "Soil Bacteria to Regulate Phoebe bournei Seedling Growth and Sustainable Soil Utilization under NPK Fertilization" Plants 10, no. 9: 1868. https://doi.org/10.3390/plants10091868
APA StyleYang, Z.-J., Wu, X.-H., Huang, L.-M., Xie, W.-W., Chen, Y., El-Kassaby, Y. A., & Feng, J.-L. (2021). Soil Bacteria to Regulate Phoebe bournei Seedling Growth and Sustainable Soil Utilization under NPK Fertilization. Plants, 10(9), 1868. https://doi.org/10.3390/plants10091868






