Drought Tolerance Responses in Vegetable-Type Soybean Involve a Network of Biochemical Mechanisms at Flowering and Pod-Filling Stages
Abstract
:1. Introduction
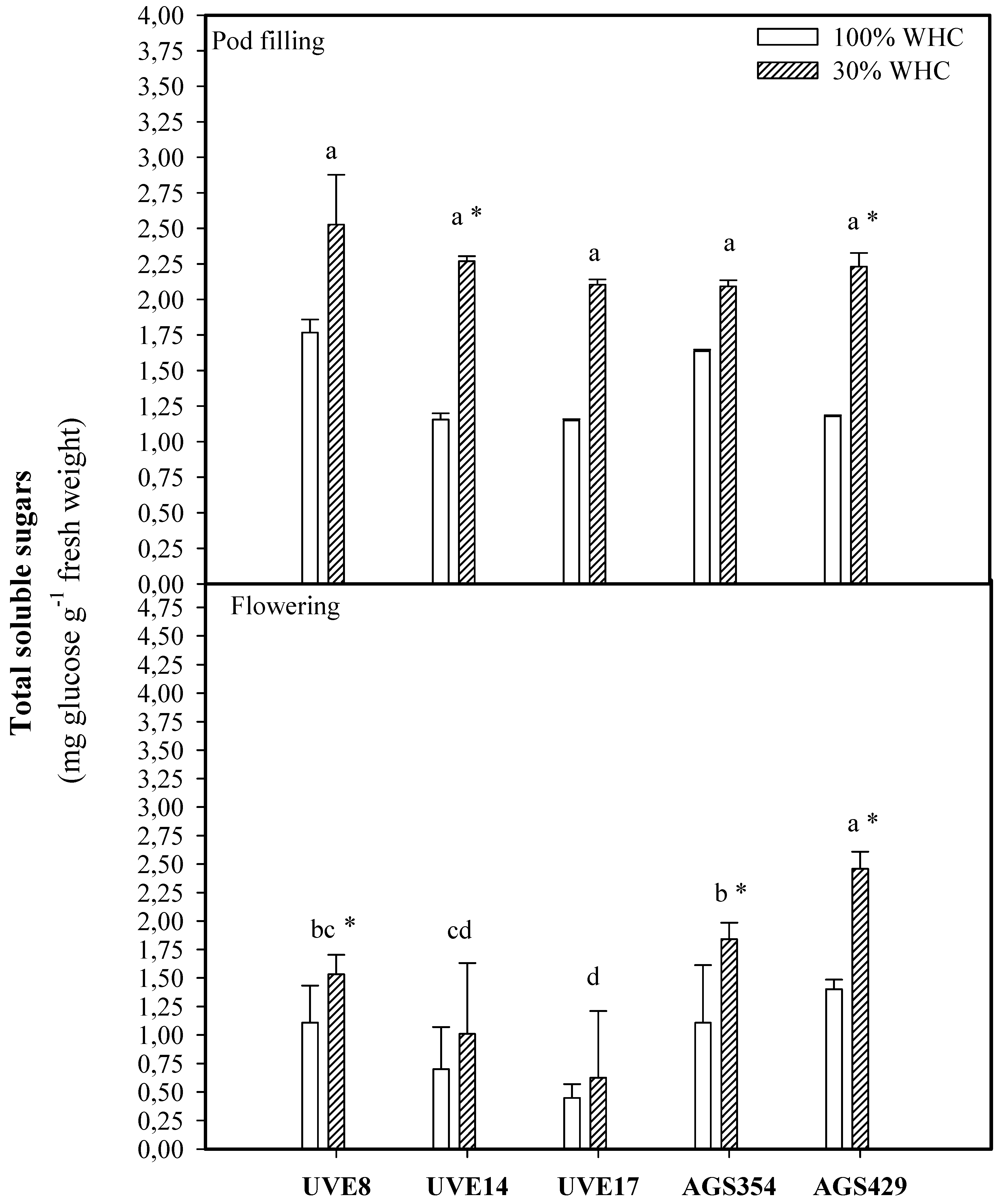
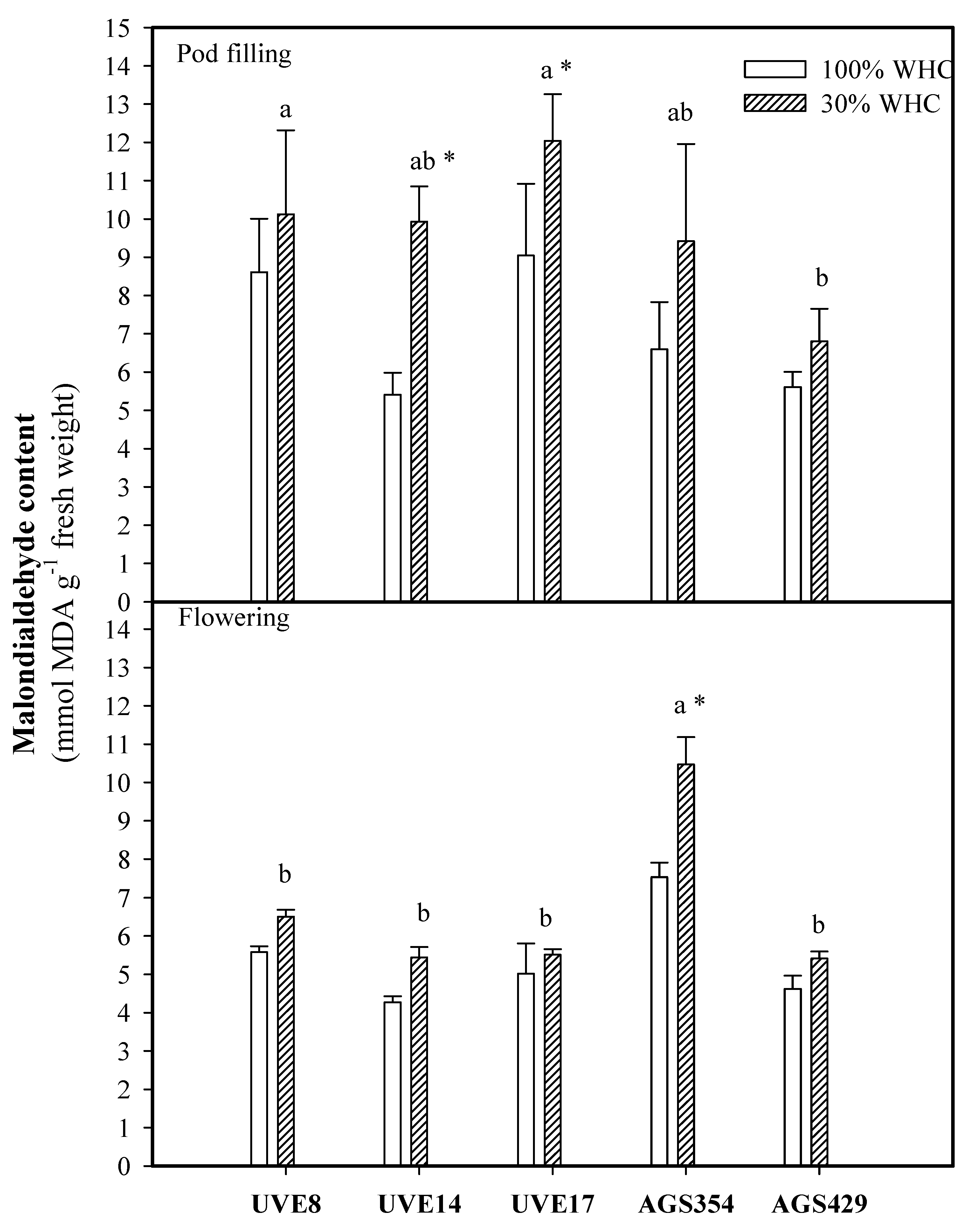
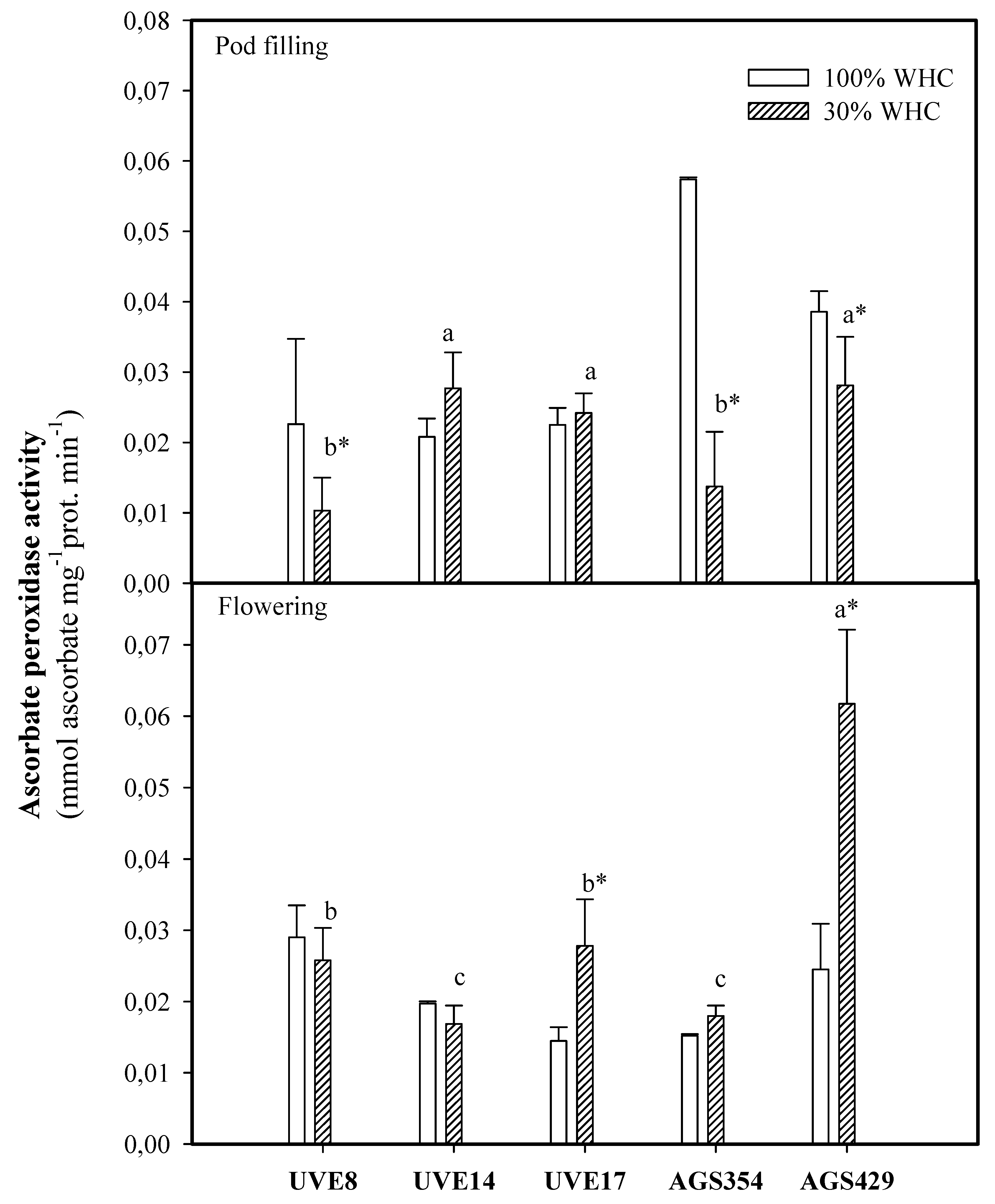
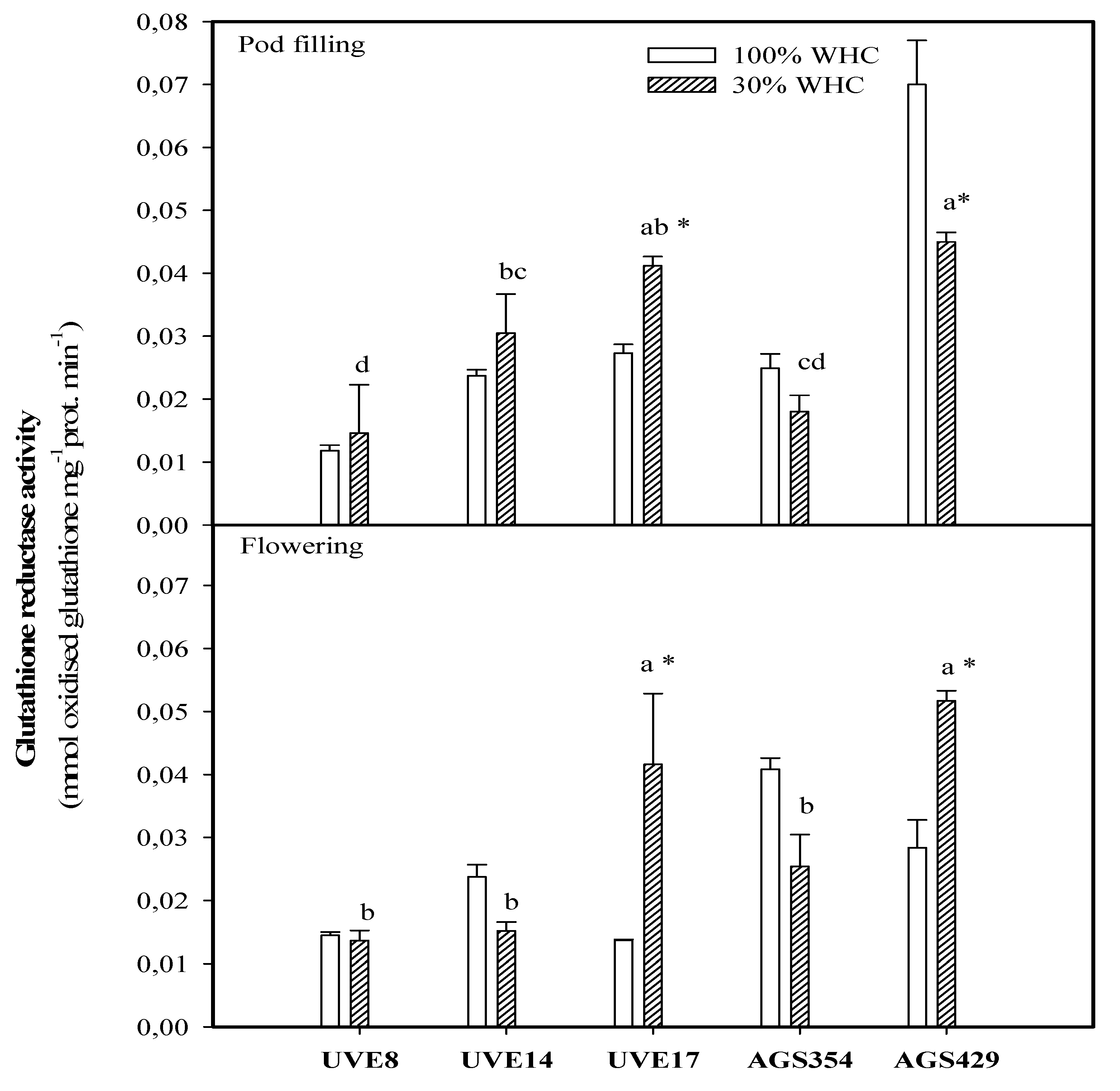
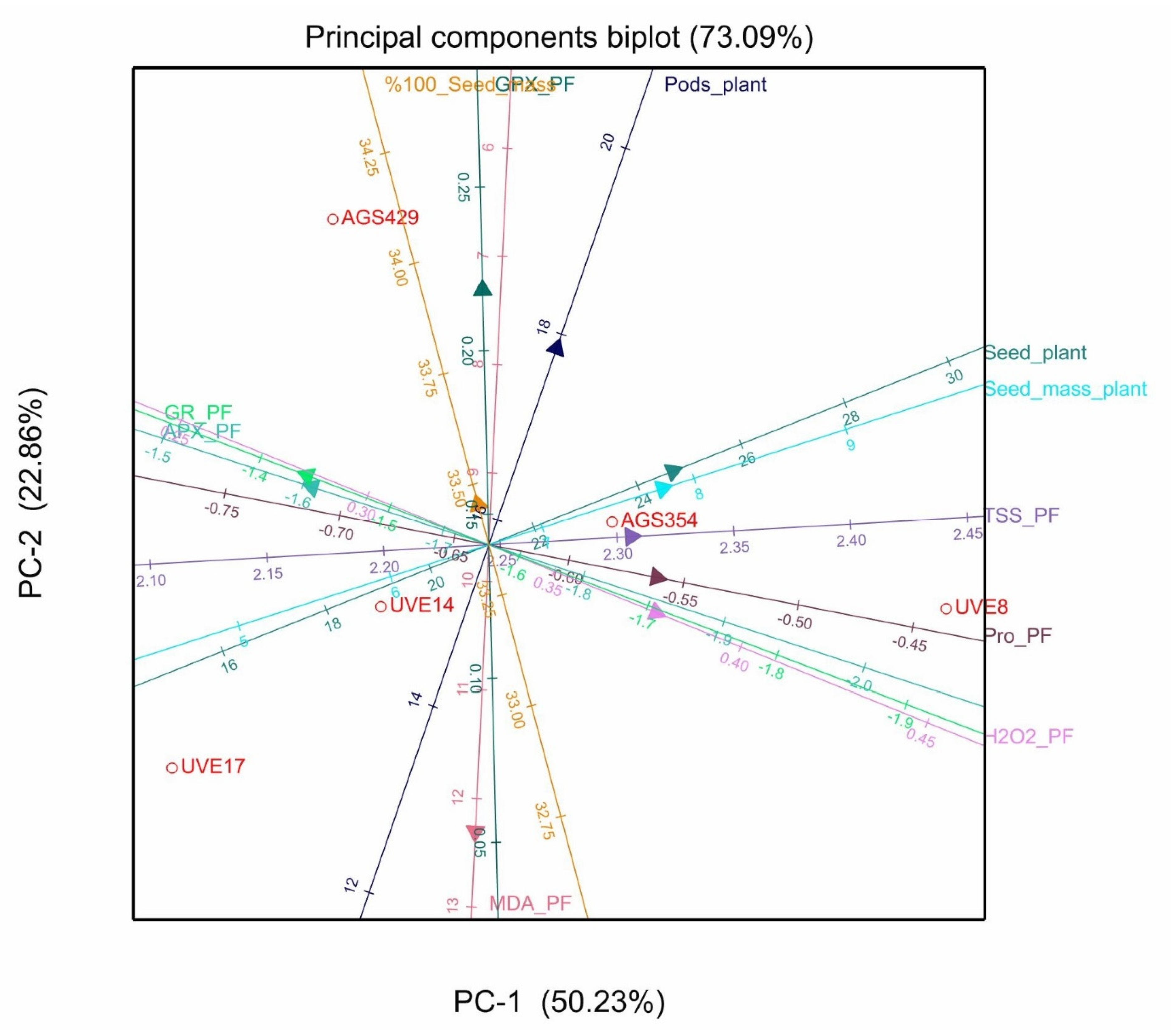
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Design
4.2. Proline Assay
4.3. Total Soluble Sugars Assay
4.4. Lipid Peroxidation
4.5. Hydrogen Peroxide Assay
4.6. Enzyme Extract Preparation
4.7. Enzyme Assays and Protein Content
4.8. Yield Parameters
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanism and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Basal, O.; Szabo, A. Physiology yield and quality of soybean as affected by drought stress. Asian J. Agric. Biol. 2020, 3, 247–253. [Google Scholar] [CrossRef]
- Djanta, M.K.A.; Agoyi1, E.E.; Agbahoungba1, S.; Quenum, F.J.-B.; Chadare, F.J.; Assogbadjo, A.E.; Agbangla, C.; Sinsin, B. Vegetable soybean, edamame: Research, production, utilization and analysis of its adoption in Sub-Saharan Africa. J. Hort. For. 2020, 12, 1–12. [Google Scholar]
- Masuda, T.; Goldsmith, P.D. World Soybean Production: Area harvested, yield, and long-term projections. Int. Food Agribus. Manag. Rev. 2009, 12, 143–162. [Google Scholar]
- Ceccherini, G.; Russo, S.; Ameztoy, I.; Marchese, A.F.; Carmona-Moreno, C. Heat waves in Africa 1981–2015, observations and reanalysis. Nat. Hazards Earth Syst. Sci. 2017, 17, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Demirevska, K.; Zasheva, D.; Dimitrov, R.; Simova-Stoilova, L.; Stamenova, M.; Feller, U. Drought stress effects on Rubisco in wheat: Changes in the Rubisco large subunit. Acta Physiol. Plant 2009, 31, 1129–1138. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, A.S.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Arora, A.; Sairam, R.K.; Sriuastava, G.C. Oxidative stress and antioxidative system in plants. Curr. Sci. 2002, 82, 1227–1238. [Google Scholar]
- Xu, P.L.; Guo, Y.K.; Bai, J.G.; Shang, L.; Wang, X.J. Effects of long-term chilling on the ultrastructure and antioxidative activity in leaves of two cucumber cultivars under low light. Physiol. Plant 2008, 132, 467–478. [Google Scholar] [CrossRef] [Green Version]
- Mwenye, O.J. Root Properties and Proline as Possible Indicators for Drought Tolerance in Soybean. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2018. [Google Scholar]
- Moloi, M.J.; Mwenye, O.J.; van der Merwe, R. Differential involvement of ascorbate and Guaiacol peroxidases in soybean drought resistance. S. Afr. J. Sci. 2016, 112, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Vam, R.P. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Hayat, S.; Hayat, Q.; Alyemeni, M.A.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furlan, A.L.; Bianucci, E.; Giordano, W.; Castro, S.; Becker, D.F. Proline metabolic dynamics and implications in drought tolerance of peanut plants. Plant Physiol. Biochem. 2020, 151, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.K.A.; Giridhar, P. Variations in Physiological Response, Lipid Peroxidation, Antioxidant Enzyme Activities, Proline and Isoflavones Content in Soybean Varieties Subjected to Drought Stress. Proc. Natl. Acad. Sci. USA India Sect. B Biol. Sci. 2015, 85, 35–44. [Google Scholar] [CrossRef]
- Mwenye, O.J.; van Rensburg, L.; van Biljon, A.; van der Merwe, R. The role of proline and root traits on selection for drought-stress tolerance in soybeans: A review. S. Afr. J. Plant Soil 2016, 33, 245–255. [Google Scholar] [CrossRef]
- Van der Merwe, R.; Tyawana, S.; Van der Merwe, J.; Mwenye, O. Evaluation of drought tolerance indices in vegetable-type soybean. Mol. Soc. Cient Gal. 2018, 18, 19–31. [Google Scholar]
- Andrade, E.R.; Ribeiro, V.N.; Azevendo, C.V.G.; Chiorato, A.F.; Williams, T.C.R.; Carbonell, S.A.M. Biochemical indicators of drought tolerance in common bean (Phaseolus vulgaris L.). Euphytica 2016, 210, 277–289. [Google Scholar] [CrossRef]
- Farahat, M.; Ibrahim, M.M.S.; Taha, L.S.; El- Quesni, E.M.F. Response of vegetative growth and some chemical constituents of Cupressuss empervirens L. to foliar application of ascorbic acid and zinc at Nubaria. World J. Agric. Sci. 2007, 3, 282–288. [Google Scholar]
- Cramer, G.R.; Ergul, A.; Grimplet, J.; Tillett, R.L.; Tattersall, E.A.; Bohlman, M.C.; Vincent, D.; Sonderegger, J.; Evans, J.; Osborne, C.; et al. Water and salinity stress in grapevines: Early and late changes in transcript and metabolite profiles. Funct. Integr. Genom. 2007, 7, 111–134. [Google Scholar] [CrossRef]
- Cunhua, S.; Wei, D.; Xiangling, C.; Xinna, X.; Yahong, Z.; Dong, S.; Jianjie, S. The effects of drought stress on the activity of acid phosphatase and its protective enzymes in pigweed leaves. Afr. J. Biotech. 2010, 9, 825–833. [Google Scholar]
- Guler, N.S.; Pehlivan, N. Exogenous Low-Dose Hydrogen Peroxide Enhances Drought Tolerance of Soybean (Glycine Max L.) through Inducing Antioxidant System. Biol. Fut. 2016, 67, 169–183. [Google Scholar]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Brestic, M.; Afrin, S.; Sakil, M.A.; Hossain, M.T.; Hossain, M.A.; Hossain, M.A. Exogenous salicylic acid and hydrogen peroxide attenuate drought stress in rice. Plant Soil Environ. 2020, 66, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.M.; Qian, P.; Xin, W.; Li, H.Y.; Burritt, D.J.; Fujita, M.; Tran, L.S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Selote, D.S.; Khanna-Chopra, R. Drought-induced spikelet sterility is associated with an inefficient antioxidant defense in rice panicles. Plant Physiol. 2004, 121, 462–471. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ünyayar, S.; Çekiç, F.Ö. Changes in antioxidative enzymes of young and mature leaves of tomato seedlings under drought stress. Turk. J. Biol. 2006, 29, 211–216. [Google Scholar]
- Karam, F.; Masaad, R.; Sfeir, T.; Mouzer, O.; Rouphael, Y. Evapotranspiration and seed yield of field grown soybean under deficit irrigation conditions. Agric. Water Manag. 2005, 75, 226–244. [Google Scholar] [CrossRef]
- Dogan, E.; Kirnak, H.; Copur, O. Deficit irrigations during soybean reproductive stages and CROPGRO-soybean simulations under semi-arid climatic conditions. Field Crop Res. 2007, 103, 154–159. [Google Scholar] [CrossRef]
- Westgate, M.E.; Peterson, C.M. Flower and pod development in water-deficient soybean (Glycine max L. Merr.). J. Exp. Bot 1993, 258, 109–117. [Google Scholar] [CrossRef]
- Carill0, P.; Gibon, Y. Protocol: Extraction and Determination of Proline. 2011. Available online: https://www.researchgate.net/publication/211353600_Protocol_Extraction_and_determination_of_proline (accessed on 3 March 2020).
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water stress-induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Pukacka, S.; Ratajczak, E. Production and scavenging of reactive oxygen species in Fagus sylvatica seeds during storage at varied temperature and humidity. J. Plant Physiol. 2005, 162, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.P.; Mishra, R.K.; Singhal, G.S. Changes in the activities of antioxidant enzymes during exposure of intact wheat leaves to strong visible light at different temperatures in the presence of protein synthesis inhibitors. Plant Physiol. 1993, 102, 903–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zieslin, N.; Ben-Zaken, R. Peroxidase, phenylalanine ammonia-lyase and lignification in peduncles of rose flowers. Plant Physiol. Biochem. 1991, 29, 147–151. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- VSN International. Genstat for Windows, 19th ed.; VSN International: Hemel Hempstead, UK, 2017. [Google Scholar]




| Flowering | Pod Filling | |||||
|---|---|---|---|---|---|---|
| Variate | Cultivar (C) | Treatment (T) | C × T | Cultivar (C) | Treatment (T) | C × T |
| APX | 0.000697 *** | 0.000669 *** | 0.000429 *** | 0.111300 *** | 0.2875 *** | 0.146200 *** |
| GPX | 0.174360 *** | 0.026590 | 0.102130 *** | 0.114200 *** | 0.0447 *** | 0.009780 *** |
| GR | 0.193300 *** | 0.063000 | 0.127300 *** | 0.331300 *** | 0.000004 | 0.041500 * |
| H2O2 | 4.675600 *** | 1.548600 ** | 0.075900 | 0.051778 *** | 0.082577 *** | 0.006332 |
| MDA | 0.068490 *** | 0.053066 ** | 0.002256 | 15.70900 ** | 53.027 *** | 2.737000 |
| Proline | 0.025750 | 0.006220 | 0.102680 | 0.191190 *** | 0.1627 *** | 0.017870 |
| TSS | 1.768700 *** | 2.189700 *** | 0.188200 * | 0.256100 | 5.6472 *** | 0.106800 |
| Flowering | Pod Filling | |||||||
|---|---|---|---|---|---|---|---|---|
| 100 SM | TPP | TSMP | TSP | 100 SM | TPP | TSMP | TSP | |
| APX | −0.2215 | 0.4857 | −0.1685 | −0.0100 | 0.2132 | 0.1394 | −0.5640 * | −0.5636 * |
| GPX | −0.2366 | 0.4414 | −0.2385 | −0.0793 | 0.1640 | 0.2178 | −0.0132 | 0.0906 |
| GR | −0.3062 | 0.2359 | −0.4766 | −0.4130 | 0.0942 | −0.0336 | −0.6558 ** | −0.6194 * |
| H2O2 | −0.0122 | 0.4852 | 0.7846 ** | 0.6993 ** | −0.0812 | 0.0417 | 0.4724 | 0.5615 * |
| MDA | 0.3020 | 0.3745 | 0.7146 | 0.4747 | −0.1244 | 0.0167 | 0.0489 | 0.1078 |
| Proline | −0.3315 | −0.2202 | 0.0749 | 0.2004 | 0.0111 | 0.2079 | 0.4955 | 0.6217 * |
| TSS | 0.0541 | 0.515 * | 0.4012 | 0.3894 | 0.2382 | 0.4506 | 0.4900 | 0.4846 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moloi, M.J.; van der Merwe, R. Drought Tolerance Responses in Vegetable-Type Soybean Involve a Network of Biochemical Mechanisms at Flowering and Pod-Filling Stages. Plants 2021, 10, 1502. https://doi.org/10.3390/plants10081502
Moloi MJ, van der Merwe R. Drought Tolerance Responses in Vegetable-Type Soybean Involve a Network of Biochemical Mechanisms at Flowering and Pod-Filling Stages. Plants. 2021; 10(8):1502. https://doi.org/10.3390/plants10081502
Chicago/Turabian StyleMoloi, Makoena Joyce, and Rouxlene van der Merwe. 2021. "Drought Tolerance Responses in Vegetable-Type Soybean Involve a Network of Biochemical Mechanisms at Flowering and Pod-Filling Stages" Plants 10, no. 8: 1502. https://doi.org/10.3390/plants10081502






